All living organisms experience force (Figure 1). These forces exist at the nanoscale level through to the tissue level and range from piconewtons upward to several orders of magnitude higher. Force plays an intimate role in virtually every cellular and tissue-level process and when disrupted promotes pathologies that include cardiovascular disease and cancer. Over the past decade there has been a virtual explosion of studies aimed at understanding the role of mechanics in regulating basic subcellular processes including chromatin organization and cell division, protein trafficking and cell adhesion, and cellular level behaviors that include migration, as well as tissue-level phenotypes that direct development and morphogenesis and underlie disease. This special issue of Molecular Biology of the Cell acknowledges the growing interest by the cell biology community in the critical role of force in cellular and tissue-level processes.
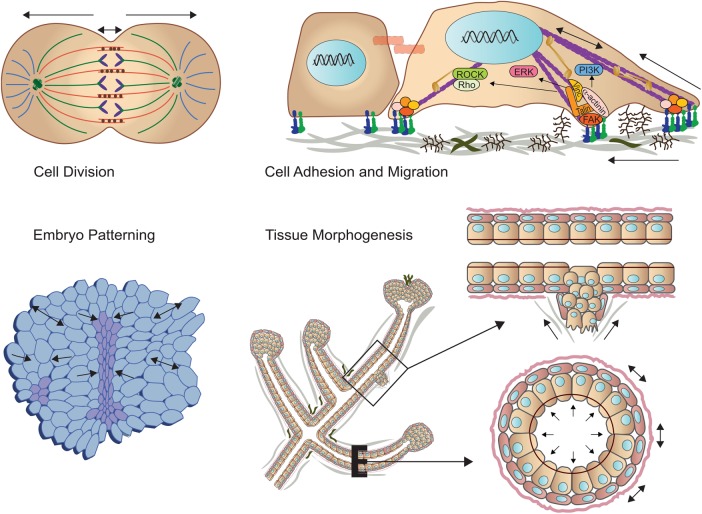
FIGURE 1:
Mechanical force across length scales regulates cell behavior and tissue development. (Top left) Spindle forces are critical for cell division. (Top right) The stiffness of the extracellular matrix promotes focal adhesion assembly to modulate cell migration. (Bottom left) Cell–cell force dictates embryonic patterning. (Bottom right) Tissue-level forces modulate branching morphogenesis and when corrupted can foster cell invasion.
Cells exist within the context of a three-dimensional microenvironment in which they are constantly experiencing a myriad of mechanical and physical cues, and in turn they exert forces on their environment that modulate their behavior. From simple bacteria and archaea to higher-level eukaryotes, survival depends on the ability of an organism to respond to environmental pressures that include a range of mechanical forces. The importance of force in a single-cell organism is addressed in the article by Helmke and colleagues, in which the authors summarize a series of studies they executed aimed at exploring motility regulation in live Toxoplasma gondii (Stadler et al., 2017). In their studies the authors adhered a microsphere to the surface of an immobilized parasite and were thereby able to use a laser trap to directly measure cortical force generation and polarization that provided critical insight into the function of the motility apparatus. Given that these parasites belong to the apicomplexan phylum of intracellular parasites that cause diseases such as toxoplasmosis, malaria, and cryptosporidiosis, this work and future studies using this approach have strong potential to provide important information for developing drugs that could specifically target these organisms to eradicate these diseases. Similarly, in the article titled “Forces that shape fission yeast cells” Fred Chang discusses the importance of cellular mechanics in the fission yeast Schizosaccharomyces pombe, where his work builds upon the view that cell shape arises from the physical properties of an elastic cell wall inflated by internal turgor pressure (Chang, 2017). Chang’s foray into the realm of cell mechanics has provided unique insight into fundamental biological processes linked to force at the single-cell level that regulate yeast growth as well as cytokinesis and endocytosis. The critical role of mechanics in single-cell-organism function is addressed by Kimberly Fong and colleagues, who explored the role of force in regulating microtubule attachment to yeast centrosomes (Fong et al., 2017). Fong and colleagues purified spindle pole bodies from budding yeast and used laser trapping to manipulate single attached microtubules in vitro. Their experiments revealed that spindle pole body–microtubule attachments are extraordinarily strong, and their work identified calmodulin as a major contributor to spindle pole body mechanical integrity, implicating spindle pole binding to microtubule attachments as a key regulator of spindle integrity in mitotic cells.
Consistent with an important role for mechanics in fundamental cellular processes, in the article by Sophie Dumont and Timothy Mitchison’s group, the authors report on work in mammalian cells that addresses the role of microtubule forces mediated by the spindle apparatus through contact at the cell cortex (Guild et al., 2017). They show that a simple change in cell confinement can trigger centrosome separation and increase spindle steady-state length at metaphase. In their studies they found that metaphase and anaphase spindles elongate at the same rate when confined, suggesting that similar elongation forces can be generated independent of biochemical and spindle structural differences. Their data suggest that promoting lateral cortex–microtubule contacts increases dynein-mediated force generation and is sufficient to drive spindle elongation. Moving up length scales, John Marko from the Banigan group addresses the distinct roles of chromatin and lamin A in regulating nuclear mechanics, which is critical for maintaining proper genome organization and gene expression (Stephens et al., 2017). By micro-manipulating single isolated nuclei, they found that chromatin governs the response to small extensions and that eu-/heterochromatin levels modulate nuclear stiffness, whereas the level of lamin A/C controls nuclear strain stiffening at large extensions. By simulating the nucleus as a polymeric shell with a cross-linked polymer interior, Stephens and colleagues have developed a framework for understanding the differential effects of chromatin and lamin A/C in cell nuclear mechanics and the impact of their alterations in diseases including heart disease, progeria, and cancer.
Virtually all organisms have evolved specific structures that are tailored to respond to physical force. Consistently, all cells, including single-cell organisms, plant cells, and mammalian cells, possess the machinery to recognize and respond to many types of mechanical forces such as shear, tensile, and compressive stress and environmental stiffness. A mechanistic comprehension of how cells sense mechanical cues to influence cellular systems across length scales is central to understanding the processes maintaining tissue homeostasis that regulate fundamental biological processes and development and homeostasis and that are hijacked in disease states. Mechanotransduction is the process by which cells detect and integrate mechanical cues through biochemical effectors and translate these signals to alter cellular behavior. The machinery that cells have evolved to sense differences in mechanical loads include mechanosensitive transmembrane proteins such as integrins, discoidin domain receptors, growth factor receptors, and ion channels, as well as more general mechanisms whereby membrane deformations and cytoskeletal remodeling affect membrane domain organization. Mechanical cues are sensed as a change in strain that alters protein conformation or enhances protein–protein interactions or membrane organization.
For the altered molecular state of a protein to induce a change in cell behavior, the mechanical cue needs to be amplified within the cell to alter the activity of enzymes and stimulate signaling mechanisms that alter gene transcription to elicit a sustained cellular response, such as growth or migration or survival. In many cases, intracellular signaling cascades adjust the cell’s reciprocal tension by altering cytoskeleton organization and actomyosin contractility through the action of kinases and Rho-GTPases, or by remodeling the local environment via the secretion of ECM-modifying enzymes. Our understanding of the molecular mechanisms that regulate mechanotransduction remain rudimentary, and they are an area of active investigation. To address this deficiency, in the article from the Garcia group (Zhou et al., 2017) the authors assessed coupling between substrate stiffness and actomyosin contractility to force transmission through vinculin–paxillin recruitment at single focal adhesions to clarify how force stabilizes vinculin’s active conformation to promote force transfer. In their studies, they report that vinculin residence time at a single focal adhesion varies linearly with applied force for stiff substrates but is disrupted on soft substrates and after contractility inhibition. By contrast, they note that paxillin residence time at focal adhesions is independent of local applied force and substrate stiffness and instead is regulated by cytoskeletal contractility in cells on a soft substrate. They go on to show that substrate stiffness and cytoskeletal contractility determine whether vinculin and paxillin turnover dynamics correlate with each other at single focal adhesions. Their analysis sheds insight into how force, substrate stiffness, and focal adhesion dynamics are coupled.
Embryogenesis and tissue development depend on the ability of cells to impart forces on their surroundings so that the cells can efficiently migrate and remodel their extracellular matrix. Cell adhesion-dependent migration, in turn, depends on rigidity adaptation that is mediated, at least in part, through integrin-mediated adhesion to the extracellular matrix. In the article by González-Tarragó and colleagues, the link between the ECM component fibronectin and integrin α5β1 and ZO-1 was assessed in migrating cellular monolayers (González-Tarragó et al., 2017). In their article, they report that the α5β1/ZO-1 complex decreases the resistance to force of α5β1 integrin–fibronectin adhesions located at the edge of migrating cell monolayers, while also increasing α5β1 recruitment. Consistent with a molecular clutch model of adhesion, they found that ZO-1 leads to a decrease in the density and intensity of adhesions in cells at the edge of migrating monolayers. Their findings highlight a new mode of integrin regulation mediated through modification of the mechanical properties of integrin–ECM links, which could be harnessed by cells to control adhesion and migration. Complementing this work, the studies reported by Ahn and colleagues address the impact of force in rear-polarized mechanisms in the cells migrating at the leading edge that control directional migration (Connacher et al., 2017). In this article the authors address the function of a new intracellular complex, named the “Wnt5a-receptor-actomyosin polarity (WRAMP) structure,” which they found coordinates the polarized localization of MCAM, actin, and myosin IIB, in a Wnt5a-induced manner. In their current article, the authors report that cells undergoing prolonged migration assemble WRAMP structures stably polarized at the rear of the cell and that the presence and localization of these structures are strongly associated with enhanced speed and persistence of directional movement. Their work suggests that WRAMP structures represent a rear-directed cellular mechanism to control directional migration and that the ability of WRAMP structures to form dynamically within cells may control changes in direction during extended migration.
Cells within multicellular tissues interact with a three-dimensional extracellular matrix, and cells embedded within hydrogels are able to recapitulate cell and tissue phenotypes more faithfully. Nevertheless, to date the vast majority of work and models in mammalian organisms that aim to clarify the molecular level role of cell and tissue mechanics are based on studies of cells adhering to flat surfaces that transmit cytoskeletally generated forces to their surroundings. In the provocative and timely article by the Dunn group, time-lapse, three-dimensional imaging and quantitative image analysis of fibroblasts embedded within a three-dimensional fibrin matrix are used to determine how the actin cytoskeleton becomes mechanically coupled to the surrounding matrix (Owen et al., 2017). Intriguingly, their studies showed that α-actinin puncta within actomyosin bundles move more quickly than paxillin-rich adhesion plaques, which in turn move more quickly than the local matrix, consistent with predictions of the molecular clutch model. They also found that a subset of stress fibers continuously elongated at their attachment points to adhesions, providing stable yet structurally dynamic coupling to the ECM. The resulting dynamic equilibrium they document could explain how cells maintain stable, contractile connections within a three-dimensional extracellular matrix during cell migration and provides a plausible means by which fibroblasts contract provisional matrices during wound healing.
Moving up length and time scales, cells in multicellular tissues are subject to a myriad of forces, including compressive, tensile, fluid shear stress, and hydrostatic pressure, each of which plays an intricate part in the shaping, development, and maintenance of the tissue. The importance of tissue mechanics in development is illustrated by the elegant studies reported in the article by Wirshing, which highlights the role of myosin actomyosin bundle formation and organization in spermathecal cells during ovulation (Wirshing and Cram, 2017). Wirshing and colleagues used four-dimensional confocal microscopy of live animals to observe changes to spermathecal actomyosin network organization during cell stretch and contraction. They concluded that myosin drives actomyosin bundle production and that myosin activity is tightly regulated during ovulation to produce an optimally organized actomyosin network in Caenorhabditis elegans spermathecae. Moving up the phyla, the article by Killion and colleagues summarizes work addressing the impact of mechanical loading on actin cytoskeletal organization and column formation in the postnatal growth plate (Killion et al., 2017). They created a mouse model in which a portion of the sciatic nerve from one hind limb was transected at postnatal day 8 to paralyze the limb, thereby preventing mechanical load through ambulation. Not only did loss of mechanical load reduce bone mineral density and growth but, in the absence of force, they observed significant alterations in collagen fiber architecture in the growth plate and perturbed organization of the actin cytoskeleton in the growth plate chondrocytes. Their studies underscored the importance of mechanical load and force on chondrocyte structure function in postnatal development.
Consistent with a critical role for mechanics in tissue development and homeostasis, diseases such as atherosclerosis, arthritis, deafness, osteoporosis, and cancer, and a number of developmental disorders, including Kartagener’s syndrome and Hutchinson–Gilford progeria syndrome, commonly result from an abnormal physiological response to extrinsic (applied) or intrinsic (cell-generated) cues. Thus the physical basis for disease can be a product of either altered tensional homeostasis owing to, for example, altered cellular level or tissue-level forces and material properties, or the perturbed cellular response to mechanical stimuli. This concept formed the basis of the work described in the article by Happe and colleagues, who developed mechanically patterned neuromuscular junctions-in-a-dish to enable studies addressing the role of neuromuscular degeneration that leads to progressive motor neuron disease due to the intimate connection between motor neurons and muscle fibers (Happe et al., 2017). A major caveat of current in vitro approaches is the inability to sustain differentiated function of neuromuscular junction in culture. Happe and colleagues developed mechanically patterned extracellular matrices with alternating soft and stiff stripes that enhanced the differentiation, fusion and myotube formation required to support acetylcholine stimulation by cocultured motor neurons with a twitch duration and frequency that recapitulated mature muscle. This system will enable future studies aimed at elucidating disease pathology and identifying drug treatments.
In summary, this current special issue on mechanobiology highlights the breadth and scope of the studies being conducted in this area by members of the cell biology community. The work crosses length scales and encompasses single-cell and multicellular organisms with data underscoring how the basic molecular mechanisms are evolutionarily conserved. Clearly, there is much to be discovered in this exciting interface between the physical sciences and cell biology.
Footnotes
REFERENCES
- Chang F. Forces that shape fission yeast cells. Mol Biol Cell. 2017;28:1819–1824. doi: 10.1091/mbc.E16-09-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connacher MK, Tay JW, Ahn NG. Rear-polarized Wnt5a-receptor-actin-myosin-polarity (WRAMP) structures promote the speed and persistence of directional cell migration. Mol Biol Cell. 2017;28:1924–1936. doi: 10.1091/mbc.E16-12-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong KK, Sarangapani KK, Yusko EC, Riffle M, Llauró A, Graczyk B, Davis TN, Asbury CL. Direct measurement of the strength of microtubule attachment to yeast centrosomes. Mol Biol Cell. 2017;28:1853–1861. doi: 10.1091/mbc.E17-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Tarragó V, Elosegui-Artola A, Bazellières E, Oria R, Pérez-González C, Roca-Cusachs P. Binding of ZO-1 to α5β1 integrins regulates the mechanical properties of α5β1–fibronectin links. Mol Biol Cell. 2017;28:1847–1852. doi: 10.1091/mbc.E17-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild J, Ginzberg MB, Hueschen CL, Mitchison TJ, Dumont S. Increased lateral microtubule contact at the cell cortex is sufficient to drive mammalian spindle elongation. Mol Biol Cell. 2017;28:1975–1983. doi: 10.1091/mbc.E17-03-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe CL, Tenerelli KP, Gromova AK, Kolb F, Engler AJ. Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation. Mol Biol Cell. 2017;28:1950–1958. doi: 10.1091/mbc.E17-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killion CH, Mitchell EH, Duke CG, Serra R. Mechanical loading regulates organization of the actin cytoskeleton and column formation in postnatal growth plate. Mol Biol Cell. 2017;28:1862–1870. doi: 10.1091/mbc.E17-02-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen LM, Adhikari AS, Patel M, Grimmer P, Leijnse N, Kim MC, Notbohm J, Franck C, Dunn AR. A cytoskeletal clutch mediates cellular force transmission in a soft, three-dimensional extracellular matrix. Mol Biol Cell. 2017;28:1959–1974. doi: 10.1091/mbc.E17-02-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler RV, White LA, Hu K, Helmke BP, Guilford WH. Direct measurement of cortical force generation and polarization in a living parasite. Mol Biol Cell. 2017;28:1912–1923. doi: 10.1091/mbc.E16-07-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens AD, Banigan EJ, Adam SA, Goldman RD, Marko JF. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol Biol Cell. 2017;28:1984–1996. doi: 10.1091/mbc.E16-09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirshing ACE, Cram EJ. Myosin activity drives actomyosin bundle formation and organization in contractile cells of the Caenorhabditis elegans spermatheca. Mol Biol Cell. 2017;28:1937–1949. doi: 10.1091/mbc.E17-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou DW, Lee TT, Weng S, Fu J, García AJ. Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin–paxillin recruitment at single focal adhesions. Mol Biol Cell. 2017;28:1901–1911. doi: 10.1091/mbc.E17-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]