Abstract
DNA immunization, although attractive, is poor for inducing mucosal immunity, thus limiting its protective value against most infectious agents. To surmount this shortcoming, we devised a method for mucosal transgene vaccination by using an M cell ligand to direct the DNA vaccine to mucosal inductive tissues and the respiratory epithelium. This ligand, reovirus protein σ1, when conjugated to polylysine (PL), can bind the apical surface of M cells from nasal-associated lymphoid tissues. Intranasal immunizations with protein σ1-PL-DNA complexes produced antigen-specific serum IgG and prolonged mucosal IgA, as well as enhanced cell-mediated immunity, made evident by elevated pulmonary cytotoxic T lymphocyte responses. Therefore, targeted transgene vaccination represents an approach for enabling DNA vaccination of the mucosa.
Most human pathogens infect the host by means of a mucosal surface. Yet, most conventional vaccines are administered peripherally, consequently varying in their ability to elicit protective mucosal responses. More recently, vaccines, particularly attenuated viruses (1, 2), have been designed for their mucosal application, because the stimulation of the mucosal compartment is necessary for optimal protective antiviral immunity. However, although vaccines are effective for stimulating appropriate host immune responses, the enthusiasm for viral vector vaccines has been compromised by unexpected complications (3). Thus, to avoid the potential side effects of live viral vectors, viable alternative vaccines or vaccine strategies are warranted. Such an alternative may lie with subunit vaccines or DNA vaccines, the latter being relatively easy to prepare and administer. Recent evidence shows that DNA vaccination can confer protective immunity against a number of infectious agents including influenza (4, 5), herpes simplex virus (6), HIV (7, 8), and Borrelia burgdorferi (9) infections. However, naked DNA immunizations have proven less than optimal for stimulating mucosal immunity (7, 10, 11) because they are administered peripherally as with conventional vaccines. On the other hand, microencapsulated DNA vaccines were shown to effectively elicit protective mucosal responses in a murine rotavirus challenge model (12). Although it is unclear whether such protection was conferred by the protective properties of microencapsulation, the ability of microspheres to interact with Peyer's patches (PP), M cells (13, 14), or a combination of both, suggests that more efficient uptake of DNA by mucosal inductive tissues would favor enhanced mucosal immunity.
When formulating vaccines, it is important to note that effective mucosal immunity often correlates with the uptake of antigen by mucosal inductive tissues, e.g., the PP in the gut and nasal-associated lymphoid tissues (NALT) in the upper respiratory tract. These inductive tissues are enveloped with specialized epithelial cells, termed M cells. M cells are responsible for antigen sampling of the lumen for eventual antigen presentation to mucosal B and T cells (15, 16). In fact, a number of pathogens, including Salmonella (17) and reovirus (18), target this specialized epithelium as a mode of entry into the host. Thus, the targeting of vaccines to M cells can be an effective method for immunization.
Intranasal (i.n.) immunization, used in our study, has proven to be an effective method for stimulating both upper and lower respiratory immunity (19–22). Moreover, this method of immunization has the ability to elicit distal mucosal immunity be it mediated by humoral or cellular immunity. Another attractive feature of i.n. immunization is that, in general, less vaccine is required to stimulate local and distal mucosal responses, that presumably minimizes the vaccine loss or degradation which would occur if vaccines were delivered orally. i.n. delivery of live vector vaccines encoding various passenger antigens has also produced protective immunity at local and distal mucosal sites. However, a disadvantage for such vaccines is the induction of mild disease, which may be problematic for immunocompromised subjects. Thus, we hypothesize that improved DNA targeting to mucosal inductive tissues should enhance mucosal and systemic responses, and to this end, we propose that adapting the M cell-targeting capabilities of live vaccine vectors to DNA vaccines will allow for this improved DNA targeting. To enable DNA immunization of the mucosa, the reovirus protein σ1 was selected because of its ability to bind to M cells (18, 23, 24). Herein, we can demonstrate enhanced mucosal IgA and cytotoxic T lymphocyte (CTL) responses to our formulated DNA vaccine when compared with naked DNA immunization.
Materials and Methods
Vaccine Preparations and Immunizations.
The production of recombinant protein σ1 and polylysine (PL) conjugate was performed as described (25, 26). For our immunizations, both pCMVLuciferase (pCMVLuc) and pCMV-β-galactosidase (pCMV-β-gal; Life Technologies, Grand Island, NY) were used. The pCMVLuc was constructed as described (25). BALB/c mice (Frederick Cancer Research Facility) between 6–8 weeks old were maintained under microisolation conditions; sterile food and water were provided ad libitum. Groups of mice (5 per group) were i.n. immunized at 7-day intervals on days 0, 7, and 14 with 20 μg of plasmid, pCMVLuc or pCMV-β-gal, per dose with or without 20 μg of protein σ1-PL or PL conjugates in a total volume of 20 μl. Immunizations were performed without anesthesia. An additional group of mice received three doses of protein σ1 only.
Immunofluorescent Staining for Protein σ1 Binding to M Cells.
NALTs were collected, embedded, and frozen as described (27). For immunofluorescent staining, frozen NALT was sectioned at 7 μm and air-dried for 30 min at room temperature. Sections were incubated with either 50 μg of biotinylated recombinant protein σ1 diluted in Dulbecco's PBS (dPBS), dPBS alone as a control, or with biotinylated maltose-binding protein, under rotation for 6 h at 4°C. Sections then were treated with a 1:250 streptavidin-Alexa 546 (Molecular Probes) for 30 min. Then M cells were visualized by addition of a 1:250 dilution of FITC-labeled Ulex europaeus agglutinin 1 (UEA-1; Vector Laboratories) for 30 min and subsequently washed and mounted. Hematoxylin/eosin staining also was performed to show M cells.
Anti-Luc Ab ELISA.
Serum and fecal samples were collected at the indicated time points. Fecal samples were extracted from the supernatants of 10% (wt/vol) suspension of sample and PBS. Endpoint Ab titers were determined by ELISA as described (28). To detect specific anti-Luc Abs, NUNC Maxi-sorp (Roskilde, Denmark) ELISA wells were coated overnight with 100 ng per well of Luc in PBS at room temperature. Standard ELISA procedures were followed (28, 29). Horseradish peroxidase conjugated goat anti-mouse IgG- and IgA-specific Abs (Southern Biotechnology Associates) were used to detect Luc-specific IgG and IgA Ab titers, respectively. Endpoint titers were defined as the highest reciprocal dilution of the sample giving an absorbance ≥ OD415 above 0.100 after 45 min of incubation at 25°C as measured by a Kinetics Reader model EL312 (Biotek Instruments, Winooski, VT).
Lymphocyte Isolation.
Lymphocytes were isolated from NALTs, cervical lymph nodes (CLNs), spleen, nasal passages (NPs), and small intestinal lamina propria (LP). NALT, CLN, and splenic mononuclear cell suspensions were obtained by conventional methods (28–30). To isolate mononuclear cells from NP and LP, the tissues were minced and suspended in media containing 300 units/ml of Clostridium histolyticum Type IV collagenase (Worthington), followed by several sequential 15-min digestions at 37°C in spinner flasks. After incubation, the digestion mixtures were passed through a mesh to remove undigested tissues. Mononuclear cells were subjected to Percoll (Amersham Pharmacia) density gradient centrifugation and cells interfaced between 40% and 75% Percoll (20, 31). Greater than 95% viability was noted for lymphocytes isolated from each tissue as determined by trypan blue exclusion.
ELISPOT Assays.
An Ab ELISPOT assay was performed as described (20, 31). Nitrocellulose-bottomed microtiter plates (Millititer HA; Millipore) were coated with 0.2 μg per well of Luc overnight at room temperature and blocked as described (20, 31). Between 1 × 105 and 5 × 105 cells, with the exception of the NPs that were normally loaded at 10-fold less concentration, were added to the microtiter wells. The Ab-forming cell (AFC) spots were enumerated by using a dissecting binocular microscope.
The T cell cytokine ELISPOT assay was performed as described (23, 28, 29). Immune CLN, PP, and splenic CD4+ T cells were restimulated in vitro with or without 80 μg/ml of β-gal for 2 days. After in vitro restimulation, 2.4 × 105 CD4+ T cells per well were added to the anti-cytokine Ab-coated nitrocellulose-based microtiter wells for about 20 h at 37°C in a humidified 5% CO2 incubator. Cytokine-forming cells (CFCs) were enumerated as previously described.
CTL Assay.
Lung mononuclear cells were isolated as described (20, 29). Both freshly isolated lung and splenic mononuclear cells were assessed for β-gal-specific CTL activity by using BC-β-gal, a fibroblast cell line (H-2d) expressing β-gal as target cells. Targets were mitomycin C-treated and extensively washed with complete media. Targets were subsequently labeled with Na251CrO4 (Dupont/NEN) for 1 h at 37°C. Then cells were washed 3 times in complete media. Effector cells were incubated with varying ratios of labeled targets in U-bottom 96-well microtiter dishes (Corning–Costar, Oneonta, NY) for 4 h at 37°C. After incubation, cells were spun, and supernatants were assessed for released 51Cr. Specific cytolysis was determined as the amount of 51Cr released in the presence of effector cells corrected for spontaneous 51Cr release divided by maximal (detergent-induced) release corrected for spontaneous 51Cr release. For antigen restimulation, splenic mononuclear cells were restimulated with mitomycin C-treated BC-β-gal cells at 5:1 in complete media for 5 days at 37°C. Cells were assessed for CTL activity as described above.
Statistical Analysis.
Statistical differences between experimental parameters were determined significant by using the Student's t test and one-way ANOVA.
Results
Protein σ1-Mediated Gene Transfer in Vitro.
A number of enteric pathogens seek entry into the host via the small intestinal PP presumably targeting the specialized epithelium containing M cells. Mucosal immunity is optimally stimulated where there is efficient vaccine uptake by M cells for presentation to mucosal inductive tissues. This attribute demonstrates why live vector vaccines like Salmonella are effective in promoting mucosal immunity to passenger antigens. To devise a mucosal delivery system featuring the inherent ability to target M cells without an overt disease, reovirus hemagglutinin, protein σ1, was selected as a means to target M cells for improved uptake of DNA vaccines because the reovirus mode of infection is M cell-dependent (18). To test the ability of the recombinant protein σ1 to bind M cells, and thus mediate respiratory immunity, frozen sections of murine NALT were incubated with biotinylated recombinant protein σ1 or biotinylated maltose-binding protein, and bound ligand was detected on addition of fluorochrome. As depicted in Fig. 1, only protein σ1 and not the maltose binding-protein bound to the M cells' apical surface, which was evident by the colocalization with an M cell-specific lectin, UEA-1 (ref. 32; Fig. 1). Thus, these data suggest that protein σ1 can serve as an M cell ligand.
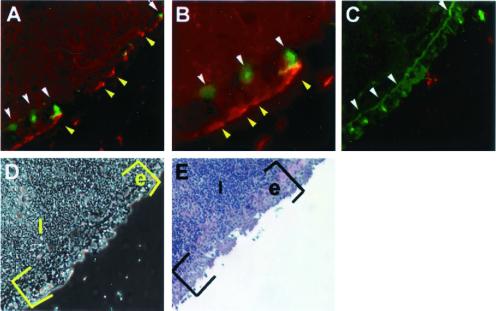
Figure 1.
Recombinant protein σ1 and UEA-1 bind NALT M cells. A frozen section of normal murine BALB/c NALT was reacted with biotinylated recombinant protein σ1 or biotinylated recombinant maltose-binding protein (50 μg/ml) for 6 h at 4°C. After washing, the section was reacted with streptavidin-Alexa 546 and FITC-UEA-1 at 4°C. (A) Protein σ1 (yellow arrows) and UEA-1 (white arrows) binding to NALT M cells at ×180. (B) High magnification (×≈360) reveals protein σ1 (red) binding to the apical surface of an M cell (bright green), as well as localizing within the M cell itself (yellow). (C) Biotinylated maltose binding fails to bind to the NALT, but FITC-UEA-1 still recognizes NALT M cells (white arrows). (D) Phase contrast image of NALT depicted in A and B; (E) Hematoxylin/eosin staining of NALT depicting M cells.
Ab Responses Induced by i.n. Administration of Protein σ1-PL-pCMVLuc Complexes.
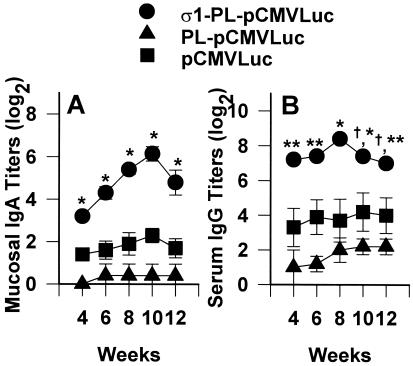
Because recombinant protein σ1 binds to NALT M cells, we hypothesized that the protein σ1-PL conjugate should facilitate mucosal DNA immunization. To test this hypothesis, Ab responses including mucosal IgA and serum IgG were analyzed after i.n. installation with a eukaryotic expression plasmid encoding Luc, referred to as pCMVLuc, with or without the addition of protein σ1. Immune IgA copro-Abs were substantially induced by protein σ1-PL-pCMVLuc (Fig. 2A). Antigen-specific mucosal IgA could be detected in the fourth week, with peak levels between 8–10 weeks, with the mean titer as much as 14-fold and 50-fold greater than that induced by naked DNA immunization or PL complexed DNA, respectively. The observed elevations in mucosal IgA induced by protein σ1-PL-pCMVLuc were maintained for at least 18 weeks after immunization, whereas the mucosal IgA induced by naked DNA or PL-complexed DNA subsided to background levels by the ninth week after initial immunization. Serum IgG responses also were elicited in the fourth week with peak titers attained by 8 weeks (Fig. 2B). The peak level of serum IgG was significantly increased by protein σ1-PL-DNA conjugates when compared with mice i.n. immunized only with DNA (P ≤ 0.01) or PL-DNA complexes (P ≤ 0.013). Thus, the elevated mucosal immune responses suggested that protein σ1-PL conjugates effectively facilitated the uptake of plasmids by mucosal inductive tissue such as NALT.
Figure 2.
Protein σ1-PL conjugates improve the efficacy of DNA immunization of nasal mucosa by increasing copro-IgA (A) and serum IgG (B). Data depict the mean endpoint titers (± SE) for mice immunized with protein σ1-PL-pCMVLuc, PL-pCMVLuc, or uncomplexed pCMVLuc (5 mice per group) at days 0, 7, and 14. Preimmune copro-IgA and serum IgG were < 21. Significant differences between protein σ1-PL-pCMVLuc and PL-pCMVLuc or pCMVLuc only were determined. *, P ≤ 0.001; **, P < 0.013; †, not significant.
Ab-Producing Cell Responses After i.n. Administration with Protein σ1-PL-pCMVLuc.
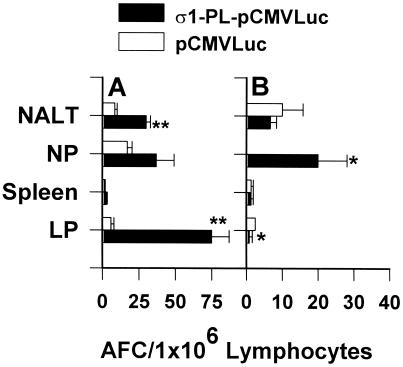
Mucosal and systemic AFCs were detected by using an ELISPOT assay 6 weeks after the initial immunization (Fig. 3). IgA anti-Luc AFCs were detected in immune NALT, representing ≈3-fold enhancement compared with mice immunized only with naked DNA (P < 0.001). IgA anti-Luc AFC responses were also greatly elevated in intestinal LP where IgA AFC responses were detected as 10-fold greater than those in mice i.n. immunized with naked DNA. Elevations in IgA AFC responses were observed in the NP but were not significantly enhanced from those IgA responses observed in naked DNA-immunized mice. Minimal IgA AFC were found in the spleen for either immunization group. IgG anti-Luc AFCs were significantly enhanced in NP (P = 0.013), and no IgG AFCs were detected in the naked DNA-immunized group. No significant differences were detected in induced IgG AFCs in NALT by the DNA immunizations. Minimal IgG AFC responses were detected in spleen and intestinal LP. This evidence suggests that i.n. immunization with protein σ1-PL-pCMVLuc induces effective local and distal mucosal immunity.
Figure 3.
i.n. immunization with protein σ1-PL-pCMVLuc enhances AFC responses when compared with immunizations with naked pCMVLuc. Mice received 3 doses of protein σ1-PL-pCMVLuc or pCMVLuc, and were assessed for AFC responses in mucosal and systemic tissues by Luc-specific ELISPOT 6 weeks after the first dose. (A) IgA AFCs were significantly elevated in NALT and small intestinal LP compared with the naked DNA immunization group. (B) IgG AFCs were elevated in NPs. Both IgG and IgA AFCs were minimally affected in spleen. Depicted is the mean of three experiments (± SD), and each experiment represents 5 mice per administration group. *, P ≤ 0.025; **, P < 0.001.
T Helper (Th) Cell Cytokine Responses Supporting the Ab Responses Induced by Protein σ1-PL Conjugate.
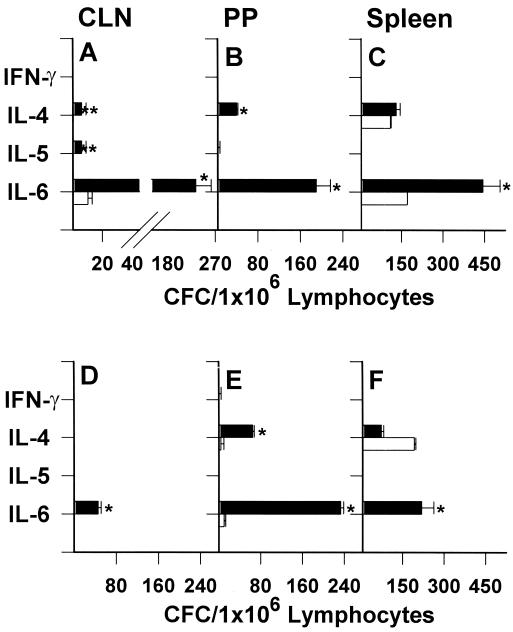
Because i.n. immunization with protein σ1-PL-DNA induced both mucosal and systemic immune responses, it was important to establish the nature of Th cell cytokine responses supporting antigen-specific Ab responses. Mice were i.n. immunized with either protein σ1-PL-pCMV-β-gal or pCMV-β-gal as previously described. After in vitro stimulation with or without β-gal antigen, Th2-type cytokine-secreting CLN, PP, and splenic CD4+ T cells for IL-4 and IL-6 were significantly elevated (Fig. 4 A–C). Some IL-5 CFCs could be detected in CLNs. However, mice i.n. immunized with pCMV-β-gal (Fig. 4 D–F) showed no IL-4 or IL-5 CFCs, and minimal IL-6 CFC by β-gal-pulsed CLNs. Elevations in IL-4 and IL-6 PP CFCs were noted similar to protein σ1-PL-pCMV-β-gal-immunized mice. Only significant increases in IL-6 CFCs were detected for the β-gal-pulsed splenic cultures. No IFN-γ CFCs were detected in any of the β-gal-restimulated cultures. These studies show that i.n. administration of protein σ1-PL-DNA preferentially induces Th2-type cytokine responses to support antigen-specific Abs.
Figure 4.
i.n. immunization with protein σ1-PL-DNA elicits Th2 cell-dependent responses. BALB/c mice were i.n. immunized 3 times at weekly intervals with either protein σ1-PL-pCMV-β-gal (A–C) or pCMV-β-gal (D–F), and 4 weeks subsequent the last dose, mononuclear cells from CLN (A, D), PP (B, E), and spleens (C, F) were harvested and left unrestimulated (open bars) or restimulated with 80 μg/ml β-gal (filled bars) for 2 days. CFCs were enumerated by cytokine-specific ELISPOT assay. β-gal restimulation of protein σ1-PL-DNA vaccinated mice induced increases in CLN IL-4, IL-5, and IL-6, PP IL-4 and IL-6, splenic IL-6, and no IFN-γ CFC in any of the tissues. β-gal restimulation of DNA vaccinated mice showed increases in only CLN IL-6, PP IL-4 and IL-6, and splenic IL-6 CFC. Data are representative of three experiments (± SD). *, P ≤ 0.007; **, P = 0.021.
Specific CTLs Induced by i.n. Immunization with Protein σ1-PL-pCMV-β-Gal.
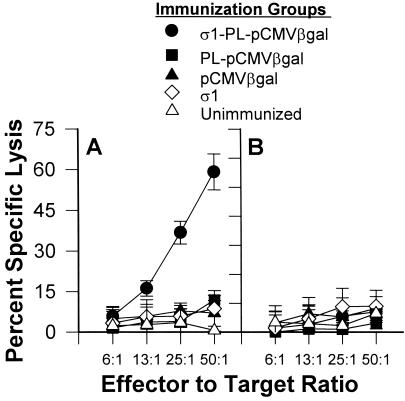
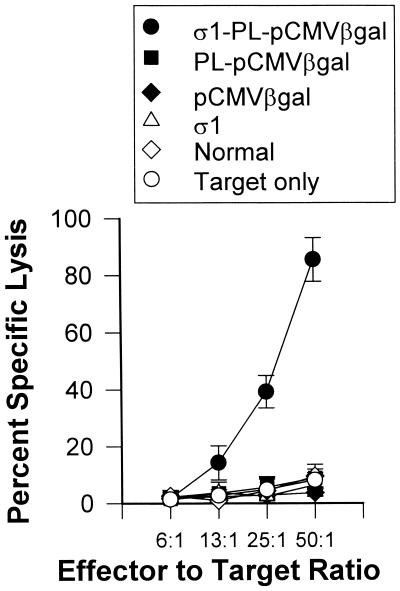
Specific CTLs can be induced by mucosal immunization by means of the i.n. route when CTL epitope peptide or plasmid DNA is appropriately delivered. Mice receiving 3 doses of protein σ1-PL-pCMV-β-gal were evaluated 6 weeks after the first dose. Mononuclear cell suspensions were prepared from spleens and lungs to determine the level of ex vivo CTL activity. Elevated lung CTL responses were detected in a dose-dependent fashion in those mice i.n. immunized with protein σ1-PL-pCMV-β-gal, but not those mice i.n. immunized with naked DNA or PL-pCMV-β-gal (Fig. 5A). No splenic anti-β-gal CTL responses were detected for any of the immunized groups (Fig. 5B). Likewise, no CTL activity was detected for normal lymphocytes or for mice immunized only with protein σ1. Splenocytes restimulated with β-gal-expressing cells resulted in enhanced cytolysis from protein σ1-PL-pCMV-β-gal immunized mice but not from mice i.n. immunized with pCMV-β-gal, PL-pCMV-β-gal, or with only protein σ1 (Fig. 6). Thus, these results demonstrate that the attachment of protein σ1 greatly enhances mucosal IgA as well as mucosal and systemic CTL responses.
Figure 5.
i.n. immunization with protein σ1-PL-pCMV-β-gal induces pulmonary β-gal-specific CTLs. Five groups of BALB/c mice were i.n. immunized with protein σ1-PL-pCMV-β-gal, PL-pCMV-β-gal, pCMV-β-gal, or protein σ1, or were left unimmunized, and evaluated for β-gal-specific CTL responses. Freshly isolated lung (A) and splenic (B) mononuclear cells were assessed for cytolytic activity, and only the lung effector cells from the protein σ1-PL-pCMV-β-gal-immunized mice showed cytolysis of 51Cr-loaded fibroblasts expressing β-gal (BC-β-gal) targets. Data are representative of three experiments.
Figure 6.
In vitro β-gal-restimulated splenic mononuclear cells from protein σ1-PL-pCMV-β-gal-immunized mice showed elevated CTL responses, but not cells from mice immunized with PL-pCMV-β-gal, pCMV-β-gal, protein σ1, or unimmunized mice. Data are representative of three experiments.
Discussion
In an effort to facilitate DNA immunization of the mucosa, we have incorporated into our vaccine formulation an M cell-targeting molecule, much like live vector vaccines, to enable immunity at mucosal inductive sites, and possibly, the respiratory epithelium. As demonstrated, the recombinant reovirus protein σ1 could bind to NALT M cells, and when covalently attached to PL for complexing with the vaccinating DNA, it enhanced both humoral and cell-mediated immune responses against the transgene vaccine when compared with i.n. immunization with naked DNA. Mucosal IgA responses were induced at both local and distal mucosal tissues as evidenced by the mucosal secretions and by antigen-specific B cells derived from mucosal tissues. In fact, the magnitude of these mucosal IgA responses is similar to those obtained with first-generation Salmonella vaccine vectors (30). Importantly, prolonged mucosal IgA responses were observed, which represent an advantage for this method of DNA delivery when compared with conventional DNA immunization (7, 10, 11). In addition, the prolonged IgA responses resembled the observed time course described for oral immunization with microencapsulated DNA (18, 33, 34). As formulated, the mucosal delivery of the protein σ1-PL-DNA favored Th2-type cytokine responses consistent with previous reports that vaccination of mucosal surfaces favors Th2-type development (12, 35, 36). The reason for this preferential Th2-type bias is unclear, because a recent report showed that i.n. DNA immunization with carboxymethylcellulose polymer showed mixed Th cell responses (37). Our results clearly signify a different mechanism of effecting mucosal immunity, perhaps favoring Th2 cell development.
Evaluations of cell-mediated immunity showed that the mice immunized with protein σ1-PL-pCMV-β-gal produced elevated pulmonary CTL responses against β-gal-expressing target cells. Mice immunized with pCMV-β-gal, PL-pCMV-β-gal, or only protein σ1 showed no induced CTL responses by pulmonary or splenic effector cells. Although the restimulated splenic effector cells from the protein σ1-PL-pCMV-β-gal-immunized mice could show elevated specific killing after in vitro culture, no CTL activity was detected from freshly isolated splenocytes. This evidence suggests that the extent of priming in the systemic compartment was not as effective as in the mucosal compartment. Alternatively, if the restimulation cultures are representative of a challenge stimulation, then perhaps the systemic compartment was primed but did not retain the effector CTLs.
As evidenced in this study, the mucosal transgene vaccination with our formulated DNA was quite effective. Clearly, the addition of the M cell ligand protein σ1 enhanced the mucosal IgA responses when compared with naked DNA immunizations in this report and others (10, 11). The results from this study not only suggest that NALT M cells are targeted by our protein σ1, but also do not exclude the possibility that the nasal epithelium may be transfected. Nonetheless, improved accessibility to mucosal inductive tissues or the drainage of antigen to such sites will favor the development of mucosal immunity. However, one consequence of repeated protein σ1-mediated vaccination is the development of anti-protein σ1 Abs. Such an induced Ab response, or preexisting Abs derived from reovirus infection, may interfere with successful targeting of this vaccine vector.
Past studies have shown that soluble proteins in conjunction with mucosal adjuvants that bind M cells, e.g., cholera toxin or heat-labile toxin, will stimulate mucosal responses. Likewise, Salmonella vaccine vectors, adept at infecting mucosal inductive tissues by means of interaction with M cells, can promote elevated mucosal responses against passenger antigens. To adapt such delivery, M cell ligands should direct immunization to mucosal inductive tissues that should then enable interactions with mucosal B and T cells. In addition to its M cell-binding properties, protein σ1 has been described recently as possessing glycosyl hydrolase activity, which allows penetration through the mucus barrier (38). This characteristic may contribute to its effectiveness at accessing mucosal inductive tissues.
To address the shortcomings of conventional DNA immunization, other modes of delivering DNA to mucosal surfaces have been devised. Live Salmonella and Shigella vaccine vectors (39, 40), cationic lipid (41), carboxymethylcellulose polymer (37), and microspheres (12–14, 33, 34) have been used to enhance mucosal DNA immunization. Alternatively, we developed reovirus protein σ1-PL conjugates with the same goal in mind. Because gene transfer facilitated by protein σ1 is by means of a receptor-mediated mode, recombinant protein σ1-PL conjugates may permit more readily the assimilation of the DNA vaccine for protein expression.
In conclusion, reovirus protein σ1-PL conjugates lack the risk of a live vector system but remain efficient in inducing an immunity by means of the i.n. route. These results make this system more attractive in the development of new applications for DNA immunization. This approach may be especially useful for DNA immunization against upper respiratory pathogens, such as influenza, or sexually transmitted diseases, such as HIV.
Acknowledgments
This work was supported by U.S. Public Health Service Grants DE 13812, AI 42673, S10 RR11877, and was supported in part by Montana Agricultural Station and U.S. Department of Agriculture Formula funds. This is Montana Agricultural Station manuscript number J-2000-18.
Abbreviations
- AFC
Ab-forming cells
- β-gal
β-galactosidase
- CFC
cytokine-forming cells
- CLN
cervical lymph node
- i.n.
intranasal
- LP
lamina propria
- Luc
luciferase
- NALT
nasal-associated lymphoid tissue
- NP
nasal passages
- PP
Peyer's patches
- PL
polylysine
- UEA-1
Ulex europaeus agglutinin 1
- CTL
cytotoxic T lymphocyte
- Th
T helper
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rennels M B, Glass R I, Dennehy P H, Bernstein D I, Pichichero M E, Zito E T, Mack M E, Davidson B L, Kapikian A Z. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 2.Belshe R B, Gruber W C, Mendelman P M, Mehta H B, Mahmood K, Reisinger K, Treanor J, Zangwill K, Hayden F G, Bernstein D I, et al. J Infect Dis. 2000;181:1133–1137. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. J Am Med Assoc. 1999;282:2113–2114. [Google Scholar]
- 4.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson H L, Hunt L A, Webster R G. Vaccine. 1993;11:957–960. doi: 10.1016/0264-410x(93)90385-b. [DOI] [PubMed] [Google Scholar]
- 6.Gallichan W S, Rosenthal K L. J Infect Dis. 1998;177:1155–1161. doi: 10.1086/515286. [DOI] [PubMed] [Google Scholar]
- 7.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, et al. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 8.Boyer J D, Wang B, Ugen K E, Agadjanyan M, Javadian A, Frost P, Dang K, Carrano R A, Ciccarelli R, Coney L, et al. J Med Primatol. 1996;25:242–250. doi: 10.1111/j.1600-0684.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 9.Simon M M, Gern L, Hauser P, Zhong W, Nielsen P J, Kramer M D, Brenner C, Wallich R. Eur J Immunol. 1996;26:2831–2840. doi: 10.1002/eji.1830261206. [DOI] [PubMed] [Google Scholar]
- 10.Feltquate D M, Heaney S, Webster R G, Robinson H L. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 11.Noll A, Bucheler N, Bohn E, Schirmbeck R, Reimann J, Autenrieth I B. Eur J Immunol. 1999;29:986–996. doi: 10.1002/(SICI)1521-4141(199903)29:03<986::AID-IMMU986>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen S C, Jones D H, Fynan E F, Farrar G H, Clegg J C, Greenberg H B, Herrmann J E. J Virol. 1998;72:5757–5761. doi: 10.1128/jvi.72.7.5757-5761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eldridge J H, Staas J K, Meulbroek J A, McGhee J R, Tice T R, Gilley R M. Mol Immunol. 1991;28:287–294. doi: 10.1016/0161-5890(91)90076-v. [DOI] [PubMed] [Google Scholar]
- 14.Jepson M A, Simmons N L, O'Hagan D T, Hirst B H. J Drug Target. 1993;1:245–249. doi: 10.3109/10611869308996082. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Owen R L. In: Handbook of Mucosal Immunology. Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. San Diego: Academic; 1994. pp. 11–26. [Google Scholar]
- 16.Neutra M R, Phillips T L, Mayer E L, Fishkind D J. Cell Tissue Res. 1987;247:537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein D L, O'Neill B L, Hone D M, Metcalf E S. Infect Immun. 1998;66:2310–2318. doi: 10.1128/iai.66.5.2310-2318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf J D, Rubin R, Finberg R. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- 19.Tamura S, Ito Y, Asanuma H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. J Immunol. 1992;149:981–988. [PubMed] [Google Scholar]
- 20.van Ginkel F W, Liu C, Simecka J W, Dong J Y, Greenway T, Frizzell R A, Kiyono H, McGhee J R, Pascual D W. Hum Gene Ther. 1995;6:895–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- 21.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergquist C, Johansson E L, Lagergard T, Holmgren J, Rudin A. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee P W K, Hayes E C, Jolik W K. Virology. 1981;108:156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- 24.Amerongen H M, Wilson G A, Fields B N, Neutra M R. J Virol. 1994;68:8428–8432. doi: 10.1128/jvi.68.12.8428-8432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Boysun M J, Csencsits K L, Pascual D W. Gene Ther. 2000;7:61–69. doi: 10.1038/sj.gt.3301046. [DOI] [PubMed] [Google Scholar]
- 26.Wagner E, Zenke M, Cotton M, Beug H, Birnstiel M L. Proc Natl Acad Sci USA. 1990;87:3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Csencsits K L, Jutila M A, Pascual D W. J Immunol. 1999;163:1382–1389. [PubMed] [Google Scholar]
- 28.Pascual D W, Hone D M, Hall S, van Ginkel F W, Yamamoto M, Walters N, Fujihashi K, Powell R J, Wu S, VanCott J L, et al. Infect Immun. 1999;67:6249–6256. doi: 10.1128/iai.67.12.6249-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Ginkel F W, McGhee J R, Liu C, Simecka J W, Yamamoto M, Frizzell R A, Sorscher E J, Kiyono H, Pascual D W. J Immunol. 1997;159:685–693. [PubMed] [Google Scholar]
- 30.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 31.Ascón M A, Hone D M, Walters N, Pascual D W. Infect Immun. 1998;66:5470–5476. doi: 10.1128/iai.66.11.5470-5476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster N, Clark M A, Jepson M A, Hirst B H. Vaccine. 1998;16:536–541. doi: 10.1016/s0264-410x(97)00222-3. [DOI] [PubMed] [Google Scholar]
- 33.Jones D H, Corris S, McDonald S, Clegg J C S, Farrar G H. Vaccine. 1997;15:814–817. doi: 10.1016/s0264-410x(96)00266-6. [DOI] [PubMed] [Google Scholar]
- 34.Jones D H, Clegg J C S, Farrar G H. Dev Biol Stand. 1998;92:149–155. [PubMed] [Google Scholar]
- 35.Xu-Amano J, Aicher W K, Taguchi T, Kiyono H, McGhee J R. Intern Immunol. 1992;4:433–445. doi: 10.1093/intimm/4.4.433. [DOI] [PubMed] [Google Scholar]
- 36.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, et al. J Immunol. 1995;155:4621–4628. [PubMed] [Google Scholar]
- 37.Hamajima K, Sasaki S, Fukushima J, Kaneko T, Xin K Q, Kudoh I, Okuda K. Clin Immunol Immunopathol. 1998;88:205–210. doi: 10.1006/clin.1998.4566. [DOI] [PubMed] [Google Scholar]
- 38.Bisaillon M, Sénéchal S, Bernier L, Lemay G. J Mol Biol. 1999;286:759–773. doi: 10.1006/jmbi.1998.2495. [DOI] [PubMed] [Google Scholar]
- 39.Shata M T, Stevceva L, Agwale S, Lewis G K, Hone D M. Mol Med Today. 2000;6:66–71. doi: 10.1016/s1357-4310(99)01633-0. [DOI] [PubMed] [Google Scholar]
- 40.Fennelly G J, Khan S A, Abadi M A, Wild T F, Bloom B R. J Immunol. 1999;162:1603–1610. [PubMed] [Google Scholar]
- 41.Klavinskis L S, Gao L, Barnfield C, Lehner T, Parker S. Vaccine. 1997;15:818–820. doi: 10.1016/s0264-410x(96)00278-2. [DOI] [PubMed] [Google Scholar]