Abstract
Adult neurogenesis mainly occurs at the subventricular zone (SVZ) on the walls of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG). However, the majority of newborn neurons undergo programmed cell death (PCD) during the period of proliferation, migration, and integration. Stroke activates neural stem cells (NSCs) in both SVZ and SGZ. This process is regulated by a wide variety of signaling pathways. However, the newborn neurons derived from adult neurogenesis are insufficient for tissue repair and function recovery. Thus, enhancing the endogenous neurogenesis driven by ischemia and promoting the survival of newborn neurons can be promising therapeutic interventions for stroke. Here, we present an overview of the process of adult neurogenesis and the potential of stroke-induced neurogenesis on brain repair.
1. Introduction
Stroke is one of the leading causes of morbidity and mortality worldwide. In addition, about two-thirds patients had neurologic impairment and disability, based on a population study of follow-up of stroke survivors at five years [1]. Therefore, poststroke rehabilitation becomes a major therapeutic focus for most poststroke patients. Unfortunately, the currently available therapies are only rarely successful in improving recovery from neurological deficits. It is well established that de novo neurogenesis mainly occurs at two distinct regions in the adult brain: the SGZ of the dentate gyrus of the hippocampus and the SVZ adjacent to the lateral ventricle [2, 3]. In pathological conditions such as stroke, increased neurogenesis has been reported in adult animal models and even in stroke patients [4]. The proliferated neural progenitor cells migrate to the injured striatum and cortex; however, most of them failed to survive and rewire the brain. Taking advantage of the neurogenic capacity of the brain and improving the survival of endogenous neuroprogenitor cells shed light on the restorative therapies for stroke and other brain insults. Here, we review adult neurogenesis from a comprehensive perspective and summarize the current status of research on neurogenesis in poststroke therapy.
2. Adult Neurogenesis
Adult neurogenesis (AN) is a process that is continuously producing new neurons which integrate into existing circuits in adult age and have different mechanisms compared with fetal and early postnatal development [5]. AN was first demonstrated by Altman and Das in a rat brain in 1965 [6]. They injected thymidine-H3 into adult rats and cats to tag the newborn cells and found that the labeled glia cells and neurons are present in various regions of the normal adult mammalian brain. In the 1990s, bromodeoxyuridine (BrdU) was applied to label newborn cells in neurogenesis research. With the application of this new technique, two areas of the adult neurogenesis were found: the DG and the SVZ. In the DG, new neurons continue to be generated from NSCs in the SGZ. NSCs also reside and proliferate in the SVZ and differentiate into neuroblasts. These neuroblasts migrate through the rostral migratory stream (RMS) to the olfactory bulb (OB) and integrate into OB circuits. Recently, some noncanonical sites of adult neurogenesis, such as neocortex, striatum, corpus callosum, amygdala, and hypothalamus, have been found in different species [7].
The process of maturation of new neurons encompasses the proliferation of resident NSCs and their subsequent differentiation, migration, survival, and functional integration into the preexisting circuitry [8]. AN is mediated by a series of physiological and pathological processes at all these stages. Moreover, programmed cell death (PCD) plays critical roles in regulating the process from NSC proliferation to the integration of neural circuits. We focus on current knowledge of the main neurogenic sites (SVZ and SGZ) of AN with their specificities and address the potential roles of PCD as a regulatory strategy.
2.1. AN in SVZ and SGZ
2.1.1. SVZ
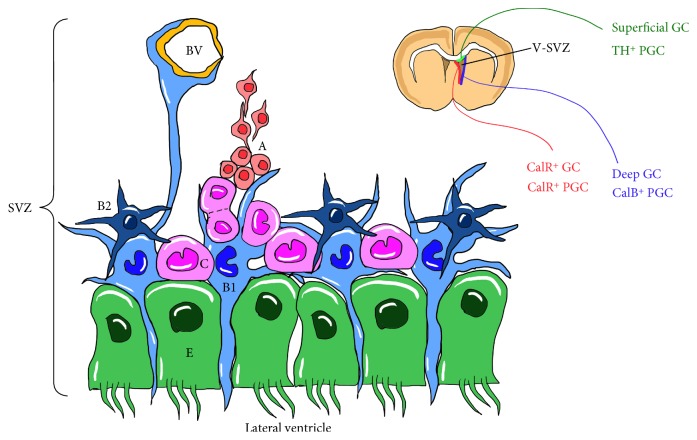
In mammalian animals, new OB neurons are derived from SVZ, on the walls of the lateral ventricles. The SVZ have five main cell types: B1 astrocytes (type B1 cells), B2 astrocytes (type B2 cells), transit-amplifying cells (type C cells), neuroblasts (type A cells), and ependymal cells (type E cells) (Figure 1). Microglia and oligodendrocyte precursor cells (OPCs) also reside in the SVZ. Type B2 cells and ependymal cells are important for maintaining and regulating the niche of SVZ. Type B1 cells lie atop ependymal cells and extend their processes further to blood capillary [9]. Besides, most B1 astrocytes contact the ventricle by extending a thin cellular process between ependymal cells [2]. Type C cells are shaped like a smooth ellipse and have large nuclei with deep invaginations [2]. Type A cells have an elongated cell body with smooth contours. They have one or two processes and join to other type A cells by small junctional complexes. Nestin, SRY-box 2 (Sox2), and brain lipid-binding protein (BLBP) have been considered as NSC markers. Distal-less homeobox 2 (DLX2), epidermal growth factor receptor (EGFR), and mammalian achaete-scute homolog 1 (MASH1) are mainly expressed on type C cells [10]. Doublecortin (DCX), β-III-tubulin (TuJ1), and polysialylated neural cell adhesion molecule (PSA-NCAM) are the unique markers of type A cells [2].
Figure 1.
Neurogenesis in SVZ. The SVZ is shown in the left. Type B1 cells (B1, blue) lie atop ependymal cells (E, green) and extend their processes to blood capillary (BV). Type B1 cells divide to produce type C cells (C, pink). Type C cells then give rise to type A cells (A, red). Type B2 cells (B2, dark blue) also reside in the SVZ. The coronal section in the upper right is shown the diversity of newborn OB interneurons. Deep GCs and CalB+ PGCs are derived from ventral NSCs, whereas superficial GCs and TH+ PGCs are derived dorsal NSCs. NSCs from the medial wall produce CalR+ GCs and CalR+ PGCs.
NSCs in the SVZ correspond to type B1 cells. Asymmetric division of type B1 cells produce self-renewed type B1 cells and type C cells [11, 12], which symmetrically divide into type A cells [13]. After birth in SVZ, type A cells form elongated, chain-like aggregates, which are ensheathed by astrocytes [14–16]. These neuroblasts migrate through RMS at the anterior SVZ [17]. The migration of neuroblasts follows a salutatory manner: first, a leading process extended; then, swelling formation and centrosome migration; and last, somal translocation [16, 18–20]. The RMS carries the neuroblasts into the OB where these neuroblasts detach from the RMS and then migrate radially to the outer layer and differentiate into various subtypes of olfactory neurons.
There are two principal types of adult-born OB neurons: periglomerular cells (PGCs) in the glomerular layer (GL) and granule cells (GCs) in the granule cell layer (GCL). Deep GCs and calbindin (CalB)+ PGCs are derived from ventral NSCs, whereas superficial GCs and tyrosine hydroxylase (TH)+ PGCs are derived from dorsal NSCs. NSCs from medial wall produce calretinin (CalR)+ GCs and CalR+ PGCs [21] (Figure 1).
2.1.2. SGZ
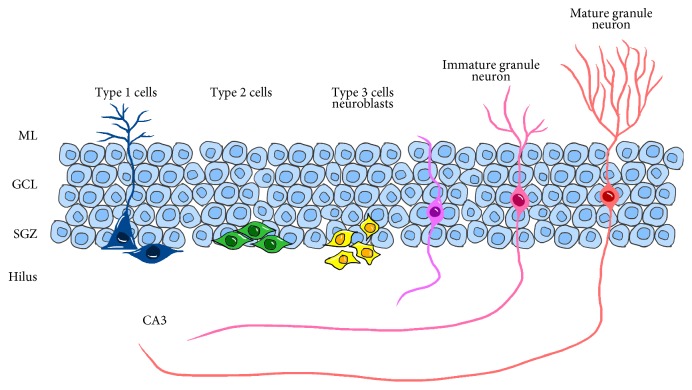
The adult neurogenic niche of the hippocampus resides in the SGZ, a thin band of cells lying between the hilus cells and the granule cell layer in the DG. NSCs first develop into radical astrocytes (type 1 cells) that, in turn, generate intermediate neural progenitors (type 2 cells). These cells are immature neuroblasts that can be further differentiated into neuroblasts. Neuroblasts can be further divided into more differentiated cells (type 3 cells) [22, 23]. Type 3 cells progressively acquire characteristics of neurons. During the stage of immature to mature, elaborate dendritic arborization grows to the middle of the molecular layer and axon elongate toward CA3 [23] (Figure 2).
Figure 2.
Neurogenesis in SGZ. The SGZ is a thin band of tissue that lies between the granule cell layer (GCL) and the hilus cells in the DG. Type 1 cells are triangular-shaped NSCs and usually extend a strong apical process into the molecular layer (ML). Type 1 cells (blue) generate type 2 cells (green). Type 2 cells are immature neuroblasts that can be further differentiating into type 3 cells (yellow). Type 3 cells progressively acquire characteristics of granule neurons. During the stage of immature (pink) to mature (red), large parts of the dendritic tree and axon elongate toward CA3.
Type 1 cells are located in the SGZ and have a triangular-shaped soma. A strong apical process extended into the molecular layer of DG is the typical characteristic of type 1 cells. Type 1 cells have some astrocyte features that may contact blood vessels through the end-feet [22]. Recently, another class of type 1 cells has been identified. These newly identified type 1 cells are characterized by short, horizontal processes [24]. Type 2 cells have a unique morphology that is distinct from type 1 cells: they lack the strong apical process and have a round or ovoid nucleus. Type 3 cells have variable morphologies. The processes of type 3 cells are short and the orientations alter from horizontal to vertical (Figure 2). Type 1 cells express GFAP, nestin [25, 26], BLBP, and Sox2. Type 2 cells have both neural and glial features that express neuronal (DCX and PSA-NCAM) and glial marker (nestin, BLBP) [27]. DCX, PSA-NCAM, NeuroD, and Prox1 are mainly expressed on type 3 cells.
2.2. PCD for the Regulation of Adult Neurogenesis
PCD is the death of a cell in any form, mediated by an intracellular program that mainly occurs during embryo/adult development and in some pathologic conditions [28]. The majority of adult-born neurons are eliminated by apoptosis. There are three main functions of PCD during adult neurogenesis: (1) regulate of the size of the NSC pool, (2) correct the errors during proliferation and migration, and (3) form correct synaptic contacts. The roles of all three functions are to optimize the neural system.
Neuroblasts from SVZ migrate through RMS to the OB and differentiate into GCs and PGCs. GCs are mature at 15–30 days and PGCs at 4 weeks after birth. There are around 30,000 newborn interneurons integrated into OB neural circuits daily in adult mice [10, 14, 29, 30]. However, 50% of NSCs, neuroblasts, and newborn interneurons undergo apoptosis to eliminate redundant and false connected cells. The survivals integrate into neural circuits and persist up to 19 months [30, 31]. Hippocampal NSCs proliferate and differentiate into granule neurons in the DG. In addition, about 30–70% of the newborn cells die of PCD in the first 2 weeks after birth. The remaining forms functional synapses on CA3 pyramidal neurons at 2 weeks after birth, and this projection becomes stable at 4 weeks [32]. About 4 weeks after birth, dendritic processes of newborn neurons extend toward and into the molecular layer and an axon project into the hilar area [33]. At 2 months, the number of DG neurons in bax−/− mice has no difference compared with that in wild-type (WT) mice, whereas at 12 months, the number of DG neurons is doubled in bax−/− mice [34]. In adult humans, 700 new neurons are added to the hippocampus per day and with a continuous decline during aging [35]. These data indicate that PCD is important for the renewal of neural circuits and occurs at all stages during adult neurogenesis.
2.2.1. PCD of NSCs
Growth factors secreted in the SVZ niche are essential for the survival of NSCs. Thus, NSCs that lack neurotrophic signals are more sensitive to apoptosis stimuli. NSCs from adult bax−/−bak−/− mice show resistance to a series of apoptotic stimuli and are accumulated in the SVZ and SGZ. Bax single-deficient NSCs are resistant to apoptosis induced by staurosporine [36]. While in Mcl1 conditional knockout mice, NSCs in the SVZ are more vulnerable to apoptotic cell death. However, overexpression of Mcl-1 reduces the apoptotic rate by about 50% in NSCs from the SVZ [37]. Regarding all above results, Bcl-2 family proteins play an essential role for the apoptosis of NSCs and regulate the size of the NSC pool. In the DG, bim or puma deficiency significantly enhanced the survival of adult-born cells but have no change on NSC differentiation [38]. Puma deficiency also increases the survival of SVZ NSCs. Puma is required for p53-induced apoptosis in NSCs of DG [39, 40]. Besides, loss of Trp53 enhances slow and fast proliferation in SVZ populations and associates with their differentiation toward neuronal and glial cell lines [41]. However, opposite results are found in the mice knockout Trp53 and p53 deficiency induces apoptotic brain lesion. These p53-deficient mice have thinner isocotex and enlarged ventricle compared with wild-type mice [42]. Therefore, the exact role and mechanism of p53 in regulating the PCD of NSCs remain unclear. Adult hippocampal NSCs undergo autophagic cell death instead of apoptosis on deprivation of insulin [43–45].
2.2.2. PCD during Migration and Integration
Errors during migration also induce apoptosis in the adult-born neuroblasts. In bax-deficient mice, a large number of abnormal neuroblasts accumulate in the RMS [10]. A similar result exists in newborn cells with increased mTOR activity. Heterotopia and ectopic neuroblasts are observed in the RMS and the OB. Moreover, these heterotopia cells survive and integrate to the OB network. They have increased dendritic complexity, altered membrane biophysics, and increased frequency of GABAergic synaptic inputs [46]. However, the effects and functions of these heterotopia and ectopic survived interneurons are still unknown.
The most extensive apoptosis of newborn neurons occurs during the integration into neural circuits. 30–70% of immature neurons are eliminated by apoptosis during the formation of synaptic contacts [29, 31, 47]. This phenomenon can be interpreted by a neurotrophic hypothesis that the neurotrophic substance released for the survival of neurons is limited; thus, newborn neurons need to compete for these trophic signals [48, 49]. Competition for neurotrophic signals not only occurs between homogeneous neuroblasts and immature neurons but also is observed between immature neuron and preexisted mature neuron for new synaptic connections. Using fluorescent retrograde tracers and BrdU-labeling techniques, it is proved that newborn neurons in the DG extend axons into CA3 of hippocampus and may influence the normal hippocampal function [23, 31, 50, 51]. In bax−/− mice, apoptosis is inhibited in immature and mature neurons of DG, and the size of DG neurons enlarges continuously with age [34]. Synaptic connections with efferent and afferent neurons are both observed in this the DG [10]. All these results proved that the immature neurons can extend axons to mature neurons and make contacts. Thus, the apoptosis of adult-born cells is to keep the balance of mature and immature neurons and maintain the integrity of neuronal circuits [52]. However, some opposite data have shown that in bax-deficient mice, the pattern separation function of the hippocampus is enhanced. However, knockout bax seems to have no effects on other major hippocampal functions [53]. It seems that pattern separation is regulated by immature DG neurons. Other hippocampal functions, such as aligning internal spatial representation to external landmarks, are mediated by mature DG neurons [54]. A similar phenomenon has also been found in the cell replacement of OB. In bax−/− mice, the normal olfactory learning behavior is improved, and the perturbations of newborn cell migration result in imbalance of neural circuits that destroy the olfactory learning ability. Besides, the bax-deficient mice show no significant changes on olfactory sensation [55, 56]. Based on above results, we may conclude that the immature neurons and mature neurons have different roles in neural circuits, and apoptosis is the key regulator that keeps the balance of adult-born neurons and the preexisting ones.
2.2.3. PCD of Mature Neurons
Although the majority of cell types that undergo PCD are immature neurons and neuroblasts, mature neurons also have lower levels of PCD in the OB and DG. The purpose of PCD in mature neurons is to renew the preexisting neural circuits [30, 57]. At about 15–30 days after birth, newborn cells differentiate into mature neurons in the OB. Thereafter, about 50% newborn neurons undergo apoptosis. Cells that survive the first 3 months persist up to 19 months [31]. One fourth of the DG neurons born at the peak of DG development on postnatal day 6 died in the first 1 to 6 months [58]. The production of adult-born neurons and elimination of mature neurons are critical for the maintenance of a constant number of neurons in DG and for the regulation of hippocampal functions [34].
3. AN and Stroke Recovery
Adult NSCs in neurogenic regions can be activated by different stimuli such as learning [59] and running [60] and also can be activated in the disease processes including seizure [61], mechanical lesions [62], and ischemic insult [63]. These results raised the possibility that functional deficits induced by stroke may be cured through neuronal replacement by endogenous NSCs. In well-studied rodent models of stroke, cerebral ischemia and hemorrhage have been shown to stimulate proliferation of endogenous progenitor cells and differentiate into neural system cells, including neurons, astrocytes, oligodendrocytes, and ependymal cells [64]. Evidence for stroke-activated neurogenesis has also been reported in the stroke patients [65]. Accumulating evidence has convincingly demonstrated that stroke-induced neurogenesis in SVZ and SGZ and other noncanonical stem cell niches have also been confirmed in the adult brain.
3.1. Classical Neurogenic Niches after Stroke: SGZ and SVZ
In models of transient global cerebral ischemia, cerebral blood flow is reduced throughout the whole brain [66]. The hippocampus CA1 area plays an important role in cognitive processes such as learning and memory and is more sensitive to hypoxia-ischemia insults than other areas of the brain [67, 68]. Remarkable increased progenitor proliferation in hippocampal SGZ has been observed in many species, such as mice, rats, gerbils, and monkeys, after global cerebral ischemia [69–71]. Liu et al. first reported increased hippocampal neurogenesis after transient global ischemia in gerbils in 1998 [63]. Newborn cells with neuronal features were first seen 26 days after ischemia, migrated from the SVZ to the granule cell layer, and survived for at least 7 months [63]. Since this initial publication, many follow-up studies have confirmed stimulation of neurogenesis in the SGZ across various species of global ischemia [69–71]. Nakatomi et al. further revealed that ischemia-induced adult neural progenitors in DG can replace CA1 pyramidal neurons form functional synapses and integrated into the existing brain circuitry [72]. Tanaka and his colleagues visualized that the neuronal progenitor cells in the DG proliferated, migrated, and differentiated into mature neurons by retroviral vector expressing enhanced green fluorescent protein (EGFP) [73]. Increased NSC proliferation has also been reported in the SVZ following global ischemia [74]. Promoting endogenous neurogenesis in SGZ may contribute to replace the CA1 neuron loss and improve function recovery after global ischemia. However, it is also worth to point that some studies cannot reproduce the evidence that SGZ neural stem cell migrate into CA1 as previously reported by Nakatomi and coworkers. In contrast, the CA1 area merely displays gliogenesis [71, 75].
In focal brain ischemia, middle cerebral artery occlusion (MCAO) is the most frequently used focal brain ischemia model, which produces consistent infarcts in the ipsilateral hemisphere of the cerebral cortex, hippocampus, and striatum [76]. Neurogenesis was increased bilaterally in both SVZ and SGZ after unilateral MCAO, indicating that endogenous neuronal precursors might be in response to contralateral ischemia as well [77]. The vast majority of adult neurogenesis in mammalian species occurs within the SVZ. SVZ are a paired brain structure situated throughout the lateral walls of the lateral ventricles. Significant enhanced proliferations of NSCs in the SVZ were observed in the first 7–14 days after MCAO in mice [78] and rats [77, 79, 80]. A fluorescent tracing of proliferating cells in the SVZ showed that these cells directly migrate from birth site to striatum in the post-MCAO rat brain [81]. In the normal brain, most of the SVZ neuroblasts migrate through the RMS into the OB and differentiate into interneurons. Ischemia may revoke the normal migratory pattern of SVZ NSCs and lead these cells to migrate toward the injured areas and aid in spontaneous recovery [82]. In the damaged striatum, neuroblasts were continuously generated from SVZ precursors as early as 1 week and last to 16 weeks after insult [82]. The SVZ was the principal source of the neuroblasts migrated laterally toward the injured striatal regions and integrated into neuronal networks receiving synaptic input and firing action potentials after MCAO [83, 84]. Inspiringly, Kreuzberg and his colleagues found MCAO also induced SVZ-derived neuroblasts migrated to the cortex, differentiated into mature neurons, and survived for at least 35 days [78]. These results highlight the role of the SVZ NSCs in neuronal regeneration after focal cerebral ischemia and its potential as a new therapeutic target for various neurological disorders.
3.2. Adult Neurogenesis from Noncanonical Sites
Above studies have shown convincing evidence of neuroblasts migrating from the SVZ or SGZ to the ischemic areas. However, several studies have proposed the possibility that there exist other stem cell niches in the adult brain [85, 86]. In fact, this noncanonical site of adult NSCs has been found in rodent striatum [87], hypothalamus [88], neocortex [87], amygdala [87], substantia nigra [89], and brainstem [90]. In addition, endogenous brain repair occurs in, but not restricted to, neurogenic regions. It is reported that astrocytes surrounding the infarct core lesion can be activated to generate neurons [91, 92]. Pericytes and OPCs have also been reported to differentiate into neurons following brain injury [93]. These data indicate that stem cell niches are much extensive. These existed multiple neurogenesis sites may be important for brain repair after injury [94].
4. Targeting AN as Therapeutic Strategy for Stroke
AN has arisen great interest as it can be applied for new therapies to replace damaged neurons and treat severe neurological deficits after stroke and other neurological diseases. However, the endogenous neurogenesis failed in producing adequate amounts of newborn neurons that can survive and integrate to restore the function recovery. Stimulating or enhancing the endogenous neurogenesis driven by ischemia can be a promising therapeutic intervention for stroke. The vast majority of the newborn neurons die between 2 and 5 weeks, which may be caused by the unfavorable environment that is exposed to the detrimental injury niche, lacking appropriate trophic support and failed connections with other neurons after stroke [95]. Reducing endogenous toxic substances, inhibiting inflammatory responses, and promoting the release of growth factors and neurotrophic signals have been suggested as effective manipulations to improve AN.
A wide variety of signaling pathways are related to NSC activity during proliferation, migration, differentiation, and their maintenance in the adult neurogenic regions. As survival is the first factor of newborn neurons, neuroprotective agents or manipulations attempt to benefit from neuronal survival preserving the property to support neurogenesis in long term [96]. Administration of EPO (5 U/g) significantly preserves hemispheric brain volume 6 weeks after stroke and directs cell fate toward neurogenesis and away from gliogenesis [97]. The PI3 kinase/Akt pathway showed to play an important role in neuronal survival as well as adult neurogenesis. Mutation that produces constitutive activation of the Akt pathway through PTEN deletion induces a robust increase in poststroke neurogenesis [98]. Studies that targeted NOTCH, WNT, and sonic hedgehog (SHH) signaling showed the great potential of these approaches in stroke treatment [99–102]. In addition, many growth factors have been identified to protect NSCs and enhance neurogenesis after stroke. Basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), BDNF, bone morphogenetic protein (BMP), glial cell-derived neurotrophic factor (GDNF), transforming growth factor- (TGF-) α, ciliary neurotrophic factor (CNTF), and platelet-derived growth factor (PDGF) have all been proposed to play essential roles in the adult neurogenesis response to ischemia stroke [103–111]. Recently, some cytokines and hormones such as chemokine, complement, estrogen, and granulocyte-colony stimulating factor (G-CSF) have been proved to benefit against a stroke-induced brain and behavioral pathology [112–116]. The chemokine stromal-derived factor 1 (SDF1) is induced in peri-infarct blood vessels and serves as a tropic signal for migrating neuroblasts to localize to the ischemic area. Administration of SDF1 improves poststroke neuroblast migration and behavioral recovery [114]. Though great progress has been made, the search for strategies and pharmacological agents to enhance endogenous neurogenesis despite a detrimental milieu remains a challenge and is the focus of intense investigation.
5. Concluding Remarks
Majority of stroke patients suffer from serious morbidity and never regain full functional independence. The limited result of stroke treatment has driven the search for stem cell therapies directed at restoring neurological function. However, both technical and ethical issues limit the development of exogenous stem cell therapy [4]. The finding of endogenous neural stem cells in the mammalian brain is a breakthrough and provides a promising approach to repair the damaged lesion after stroke. Great efforts have been made to augment the innate neurogenic capacity of the adult brain, including increasing the survival of NSCs in the neurogenic regions, strengthening their mobilization, and integrating into damaged neural circuits. However, there are issues raised about the AN after stroke. First, little is known regarding the intrinsic properties and the modulation of the NSC fate. Additional research is needed to identify the NSC fate determinants, which modulate the differentiation of NSCs toward specific cell types. Second, the integration of newborn neurons into preexisting neural circuits and the related functional recovery should be studied and improved in future researches [117]. Third, most animal studies of stroke are performed in young adult animals; however, human stroke most frequently occurs in aged patients. Neurogenesis both in the SVZ and SGZ drops precipitously with age, and the effects of age on AN should be considered. Forth, for efficient repair, it may be necessary to provide endogenous and/or graft new cells to form synthetic extracellular matrix so that they can reform appropriate brain structure [118]. The discoveries reported in this review may pave the way for targeting AN as future therapeutic interventions for stroke as well as other central nervous system diseases.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81671130).
Conflicts of Interest
The authors declare there is no conflict of interest regarding the publication of this paper.
References
- 1.Feigin V. L., Barker-Collo S., Parag V., et al. Auckland stroke outcomes study. Part 1: gender, stroke types, ethnicity, and functional outcomes 5 years poststroke. Neurology. 2010;75(18):1597–1607. doi: 10.1212/WNL.0b013e3181fb44b3. [DOI] [PubMed] [Google Scholar]
- 2.Doetsch F., Garcia-Verdugo J. M., Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. The Journal of Neuroscience. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron H. A., Woolley C. S., McEwen B. S., Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56(2):337–344. doi: 10.1016/0306-4522(93)90335-D. [DOI] [PubMed] [Google Scholar]
- 4.Koh S. H., Park H. H. Neurogenesis in stroke recovery. Translational Stroke Research. 2017;8(1):3–13. doi: 10.1007/s12975-016-0460-z. [DOI] [PubMed] [Google Scholar]
- 5.Bonfanti L. Adult neurogenesis 50 years later: limits and opportunities in mammals. Frontiers in Neuroscience. 2016;10:p. 44. doi: 10.3389/fnins.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman J., Das G. D. Post-natal origin of microneurones in the rat brain. Nature. 1965;207(5000):953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- 7.Gage F. H. Neurogenesis in the adult brain. The Journal of Neuroscience. 2002;22(3):612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ming G. L., Song H. Adult neurogenesis in the mammalian central nervous system. Annual Review of Neuroscience. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 9.Shen Q., Goderie S. K., Jin L., et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 10.Kim W. R., Park O. H., Choi S., et al. The maintenance of specific aspects of neuronal function and behavior is dependent on programmed cell death of adult-generated neurons in the dentate gyrus. The European Journal of Neuroscience. 2009;29(7):1408–1421. doi: 10.1111/j.1460-9568.2009.06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega F., Gascon S., Masserdotti G., et al. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nature Cell Biology. 2013;15(6):602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- 12.Ortega F., Berninger B., Costa M. R. Primary culture and live imaging of adult neural stem cells and their progeny. Methods in Molecular Biology. 2013;1052:1–11. doi: 10.1007/7651_2013_22. [DOI] [PubMed] [Google Scholar]
- 13.Ponti G., Obernier K., Alvarez-Buylla A. Lineage progression from stem cells to new neurons in the adult brain ventricular-subventricular zone. Cell Cycle. 2013;12(11):1649–1650. doi: 10.4161/cc.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lois C., Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 15.Lois C., Garcia-Verdugo J. M., Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 16.Wichterle H., Garcia-Verdugo J. M., Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997;18(5):779–791. doi: 10.1016/S0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 17.Doetsch F., Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hikita T., Ohno A., Sawada M., Ota H., Sawamoto K. Rac1-mediated indentation of resting neurons promotes the chain migration of new neurons in the rostral migratory stream of post-natal mouse brain. Journal of Neurochemistry. 2014;128(6):790–797. doi: 10.1111/jnc.12518. [DOI] [PubMed] [Google Scholar]
- 19.Ota H., Hikita T., Sawada M., et al. Speed control for neuronal migration in the postnatal brain by Gmip-mediated local inactivation of RhoA. Nature Communications. 2014;5:p. 4532. doi: 10.1038/ncomms5532. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara R., Thumkeo D., Kamijo H., et al. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nature Neuroscience. 2012;15(3):373–380. doi: 10.1038/nn.3020. S1-2. [DOI] [PubMed] [Google Scholar]
- 21.Fuentealba L. C., Rompani S. B., Parraguez J. I., et al. Embryonic origin of postnatal neural stem cells. Cell. 2015;161(7):1644–1655. doi: 10.1016/j.cell.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippov V., Kronenberg G., Pivneva T., et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Molecular and Cellular Neurosciences. 2003;23(3):373–382. doi: 10.1016/S1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C., Teng E. M., Summers R. G., Jr Ming G. L., Gage F. H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. The Journal of Neuroscience. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugert S., Basak O., Knuckles P., et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6(5):445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Seri B., Garcia-Verdugo J. M., McEwen B. S., Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. The Journal of Neuroscience. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steiner B., Kronenberg G., Jessberger S., Brandt M. D., Reuter K., Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46(1):41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- 27.Steiner B., Klempin F., Wang L., Kott M., Kettenmann H., Kempermann G. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54(8):805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs Y., Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nature Reviews Molecular Cell Biology. 2015;16(6):329–344. doi: 10.1038/nrm3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biebl M., Cooper C. M., Winkler J., Kuhn H. G. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neuroscience Letters. 2000;291(1):17–20. doi: 10.1016/S0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 30.Winner B., Cooper-Kuhn C. M., Aigner R., Winkler J., Kuhn H. G. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. The European Journal of Neuroscience. 2002;16(9):1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- 31.Petreanu L., Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. The Journal of Neuroscience. 2002;22(14):6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Y., Arruda-Carvalho M., Wang J., et al. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nature Neuroscience. 2012;15(12):1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D., Gage F. H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun W., Winseck A., Vinsant S., Park O. H., Kim H., Oppenheim R. W. Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene Bax. The Journal of Neuroscience. 2004;24(49):11205–11213. doi: 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spalding K. L., Bergmann O., Alkass K., et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsten T., Golden J. A., Zong W. X., Minarcik J., Harris M. H., Thompson C. B. The proapoptotic activities of Bax and Bak limit the size of the neural stem cell pool. The Journal of Neuroscience. 2003;23(35):11112–11119. doi: 10.1523/JNEUROSCI.23-35-11112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malone C. D., Hasan S. M., Roome R. B., et al. Mcl-1 regulates the survival of adult neural precursor cells. Molecular and Cellular Neurosciences. 2012;49(4):439–447. doi: 10.1016/j.mcn.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Bunk E. C., Konig H. G., Bernas T., et al. BH3-only proteins BIM and PUMA in the regulation of survival and neuronal differentiation of newly generated cells in the adult mouse hippocampus. Cell Death & Disease. 2010;1, article e15 doi: 10.1038/cddis.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fatt M. P., Cancino G. I., Miller F. D., Kaplan D. R. p63 and p73 coordinate p53 function to determine the balance between survival, cell death, and senescence in adult neural precursor cells. Cell Death and Differentiation. 2014;21(10):1546–1559. doi: 10.1038/cdd.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D., Ou L., Clemenson G. D., Jr., et al. Puma is required for p53-induced depletion of adult stem cells. Nature Cell Biology. 2010;12(10):993–998. doi: 10.1038/ncb2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gil-Perotin S., Marin-Husstege M., Li J., et al. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. The Journal of Neuroscience. 2006;26(4):1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amson R., Lassalle J. M., Halley H., et al. Behavioral alterations associated with apoptosis and down-regulation of presenilin 1 in the brains of p53-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(10):5346–5350. doi: 10.1073/pnas.97.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu S. W., Baek S. H., Brennan R. T., et al. Autophagic death of adult hippocampal neural stem cells following insulin withdrawal. Stem Cells. 2008;26(10):2602–2610. doi: 10.1634/stemcells.2008-0153. [DOI] [PubMed] [Google Scholar]
- 44.Chung K. M., Park H., Jung S., et al. Calpain determines the propensity of adult hippocampal neural stem cells to Autophagic cell death following insulin withdrawal. Stem Cells. 2015;33(10):3052–3064. doi: 10.1002/stem.2082. [DOI] [PubMed] [Google Scholar]
- 45.Ha S., Ryu H. Y., Chung K. M., Baek S. H., Kim E. K., Yu S. W. Regulation of autophagic cell death by glycogen synthase kinase-3beta in adult hippocampal neural stem cells following insulin withdrawal. Molecular Brain. 2015;8:p. 30. doi: 10.1186/s13041-015-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lafourcade C. A., Lin T. V., Feliciano D. M., Zhang L., Hsieh L. S., Bordey A. Rheb activation in subventricular zone progenitors leads to heterotopia, ectopic neuronal differentiation, and rapamycin-sensitive olfactory micronodules and dendrite hypertrophy of newborn neurons. The Journal of Neuroscience. 2013;33(6):2419–2431. doi: 10.1523/JNEUROSCI.1840-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belvindrah R., Rougon G., Chazal G. Increased neurogenesis in adult mCD24-deficient mice. The Journal of Neuroscience. 2002;22(9):3594–3607. doi: 10.1523/JNEUROSCI.22-09-03594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oppenheim R. W., Prevette D., Yin Q. W., Collins F., MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. 1991;251(5001):1616–1618. doi: 10.1126/science.2011743. [DOI] [PubMed] [Google Scholar]
- 49.Buss R. R., Sun W., Oppenheim R. W. Adaptive roles of programmed cell death during nervous system development. Annual Review of Neuroscience. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- 50.Hastings N. B., Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. The Journal of Comparative Neurology. 1999;413(1):146–154. doi: 10.1002/(SICI)1096-9861(19991011)413:1<146::AID-CNE10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 51.Markakis E. A., Gage F. H. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. The Journal of Comparative Neurology. 1999;406(4):449–460. doi: 10.1002/(SICI)1096-9861(19990419)406:4<449::AID-CNE3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 52.Sun W., Gould T. W., Vinsant S., Prevette D., Oppenheim R. W. Neuromuscular development after the prevention of naturally occurring neuronal death by Bax deletion. The Journal of Neuroscience. 2003;23(19):7298–7310. doi: 10.1523/JNEUROSCI.23-19-07298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahay A., Scobie K. N., Hill A. S., et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J. W., Kim W. R., Sun W., Jung M. W. Disruption of dentate gyrus blocks effect of visual input on spatial firing of CA1 neurons. The Journal of Neuroscience. 2012;32(38):12999–13003. doi: 10.1523/JNEUROSCI.2608-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim W. R., Sun W. Enhanced odor discrimination learning in aged Bax-KO mice. Neuroscience Letters. 2013;548:196–200. doi: 10.1016/j.neulet.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 56.Kim W. R., Kim Y., Eun B., et al. Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. The Journal of Neuroscience. 2007;27(52):14392–14403. doi: 10.1523/JNEUROSCI.3903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi M., Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dayer A. G., Ford A. A., Cleaver K. M., Yassaee M., Cameron H. A. Short-term and long-term survival of new neurons in the rat dentate gyrus. The Journal of Comparative Neurology. 2003;460(4):563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 59.Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 60.Praag H., Kempermann G., Gage F. H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 61.Parent J. M., Yu T. W., Leibowitz R. T., Geschwind D. H., Sloviter R. S., Lowenstein D. H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. The Journal of Neuroscience. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gould E., Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80(2):427–436. doi: 10.1016/S0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 63.Liu J., Solway K., Messing R. O., Sharp F. R. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. The Journal of Neuroscience. 1998;18(19):7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gregoire C. A., Goldenstein B. L., Floriddia E. M., Barnabé-Heider F., Fernandes K. J. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia. 2015;63(8):1469–1482. doi: 10.1002/glia.22851. [DOI] [PubMed] [Google Scholar]
- 65.Jin K., Wang X., Xie L., et al. Evidence for stroke-induced neurogenesis in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yigitkanli K., Zheng Y., Pekcec A., Lo E. H., Leyen K. Increased 12/15-lipoxygenase leads to widespread brain injury following global cerebral ischemia. Translational Stroke Research. 2017;8(2):194–202. doi: 10.1007/s12975-016-0509-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y., Kimura-Ohba S., Thompson J., Rosenberg G. A. Rodent models of vascular cognitive impairment. Translational Stroke Research. 2016;7(5):407–414. doi: 10.1007/s12975-016-0486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J., Zhu R. L., Nakayama M., et al. Expression of the apoptosis-effector gene, Bax, is up-regulated in vulnerable hippocampal CA1 neurons following global ischemia. Journal of Neurochemistry. 1996;67(1):64–71. doi: 10.1046/j.1471-4159.1996.67010064.x. [DOI] [PubMed] [Google Scholar]
- 69.Takagi Y., Nozaki K., Takahashi J., Yodoi J., Ishikawa M., Hashimoto N. Proliferation of neuronal precursor cells in the dentate gyrus is accelerated after transient forebrain ischemia in mice. Brain Research. 1999;831(1-2):283–287. doi: 10.1016/S0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- 70.Kee N. J., Preston E., Wojtowicz J. M. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Experimental Brain Research. 2001;136(3):313–320. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- 71.Tonchev A. B., Yamashima T., Zhao L., Okano H. Differential proliferative response in the postischemic hippocampus, temporal cortex, and olfactory bulb of young adult macaque monkeys. Glia. 2003;42(3):209–224. doi: 10.1002/glia.10209. [DOI] [PubMed] [Google Scholar]
- 72.Nakatomi H., Kuriu T., Okabe S., et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–441. doi: 10.1016/S0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka R., Yamashiro K., Mochizuki H., et al. Neurogenesis after transient global ischemia in the adult hippocampus visualized by improved retroviral vector. Stroke. 2004;35(6):1454–1459. doi: 10.1161/01.STR.0000126480.40967.b3. [DOI] [PubMed] [Google Scholar]
- 74.Tonchev A. B., Yamashima T., Sawamoto K., Okano H. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. Journal of Neuroscience Research. 2005;81(6):776–788. doi: 10.1002/jnr.20604. [DOI] [PubMed] [Google Scholar]
- 75.Wojcik-Stanaszek L., Sypecka J., Szymczak P., et al. The potential role of metalloproteinases in neurogenesis in the gerbil hippocampus following global forebrain ischemia. PLoS One. 2011;6(7, article e22465) doi: 10.1371/journal.pone.0022465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ginsberg M. D., Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20(12):1627–1642. doi: 10.1161/01.STR.20.12.1627. [DOI] [PubMed] [Google Scholar]
- 77.Jin K., Minami M., Lan J. Q., et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kreuzberg M., Kanov E., Timofeev O., Schwaninger M., Monyer H., Khodosevich K. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Experimental Neurology. 2010;226(1):90–99. doi: 10.1016/j.expneurol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Zhang R. L., Zhang Z. G., Zhang L., Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105(1):33–41. doi: 10.1016/S0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 80.Parent J. M., Vexler Z. S., Gong C., Derugin N., Ferriero D. M. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Annals of Neurology. 2002;52(6):802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 81.Jin K., Sun Y., Xie L., et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Molecular and Cellular Neurosciences. 2003;24(1):171–189. doi: 10.1016/S1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 82.Thored P., Arvidsson A., Cacci E., et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita T., Ninomiya M., Hernandez Acosta P., et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. The Journal of Neuroscience. 2006;26(24):6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hou S. W., Wang Y. Q., Xu M., et al. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39(10):2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- 85.Dayer A. G., Cleaver K. M., Abouantoun T., Cameron H. A. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. The Journal of Cell Biology. 2005;168(3):415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pierce A. A., Xu A. W. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. The Journal of Neuroscience. 2010;30(2):723–730. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shapiro L. A., Ng K., Zhou Q. Y., Ribak C. E. Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy & Behavior. 2009;14(Supplement 1):74–80. doi: 10.1016/j.yebeh.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kokoeva M. V., Yin H., Flier J. S. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. The Journal of Comparative Neurology. 2007;505(2):209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 89.Zhao M., Momma S., Delfani K., et al. Evidence for neurogenesis in the adult mammalian substantia nigra. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(13):7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bauer S., Hay M., Amilhon B., Jean A., Moyse E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience. 2005;130(1):75–90. doi: 10.1016/j.neuroscience.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 91.Magnusson J. P., Goritz C., Tatarishvili J., et al. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346(6206):237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- 92.Duan C. L., Liu C. W., Shen S. W., et al. Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia. 2015;63(9):1660–1670. doi: 10.1002/glia.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakagomi T., Kubo S., Nakano-Doi A., et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33(6):1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- 94.Liu Z., Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Progress in Neurobiology. 2016;144:103–120. doi: 10.1016/j.pneurobio.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature Medicine. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 96.Romero J. R., Babikian V. L., Katz D. I., Finklestein S. P. Neuroprotection and stroke rehabilitation: modulation and enhancement of recovery. Behavioural Neurology. 2006;17(1):17–24. doi: 10.1155/2006/137532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gonzalez F. F., McQuillen P., Mu D., et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Developmental Neuroscience. 2007;29(4-5):321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 98.Gregorian C., Nakashima J., Belle J., et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. The Journal of Neuroscience. 2009;29(6):1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Z., Wang J., Zhao C., et al. Acute blockage of Notch signaling by DAPT induces neuroprotection and neurogenesis in the neonatal rat brain after stroke. Translational Stroke Research. 2016;7(2):132–140. doi: 10.1007/s12975-015-0441-7. [DOI] [PubMed] [Google Scholar]
- 100.Greenberg D. A., Jin K. Turning neurogenesis up a Notch. Nature Medicine. 2006;12(8):884–885. doi: 10.1038/nm0806-884. [DOI] [PubMed] [Google Scholar]
- 101.Zhang L., Chopp M., Meier D. H., et al. Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke. 2013;44(7):1965–1972. doi: 10.1161/STROKEAHA.111.000831. [DOI] [PubMed] [Google Scholar]
- 102.Wei Z. Z., Zhang J. Y., Taylor T. M., Gu X., Zhao Y., Wei L. Neuroprotective and regenerative roles of intranasal Wnt-3a administration after focal ischemic stroke in mice. Journal of Cerebral Blood Flow and Metabolism. 2017 doi: 10.1177/0271678X17702669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kitagawa H., Sasaki C., Zhang W. R., et al. Induction of glial cell line-derived neurotrophic factor receptor proteins in cerebral cortex and striatum after permanent middle cerebral artery occlusion in rats. Brain Research. 1999;834(1-2):190–195. doi: 10.1016/S0006-8993(99)01563-2. [DOI] [PubMed] [Google Scholar]
- 104.Planas A. M., Justicia C., Soriano M. A., Ferrer I. Epidermal growth factor receptor in proliferating reactive glia following transient focal ischemia in the rat brain. Glia. 1998;23(2):120–129. doi: 10.1002/(SICI)1098-1136(199806)23:2<120::AID-GLIA3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 105.Tsai P. T., Ohab J. J., Kertesz N., et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. The Journal of Neuroscience. 2006;26(4):1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chou J., Harvey B. K., Chang C. F., Shen H., Morales M., Wang Y. Neuroregenerative effects of BMP7 after stroke in rats. Journal of the Neurological Sciences. 2006;240(1-2):21–29. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 107.Lin T. N., Te J., Lee M., Sun G. Y., Hsu C. Y. Induction of basic fibroblast growth factor (bFGF) expression following focal cerebral ischemia. Brain Research. Molecular Brain Research. 1997;49(1-2):255–265. doi: 10.1016/S0169-328X(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 108.Kokaia Z., Zhao Q., Kokaia M., et al. Regulation of brain-derived neurotrophic factor gene expression after transient middle cerebral artery occlusion with and without brain damage. Experimental Neurology. 1995;136(1):73–88. doi: 10.1006/exnr.1995.1085. [DOI] [PubMed] [Google Scholar]
- 109.Tureyen K., Vemuganti R., Bowen K. K., Sailor K. A., Dempsey R. J. EGF and FGF-2 infusion increases post-ischemic neural progenitor cell proliferation in the adult rat brain. Neurosurgery. 2005;57(6):1254–1263. doi: 10.1227/01.NEU.0000186040.96929.8A. [DOI] [PubMed] [Google Scholar]
- 110.Kang S. S., Keasey M. P., Arnold S. A., Reid R., Geralds J., Hagg T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiology of Disease. 2013;49:68–78. doi: 10.1016/j.nbd.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato H., Ishii Y., Yamamoto S., et al. PDGFR-beta plays a key role in the ectopic migration of neuroblasts in cerebral stroke. Stem Cells. 2016;34(3):685–698. doi: 10.1002/stem.2212. [DOI] [PubMed] [Google Scholar]
- 112.Li J., Siegel M., Yuan M., et al. Estrogen enhances neurogenesis and behavioral recovery after stroke. Journal of Cerebral Blood Flow and Metabolism. 2011;31(2):413–425. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stokowska A., Atkins A. L., Moran J., et al. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain. 2017;140(Part 2):353–369. doi: 10.1093/brain/aww314. [DOI] [PubMed] [Google Scholar]
- 114.Ohab J. J., Fleming S., Blesch A., Carmichael S. T. A neurovascular niche for neurogenesis after stroke. The Journal of Neuroscience. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schneider A., Kruger C., Steigleder T., et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. The Journal of Clinical Investigation. 2005;115(8):2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pena I., Borlongan C. V. Translating G-CSF as an adjunct therapy to stem cell transplantation for stroke. Translational Stroke Research. 2015;6(6):421–429. doi: 10.1007/s12975-015-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inta D., Gass P. Is forebrain neurogenesis a potential repair mechanism after stroke? Journal of Cerebral Blood Flow and Metabolism. 2015;35(7):1220–1221. doi: 10.1038/jcbfm.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lindvall O., Kokaia Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harbor Perspectives in Biology. 2015;7(11) doi: 10.1101/cshperspect.a019034. [DOI] [PMC free article] [PubMed] [Google Scholar]