Abstract
Background
Cytokeratin 19, and its soluble fragment CYFRA, have been studied as markers that may be associated with response to therapy and survival in NSCLC. As a prospective correlative study of CALGB 30203, a randomized phase II trial of carboplatin/gemcitabine with eicosanoid modulators (celecoxib, zileuton or both) in advanced NSCLC, serum CYFRA levels were obtained prior to and during treatment.
Patients and Methods
Serum CYFRA levels were measured at baseline and after the first cycle of treatment using an electrochemoluminescent assay. Paired specimens were available from 88 patients. The logarithm of the initial concentration and the logarithm of the difference in concentrations, were analyzed for association with overall survival (OS) and failure free survival (FFS).
Results
Lower baseline CYFRA levels were associated with both longer overall survival and failure free survival (p<0.0001 and p=0.0003). In addition, larger reductions in CYFRA levels correlated with longer overall survival and failure-free survival (p=0.0255 and p=0.0068).
Conclusion
CYFRA and change in CYFRA were found to be reliable markers for response to chemotherapy for NSCLC; however, a precise threshold to mark response has yet to be determined.
Keywords: lung cancer, CYFRA
Background
Biologic markers have become essential in guiding treatment of many cancers, such as prostate and ovarian cancer. Identification of these markers saves time, money, and radiation exposure. Various markers such as carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), tissue polypeptide specific antigen (TPS), squamous cell carcinoma antigen (SCC) and cancer antigen 125 (CA125) have been studied in terms of their prognostic or predictive implications in lung cancer or as a method of assessing response to therapy.1,2 However, to date, no serum marker is currently recommended for routine clinical practice in NSCLC. One of the most promising markers for NSCLC is the soluble fragment of cytokeratin 19. Simple epithelium, such as bronchial epithelium, is composed of intermediate filaments that give the cell its structure and strength. In malignant tissues, the intermediate filament known as cytokeratin 19 and the C-terminus of cytokeratin 19 (CYFRA 21-1,CYFRA) are released into circulation by a cleaving enzyme, caspase-3, and apoptosis.3 For almost two decades, research has evaluated whether the serum levels of these filaments may relate to prognosis. In 2003, Vollmer et al. summarized the research done before 1999 and reported a trial completed at four CALGB institutions evaluating the levels of CYFRA in 58 patients with stage III and IV NSCLC treated with chemotherapy.4 In this study, higher initial CYFRA concentrations predicted a worse prognosis and that the ratio of logarithm of CYFRA before and after one cycle of chemotherapy correlated with prognosis. Both the initial natural logarithm of serum CYFRA and presence of >27% drop in CYFRA were significantly related to subsequent survival. As part of a prospective CALGB study evaluating chemotherapy and eicosanoid modulation in advanced NSCLC (CALGB 30203), we sought to confirm these findings. The evaluation of CYFRA, including the statistical objectives, were prospectively defined in the protocol.
Patients and Methods
CALGB 30203 tested the concept of eicosanoid inhibition in advanced lung cancer and has been previously reported.5 The hypothesis was that eicosanoid inhibition in addition to standard chemotherapy would potentially increase progression free survival. Furthermore, the concept of single vs. double pathway inhibition was tested with inhibitors of COX-2 and 5-LOX as both single agents and in combination. Patients with advanced NSClC (stage IIIb (pleural effusion)/IV) with performance status 0-2, and normal organ function were randomized to receive chemotherapy (carboplatin AUC =5.5, day 1 and gemcitabine 1000 mg/m2 day 1,8) with one of three eicosanoid modulating regimens:zileuton 600 mg qid, celecoxib 400 mg bid, or both agents. Each participant signed an IRB-approved, protocol specific informed consent in accordance with Federal and institutional guidelines.
To evaluate CYFRA levels, blood was collected in a 7 ml red top tube, inverted 5 times, left at room temperature to clot for 30 minutes, and then spun at 1100-1330 g for 10 minutes in a swinging head rotor at 25 C. Serum was then removed and placed in a polypropylene tube and frozen to −20 C or colder. Shipment to the CALGB Pathology Coordinating Office was done on dry ice. Analysis of CYFRA levels was conducted on specimens at first thaw. CYFRA levels in the serum were measured by using two monoclonal antibodies to sandwich the molecule, KS 19.1 and BM 19.21. One antibody was labeled with a Ruthenium complex, which is electrochemically luminescent, and the other with a magnetic particle. When the electric potential is applied to the molecules, light is produced and measured by a photomultiplier. The coefficient of variation is 2-5%.2 Samples were analyzed at the University of Maryland by Dr. Christenson in a CLIA approved laboratory without knowledge of patient characteristics or outcomes.
Patient registration and clinical data were managed by the CALGB Statistical Center. The statistical analysis was performed at the CALGB Statistical Center. The balance of demographic and clinical variables across study arms were tested by Chi square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. Kaplan Meier curves were used to characterize overall survival (OS) and failure free survival (FFS), in which OS was defined as the time from study entry to the date of death resulting from any cause, and FFS was defined as the time from study entry to the date of disease progression or death, whichever came first. Finally, Cox regression analysis was used to assess the association of baseline CYFRA and the change of cycle-1 CYFRA relative to the baseline with survival endpoints. The logarithmic concentration values of CYFRA were used as previous studies show them to have association with outcome, and the logarithmic transformation also serves to reduce the influence of extreme CYFRA values. As the decrease of cycle-1 CYFRA value relative to its baseline is a post-treatment covariate, the survival endpoints (OS and FFS) for this analysis are redefined by starting time from the end date of cycle-1 chemotherapy.
Results
CALGB 30203 enrolled 140 patients in under one year and showed no difference in overall survival or failure free survival between the three arms. Adequate serum samples prior to therapy and after the first cycle were obtained for 88 of the 140 patients (63%). Table 1 shows patient characteristics of those patients in CALGB 30203 who had CYFRA concentrations analyzed. There were no significant differences between the arms and the population for which samples were obtained for this analysis was comparable to the entire study population.
Table 1. Baseline Patient Characteristic of the 88 patients with serum CYFRA levels analyzed.
| Characteristics | CYFRA decrease =<27% (N=47) |
CYFRA decrease >27% (N=41) |
Total (N=88) |
|---|---|---|---|
| Gender: | |||
| Male | 26( 55%) | 28( 68%) | 54( 61%) |
| Female | 21( 45%) | 13 ( 32%) | 34( 39%) |
| Age: | |||
| <60 | 19( 40%) | 21( 51%) | 40( 46%) |
| 60-69 | 16( 34%) | 14(34%) | 30( 34%) |
| >=70 | 12( 26%) | 6 (15%) | 18( 21%) |
| median (min, max) | 61(41,80) | 59(49,81) | 60(41,81) |
| Race: | |||
| White | 38( 81%) | 37( 90%) | 75( 85%) |
| Black or other | 9( 19%) | 4( 10%) | 13( 15%) |
| Histology: | |||
| AdenoCA | 25( 53%) | 19( 46%) | 44( 50%) |
| Squamous | 12( 26%) | 9( 22%) | 21( 24%) |
| Undifferentiated | 10( 21%) | 13( 32%) | 23( 26%) |
| Performance status: | |||
| 0 | 14( 30%) | 13( 32%) | 27( 31%) |
| 1 or 2 | 33( 70%) | 28( 68%) | 61( 69%) |
| Stage | |||
| IIIB | 5( 11%) | 2( 5%) | 7( 8%) |
| IV | 39( 83%) | 37(90%) | 76(86%) |
| Recurrent | 3( 6%) | 2( 5%) | 5( 6%) |
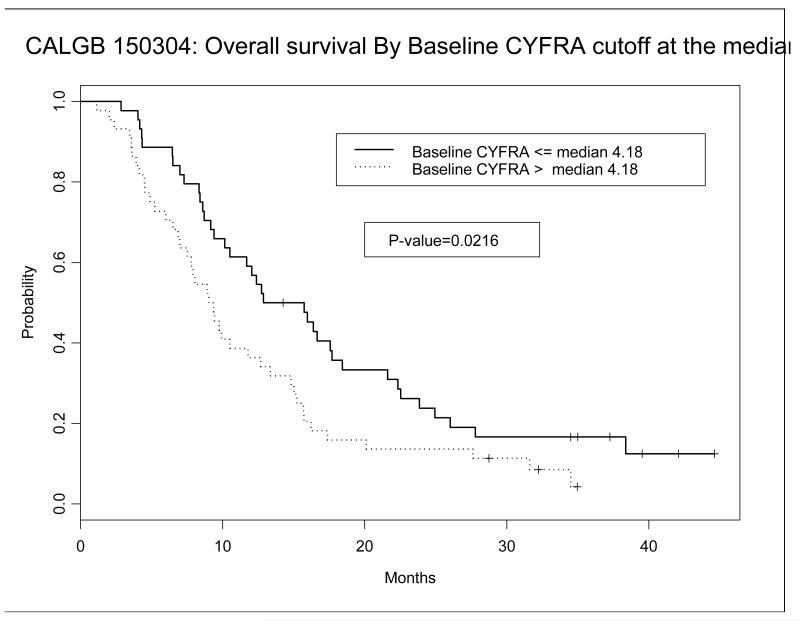
Table 2 shows the median, mean, minimum, and maximum CYFRA levels in all three arms at baseline and after cycle 1 of chemotherapy. Baseline CYFRA levels ranged from 0.44 to 204.2 ng/ml with a median CYFRA level of 4.18 ng/ml and a mean level of 12.9 ng/ml. A Kaplan Meier survival plot was constructed comparing those with initial CYFRA levels above and below the median. As shown in Figure 1, patients with initial CYFRA levels below the median had a statistically significant increase in their overall survival (p=0.0216). After log transformation, higher baseline CYFRA correlated with worse overall survival and failure free survival (p<0.0001 and p=0.003), Table 3. After cycle 1, the median CYFRA level remained steady at 4.3 but the mean CYFRA level fell to 7.1, (Wilcoxon signed test, p=0.0225, Table 2). After logarithmic transformation, a greater reduction in CYFRA from baseline to after cycle 1 correlated with longer overall and failure free survival (p=0.0255 and p=0.0068) in the multivariate analysis after adjusting for age and baseline CYFRA (Model 1, Table 3). We also confirmed the prognostic value of a greater than 27% decline in CYFRA being associated with better overall survival or failure free survival (p=0.0028 and p=0.0074), Table 3.
Table 2. CYFRA concentrations (ng/ml).
|
Median, Mean
(minimum, maximum) | |||
|---|---|---|---|
| CYFRA decrease= <27% (N=47) |
CYFRA decrease >27% (N=41) |
Total (N=88) |
|
| CYFRA Baseline | 3.7, 12.7 (0.72, 204.2) |
4.7, 13.2 (0.44, 87.0) |
4.2, 12.9 (0.44, 204.2) |
| Log CYFRA Baseline | 1.3, 1.5 (−0.33, 5.3) |
1.5, 1.6 (−0.82, 4.5) |
1.4, 1.6 (−0.82, 5.3) |
| CYFRA cycle 1 | 5.5, 10.4 (0.51, 54.4) |
1.7, 3.2 (0.66, 13.6) |
4.3, 7.1 (0.51, 54.4) |
| Log CYFRA cycle 1 | 1.7, 1.8 (−0.67, 4.0) |
0.51, 0.83 (−0.42, 2.6) |
1.5, 1.4 (−0.67, 4.0) |
| CYFRA Baseline− CYFRA cycle1 |
−1.0, 2.2 (−28.9, 154.4) |
2.4, 10.0 (−0.87, 78.8) |
0.53, 5.9 (−28.9, 154.4) |
| Log(CYFRA Baseline)− log(CYFRA cycle1) |
−0.30, −0.29 (−1.8, 1.4) |
0.70, 0.77 (−0.89, 2.6) |
0.21, 0.20 (−1.8, 2.6) |
Figure 1. Kaplan Meier Survival Curve based on baseline CYFRA above and below the median value.
Table 3. Multivariate Survival Analysis on CYFRA concentrations.
| Overall Survival (OS)# | Failure-free Survival (FFS) # | |||
|---|---|---|---|---|
| Parameter | P-value | Hazard Ratio 95% CI |
P- value |
Hazard Ratio 95% CI |
| Model 1* | ||||
| Log (baseline CYRFA) | <0.0001 | 1.68 (1.33, 2.11) | 0.0003 | 1.43 (1.23, 2.04) |
| Log (baseline CYFRA) - Log (CYFRA cycle 1) |
0.0255 | 0.73 ( 0.56, 0.96) | 0.0068 | 0.66 (0.49, 0.89) |
| Age (>65 vs. ≤65) | 0.0176 | 1.81 ( 1.11, 2.95) | 0.2360 | 1.33 (0.83, 2.15) |
| Model 2+ | ||||
| Log (baseline CYRFA) | <0.0001 | 1.55 (1.27, 1.90) | 0.0023 | 1.34 (1.10, 1.62) |
| 27% or greater decline in CYRFA from baseline to cycle 1 (yes vs. no) |
0.0028 | 0.49 (0.31, 0.78) | 0.0074 | 0.52 (0.32, 0.84) |
| Age (>65 vs. ≤65) | 0.0152 | 1.83 (1.12, 2.98) | 0.2407 | 1.33 (0.83, 2.14) |
The decrease of cycle-1 CYFFA relative to baseline is a post-treatment covariate, in this analysis, the survival endpoints, OS and FFS, were redefined to have time start at the end of cycle-1 chemotherapy.
Model 1 is the final model of Cox proportional hazards regression analysis. Log baseline CYFRA and Log (baseline CYFRA) - Log (CYFRA cycle 1) were forced into the final model for overall survival with Performance Status, Age (>65 vs <65), Treatment Arm, Sex, Race and Histology as potential variables to be selected using stepwise algorithm with entry level of 0.10 and stay level of 0.10. The model for failure-free survival is chosen to be the same as for overall survival.
Model 2 is the final model chosen by similar variable selection procedure to Model 1. The predictor “27% or greater decline in log CYRFA from baseline to cycle 1” is a binary variable with 1 denoting 27% or greater decline on logarithmic CYRFA from baseline to cycle 1. Vollmer et al. (2003) used a 27% or greater decline on raw CYRFA scale, but our analysis on their definition yields a less significant association for overall survival (p=0.1002) and failure-free survival (p=0.1797).
This decline occurred in 41 of the 88 patients tested. Our analysis also confirmed that a greater than 27% decline on a logarithmic scale is also the optimal cutoff point that yields the largest separation between the high vs. low CYFRA decline patients, Table 3. There were no statistically significant differences in CYFRA levels at cycle 1 (Wilcoxon rank sum test, p=0.3654) or changes from baseline to cycle 1 (Wilcox ran sum test, p=0.7791) related to the type of eicosanoid modulator employed. There was no relationship between a 27% decline in CYFRA and response (p = .114).
Multivariate analysis including age, sex, performance status and staging (IIIb vs. IV) was performed. Only age emerged as a significant factor. Data regarding smoking status was not collected. Logistic regression analysis (OR=1.62, 95% CI 1.09-2.40;, p-value = 0.0161) indicated that squamous patients had higher baseline CYFRA levels. However, there was no correlation between changes of CYFRA and histology (OR=0.97, p-value =0.9068). In addition, no correlation was noted between baseline CYFRA and sex ( p =0.1146), age ( p=0.0635), race ( p=0.1088), performance status(p=0.1549), histology(p= 0.1512), stage IIIb vs. IV (p=0.0765)
Discussion
This study prospectively confirms in a multicenter trial, that serum concentrations of CYFRA have prognostic value in advanced NSCLC. Higher baseline CYFRA concentrations portend worse overall and failure free survival. Additionally, this trial confirmed the significance of the cut point of a 27% reduction in log CYFRA after chemotherapy is of value in determining benefit from treatment. Other cutpoints, including 10%-75% decline, worked almost equally well (data not shown).
This information may be useful in determining whether to continue a particular chemotherapy regimen. If confirmed, this would provide a simple and inexpensive approach to assessment of response to treatment.
Of note, these findings are qualitatively similar to those of others as summarized by Vollmer (studies prior to 1999) and in Table 4 (studies after 1999).4 Prior studies have found that CYFRA elevations have correlated with stage and predicted for recurrent disease after surgery as well as for inferior survival. The significance of the current trial is that patients were part of a prospective multicenter trial employing standard entry criteria and a uniform chemotherapy regimen.
Table 4. Recent Studies of CYFRA in NSCLC from 1999-2009.
| Study | Stage | Findings |
|---|---|---|
| Localized Disease | ||
| Yeh 20027 | Stage I - IIIa | Elevations in CYFRA after surgery predicted reoccurrence |
| Muley 20038 | Stage I | In post-operative stage I patients, 3-year survival was statistically shorter with elevated CYFRA 21-1 levels above 3.3ng/ml. Consideration given for these patients to receive adjuvant chemotherapy |
| Suzuki 20079 | Stage I | Elevated CYFRA 21-1 levels in early-stage operative NSCLC predicts poor outcome and should be evaluated for possible chemotherapy |
| Advanced | ||
| Vollmer2 2003 | Stage IIIb/IV | Initial CYFRA level gives more prognostic information than stage. A drop of 27% after one cycle of chemotherapy improves prognosis |
| Barlesi 200410 | Stage IIIB/IV | CYFRA levels greater than or equal to 3.5 ng/ml correlated to poorer prognosis. CYFRA combined with CEA (carcino-embryonic antigen) and NSE (neuron specific enolase) correlated with more accurate prognosis. |
| Merle 200411 | Stage IIIB/IV | A drop of 80% in CYFRA after one cycle of chemotherapy was the most predictive of overall survival when compared with initial staging, tumor response, and surgery. |
| Ardizzoni 20063 | Advanced | Patients with CYFRA declines of 20% or more after 2 cycles had increased median survival of 5 months (6 months vs. 11 months). |
| Holdenreider 200612 | Advanced | Slower and incomplete decline in CYFRA predicted poorer outcome. |
| Nisman 200813 | Advanced | Declines of CYFRA 21-1 levels of 35% or more after 2 cycles of chemotherapy was a reliable marker for treatment efficacy and survival |
| Any Stage | ||
| Karnak 200114 | Any | CYFRA levels had a sensitivity of 65% for NSCLC, 71% for squamous cell, and 46% for adenocarcinoma. Sensitivity ranged from Stage I at 38% to Stage III at 87.5%. Specificity for all stages was 92%. |
| Kulpa 200215 | Any | CYFRA was significantly higher in advanced than early stage disease and an independent prognostic marker in early disease. |
| Hatzakis 200216 | Any | CYFRA and NSE are the most useful markers in differentiating cell type. When measured at diagnosis, CYFRA may provide prognostic information |
| Lee, 200517 | Any | In diagnosing malignant pleural effusions, CEA was the most prognostic tumor marker. CYFRA in pleural fluid was 61% sensitive and 81% specific. |
| Buccheri 200318 | Any | Use of serum cytokine markers before, during, and after treatment should be completed to access status of disease and response to treatment. There is no preference between CYFRA and tissue polypeptide antigen (TPA) |
| Hillas 200819 | Any | CYFRA levels of Induced sputum samples had a 86% sensitivity, 75% specificity, 88% positive predictive value and 72 % negative predictive value for cancer diagnosis |
There are several limitations to the current study. The number of patients studied was relatively small. In addition, as all arms utilized eicosanoid modulation, the chemotherapy regimens could be considered “non-standard”. However, all patients received standard, platinum based two drug chemotherapy. Additionally, both of the experimental agents studied, celecoxib and/or zileuton, are drugs that are commercially available and either they (or similar drugs) are commonly prescribed for patients with lung cancer. Another potentially confounding factor is a possible difference in “bulk” of disease, which was not clearly captured in the required data and for which there is no standardized approach. It is quite possible that CYFRA is a non-specific marker for tumor burden. Furthermore, this study did not evaluate nor compare CYFRA to other possible serum markers that have been utilized, such as carcinoembryonic antigen.6
In the current landscape of markers for NSCLC, CYFRA is unlikely to have the strong prognostic or predictive value of EGFR activating mutations or EML4/ALK translocations. However, it may ultimately find a role as an early marker of tumor responsiveness to therapy.
In summary, this study demonstrates the potential value of CYFRA 21-1 as both a prognostic marker in advanced NSCLC as well as an early indicator of response to chemotherapy. Further studies are warranted to compare the value of CYFRA to radiologic imaging in determining response.
Acknowledgements
The research for CALGB 15034 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, M.D., Chair) and to the CALGB Statistical Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
University of Oklahoma, Oklahoma, OK-Shubham Pant, M.D., supported by CA37447
Christiana Care Health Services, Inc. CCOP, Wilmington, DE-Stephen Grubbs, M.D., supported by CA45418
Duke University Medical Center, Durham, NC-Jeffrey Crawford, M.D., supported by CA47577
Georgetown University Medical Center, Washington, DC-Minetta C Liu, M.D., supported by CA77597
Cancer Centers of the Carolinas, Greenville, SC-Jeffrey K. Giguere, M.D, supported by CA29165
Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY-Jeffrey Kirshner, M.D., supported by CA45389
Nevada Cancer Research Foundation CCOP, Las Vegas, NV-John A. Ellerton, M.D., supported by CA35421
New Hampshire Oncology-Hematology PA, Concord, NH - Douglas J. Weckstein Rhode Island Hospital, Providence, RI-William Sikov, M.D., supported by CA08025 Roswell Park Cancer Institute, Buffalo, NY-Ellis Levine, M.D., supported by CA59518 Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC-James N. Atkins, M.D., supported by CA45808
State University of New York Upstate Medical University, Syracuse, NY-Stephen L. Graziano, M.D., supported by CA21060
University of California at San Diego, San Diego, CA-Barbara A. Parker, M.D., supported by CA11789
University of California at San Francisco, San Francisco, CA-Charles J Ryan, M.D., supported by CA60138
University of Chicago, Chicago, IL-Hedy L Kindler, M.D., supported by CA41287
University of Iowa, Iowa City, IA-Daniel A. Vaena, M.D., supported by CA47642
University of Maryland Greenebaum Cancer Center, Baltimore, MD-Martin Edelman, M.D., supported by CA31983
University of Minnesota, Minneapolis, MN-Bruce A Peterson, M.D., supported by CA16450
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO-Michael C Perry, M.D., supported by CA12046
University of North Carolina at Chapel Hill, Chapel Hill, NC-Thomas C. Shea, M.D., supported by CA47559
University of Texas Southwestern Medical Center, Dallas, TX-Debasish Tripathy, M.D.
University of Vermont, Burlington, VT-Steven M Grunberg, M.D., supported by CA77406
Washington University School of Medicine, St. Louis, MO-Nancy Bartlett, M.D., supported by CA77440
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nieder C, Andratschke N, Jeremic B, Molls M. Comparison of serum growth factors and tumor markers as prognostic factors for survival in non-small cell lung cancer. Anticancer Res. 2003 Nov-Dec;23(6D):5117–23. [PubMed] [Google Scholar]
- 2.Ardizzoni A, Cafferata M, Tiseo M, et al. Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer. Cancer. 2006;107:2842–2849. doi: 10.1002/cncr.22330. [DOI] [PubMed] [Google Scholar]
- 3.Kosacka M, Jankowska R. Comparison of cytokeratin 19 expression in tumor cells and serum CYFRA 21-1 levels in nonsmall cell lung cancer. Pol Arch Med. 2009;119:33–38. [PubMed] [Google Scholar]
- 4.Vollmer RT, Govindan R, Graziano SL, et al. Serum CYFRA 21-1 in advanced stage non-small cell lung cancer: an early measure of response. Clin Cancer Res. 2003 May;9(5):1728–33. [PubMed] [Google Scholar]
- 5.Edelman MJ, Watson D, Xiaofei W, Morrison C, Kratzke RA, Jewell S, Hodgson L, Mauer AM, Gajra A, Masters GA, Bedor M, Green MJ, Vokes EE. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–55. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 6.Cedrés S, Nuñez I, Longo M, Martinez P, Checa E, Torrejón D. Felip E Serum Tumor Markers CEA, CYFRA21-1, and CA-125 Are Associated With Worse Prognosis In Advanced Non-Small-Cell Lung Cancer (NSCLC) Clin Lung Cancer. 2011 May;12(3):172–9. doi: 10.1016/j.cllc.2011.03.019. Epub 2011 Apr 24. [DOI] [PubMed] [Google Scholar]
- 7.Yeh JJ, Liu FY, Hsu WH, et al. Monitoring cytokeratin fragment 19 (CYFRA 21-1) serum levels for early prediction of recurrence of adenocarcinoma and squamous cell carcinoma in the lung after surgical resection. Lung. 2002;180(5):273–9. doi: 10.1007/s004080000101. [DOI] [PubMed] [Google Scholar]
- 8.Muley T, Dienemann H, Ebert W. Increased CYFRA 21-1 and CEA levels are negative predictors of outcome in p-stage 1 NSCLC. Anticancer Res. 2003;23(5b):4085–93. [PubMed] [Google Scholar]
- 9.Suzuki H, Ishikawa S, Satoh H, et al. Preoperative CYFRA 21-1 levels as a prognostic factor in c-stage I non-small cell lung cancer. European Journal of Cardio-thoracic Surgery. 2007;32:648–652. doi: 10.1016/j.ejcts.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Barlesi F, Gimenez C, Torre JP, et al. Prognostic value of combination of CYFRA 21-1, CEA and NSE in patients with advanced non-small cell lung cancer. Respir Med. 2004;98(4):357–62. doi: 10.1016/j.rmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Merle P, Janicot H, Filaire M, et al. Early CYFRA 21-1 variation predicts tumor response to chemotherapy and survival in locally advanced non-small cell lung cancer patients. Int J Biol Markers. 2004;19(4):310–315. doi: 10.1177/172460080401900409. [DOI] [PubMed] [Google Scholar]
- 12.Holdenrieder S, Steiber P, Von Pawel J, et al. Early and specific prediction of the therapeutic efficay in non-small cell lung cancer patients by nucleosomal DNA and cytokeratin 19 fragments. Ann N Y Acad Sci. 2006;1075:244–57. doi: 10.1196/annals.1368.033. [DOI] [PubMed] [Google Scholar]
- 13.Nisman B, Biran H, Heching N, et al. Prognostic role of serum cytokeratin 19 fragments in advanced non-small-cell lung cancer: association of marker changes after two chemotherapy cycles with different measures of clinical response and survival. BJC. 2008;98:77–79. doi: 10.1038/sj.bjc.6604157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnak D, Ulubay G, Kayacan O, et al. Evaluation of CYFRA 21-1: A Potential Tumor Marker for Non-Small Cell Lung Carcinomas. Lung. 2001;179:57–65. doi: 10.1007/s004080000047. [DOI] [PubMed] [Google Scholar]
- 15.Kulpa J, Wojcik E, Reinfuss M, et al. Carcinoembryonic Antigen, Squamous Cell Carcinoma Antigen, CYFRA 21-1, and Neuron-specific Enolase in Squamous cell lung Cancer patients. Clinical Chemistry. 2002;48:1931–37. [PubMed] [Google Scholar]
- 16.Hatzaski K, Froudarakis M, Bouros D, et al. Prognostic Value of Serum Tumor Markers in Patients with Lung Cancer. Respiration. 2002;69:25–29. doi: 10.1159/000049366. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Chang J. Diagnostic Utility of Serum and Pleural Fluid Carcinoembryonic Antigen, Neuron-Specific Enolase, and Cytokeratin 19 Fragments in Patients With Effusions From Primary Lung Cancer. Chest. 2005;128:2298–2303. doi: 10.1378/chest.128.4.2298. [DOI] [PubMed] [Google Scholar]
- 18.Buccheri G, Torchio P, Ferrigno D. Markers in non-small cell lung cancer - clinical equivalence of two cytokeratin. Chest. 2003;124:622–632. doi: 10.1378/chest.124.2.622. [DOI] [PubMed] [Google Scholar]
- 19.Hillas G, Moschosi C, Dimakoul K, et al. Carcinoembryonic antigen, neuron-specific enolase and cytokeratin fragment 19(CYFRA 21-1) levels in induced sputum of lung cancer patients. The Scandinavian Journal of Clinical & Laboratory Investigation. 2007;68:542–547. doi: 10.1080/00365510701883172. [DOI] [PubMed] [Google Scholar]