Abstract
One of the major challenges of modern cell biology is to understand how cells are assembled from nanoscale components into micrometer-scale entities with a specific size and shape. Here I describe how our quest to understand the morphogenesis of the fission yeast Schizosaccharomyces pombe drove us to investigate cellular mechanics. These studies build on the view that cell shape arises from the physical properties of an elastic cell wall inflated by internal turgor pressure. Consideration of cellular mechanics provides new insights into not only mechanisms responsible for cell-shape determination and growth, but also cellular processes such as cytokinesis and endocytosis. Studies in yeast can help to illuminate approaches and mechanisms to study the mechanobiology of the cell surface in other cell types, including animal cells.
INTRODUCTION
The fission yeast Schizosaccharomyces pombe serves as a simple, tractable model to study the fundamental mechanisms underlying cell morphogenesis. Along with its bacterial counterpart Escherichia coli, rod-shaped eukaryotic S. pombe is one of the simplest model systems for elucidating core concepts that can be applied to more complex, larger cells (Chang and Huang, 2014; Marshall, 2014). In a field populated largely by molecular geneticists and cell biologists, my group and other labs have identified and characterized many of the intracellular molecules, including polarity factors and regulators of the actin and microtubule cytoskeleton, that organize these rod-shaped cells (Chang and Martin, 2009). However, about 10 years ago, I felt that solely studying the function of each gene product and its localization is not sufficient to address the larger questions that interest me the most: how is the shape and size of the cell determined at the micrometer scale? How are rounded shapes such as rods formed? How are the dimensions of cells determined? What are the advantages and disadvantages of certain cell shapes? It was telling that most of the mutants in key polarity programs still formed rod-shaped cells. We seemed to be missing some critical conceptual ingredient.
Nicolas Minc (a physicist postdoc at the time) pointed me to a rich literature on the physics of walled cells, which has been developed primarily in the context of plant cells. These articles posit that the shape of walled cells can be modeled using simple mechanical principles by considering the cell wall as a thin elastic shell inflated by turgor pressure, similar to a rubber balloon (Boudaoud, 2003; Dumais et al., 2006; Campas and Mahadevan, 2009). Simulations of tip growth based on these physical models predict cell shapes that are eerily similar to cellular forms seen in real life, including the familiar “rod” and pear-shaped “shmoo” shapes that appear across kingdoms (Figure 1). These models immediately inspired me to consider whether similar physical mechanisms can explain the shaping of yeast cells.
FIGURE 1:
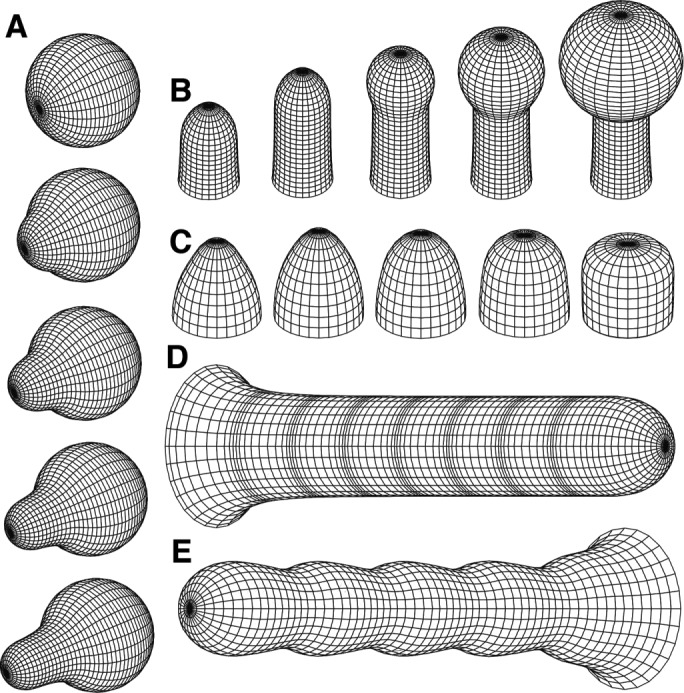
Physical models of tip growth in walled cells recapitulate cell shapes across kingdoms. These simulations depict (A) the emergence of a polarized projection from a sphere; (B) transition from tip growth to the formation of a spherical knob; (C) how changes in parameters alter tip shape; (D) periodic rates of tip growth; and (E) the formation of a beaded rod shape via periodic regulation of cell-wall properties at the growing tip. Reproduced with permission from Dumais et al. (2006).
However, in the context of yeast, this physical view was largely uncharted territory. It was not clear whether these models—developed for plant cells—could also explain the shape of yeast cells. Key parameters such as the mechanical properties of the cell wall and turgor pressure were unknown. In fact, at the time, most yeast cell biologists generally ignored the presence of the cell wall and turgor pressure in their thinking.
Why was this aspect of yeast biology so understudied? One likely reason stems from the sociological structure of science: we usually justify studying yeast as a model for studying conserved processes that are also important in human cells. Hence the perception is that it is easier to obtain funding to study highly conserved proteins such as Cdc42 and actin that have clear counterparts in humans, whereas it seems decidedly risky to focus on yeast cell walls and fungal-specific factors. (However, an equally valid justification for working on fungi is to understand fungi: fungal infections are a significant cause of death in human populations and have few effective treatments, and fungi in general play important roles in agriculture, ecology, and environmental science.) Another reason was the expertise of the field at the time: most of us, including me, were used to thinking more about proteins than about physics. Despites anxieties about moving into an unfamiliar field and concerns for my next grant renewal, I decided that in order to fully understand cell morphogenesis, we simply had to incorporate physical-based mechanisms into our thinking. Thus, working with physicists, we set off in new directions in the lab to explore the mechanics of fission yeast cells.
Here I introduce fission yeast and then describe our forays into cellular mechanics, giving a personal perspective on this scientific journey. I describe how we measured the mechanical properties of the cell and how new perspectives on cellular mechanics yield new insights into the mechanics of cytokinesis, endocytosis, and establishment of cell shape. Finally, I describe how studies of yeast mechanics may be applied to understanding the mechanobiology of mammalian and other cell types.
FISSION YEAST PRIMER
Regular size, shape, and cell-cycle periods make fission yeast a powerful model for studying cell morphogenesis and division (Figure 2). S. pombe are rod-shaped cells ∼8–14 μm in length and ∼4 μm in width. These cells have a similar aspect ratio and shape as E. coli cells but are ∼100-fold larger in volume. Fission yeast cells grow by tip extension during interphase in the cell cycle and cease growth during mitosis and cytokinesis (Figure 2). During cytokinesis, they divide medially through construction of a cell-wall septum. Under optimal growth conditions, the cell cycle of wild-type cells takes ∼2.5 h; G1 and S phases occur just around the time of cell septation and division, and much of the cell cycle is composed of a long G2 phase.
FIGURE 2:
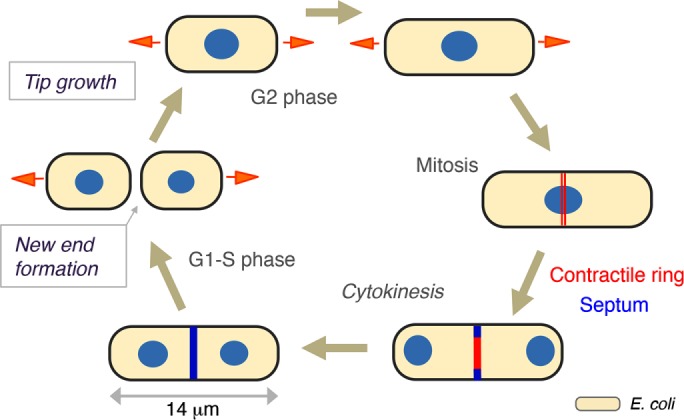
The cell cycle of fission yeast. During interphase, S. pombe cells grow from the cell tips (orange arrows) to 14 μm in length. During mitosis, the cell ceases growth, the mitotic spindle segregates chromosomes, and the actin-based contractile ring (red) assembles at the cell middle. During cytokinesis, the medial septum (blue) grows inward as the contractile ring constricts. Upon cell–cell separation, the cell wall at the septum rapidly adopts a rounded shape to form the new end. The relative size of E. coli is depicted for scale (bottom right).
Although S. pombe cells are highly regular in shape, closer inspection of these cells reveals many subtle features. Most cells have birth scars, which are circumferential ridges of cell wall left from previous cell divisions. Cells also expand slightly over generations, and so different parts of an individual cell can have slightly different widths. In addition, the shapes of the two cell ends differ slightly from each other (Abenza et al., 2015; Atilgan et al., 2015). Fission yeast undergo a specific growth program in which they initially grow from only one end (the old end) just after division and then in G2 phase initiate growth from the other end (the new end) in a process known as new-end takeoff.
Like plants, bacteria, and other fungi, fission yeast cells are encased in a cell wall that gives the cell its shape and dimensions. When a portion of the wall is digested away, the “naked” membrane-bound cell leaks out of the wall and adopts a spherical shape, leaving behind the empty rod-shaped shell of cell-wall material (a “ghost”), which maintains the rod shape. The cell wall is ∼200 nm thick and composed primarily of α, β-glucans and galactomannans, but it apparently lacks chitin (Perez and Ribas, 2004). In general, the organization of the yeast cell wall is not known, but intriguing electron micrographs of protoplasts regrowing their walls reveal bundles of glucan fibers without obvious set orientations (Osumi, 1998).
In fission yeast, many of the intracellular molecular mechanisms underlying cell polarity and cytokinesis have been elucidated (Chang and Martin, 2009; Martin and Arkowitz, 2014). Mutant screens have identified a menagerie of distinct cell shapes, ranging from round, bent, and ovoid shapes to branched T shapes (Verde et al., 1995). Analyses of the gene products represented by these mutants, as well as characterization of the microtubule and actin cytoskeletons, led to the identification of factors that affect cell shape and division. The spatial axis of the cell, which helps to specify the “middle” and “ends” of the cell, is designated in part by interphase microtubules (Chang, 2001; Piel and Tran, 2009). These dynamic microtubule bundles define the cell “middle” by positioning the nucleus in the middle of the cell through a microtubule-pushing mechanism (Tran et al., 2001). The position of the nucleus and spatial cues from cell-tip proteins are used to specify the site of cell division by localizing the anillin-like Mid1 protein (Daga and Chang, 2005). Microtubules also regulate cell polarity at the cell ends by transporting Tea proteins to the cell tips, which regulate formin-mediated actin assembly there (Martin et al., 2005). Many polarity factors, such as the small GTPase Cdc42, localize in dynamic cap distributions at cell ends to coordinate the polarized localization of actin assembly, secretion, and endocytosis (Das et al., 2012). Similarly, for cytokinesis, much is known about molecular mechanisms of assembly of the actin-based contractile ring and the regulation of cell division (Pollard and Wu, 2010). Thus, although we understand quite a bit about these intracellular factors and how they are organized spatially, how they ultimately modulate the cell wall and its mechanical properties remain relatively unknown.
DEFINING THE MECHANICAL PARAMETERS OF THE YEAST CELL
At the onset of our studies of S. pombe mechanics, surprisingly little was known about the mechanical properties of these cells. My preconception was that the cell wall was a rather static, concrete-like rigid shell. To begin testing the physical models of cell morphogenesis, we focused on measuring key parameters: turgor pressure and mechanical properties of the cell wall. We discovered that, as predicted by the models, the yeast cell wall is not like concrete but has elastic properties akin to those of rubber. We could, for instance, repeatedly bend a cell with an external force, and each time, upon release, it popped back into a straight shape (Minc et al., 2009). Addition of an osmotic agent such as sorbitol into the medium causes a decrease in turgor pressure and shrinkage of the cell and its wall (Atilgan et al., 2015); this shrinkage is reversible, as subsequent removal of sorbitol restores normal turgor pressure, and the cell wall reinflates to its original size (Nakayama et al., 2014).
A variety of methods have been developed to determine the mechanical properties of walled cells (Milani et al., 2013). In general, these measurements are challenging and often require creative approaches to suit the particular cell type. Fission yeast offers certain technical advantages for studying cellular mechanics. The simple rod shape greatly facilitates the measurement of cellular dimensions and growth rates. Experiments on turgor pressure are often complicated by cellular adaptations to changes in turgor pressure; in fission yeast, this adaptive response, which involves synthesis of glycerol that gradually restores turgor pressure, can be largely abrogated by mutating a glycerol synthesis gene, gpd1 (Minc et al., 2009). Cell geometry can be controlled in fission yeast through genetic perturbations, as well as by physical manipulation with microfabricated devices (Terenna et al., 2008; Minc et al., 2009; Piel and Tran, 2009; Zhou et al., 2015b).
In fission yeast, we have employed multiple approaches that have yielded consistent estimates of mechanical parameters. Our simplest approach has been to measure the dimensions of S. pombe cells when turgor pressure is reduced by breaking the cell or adding sorbitol to the medium (Atilgan et al., 2015). When the cell is broken, for instance, by a laser shot to the cell surface, it rapidly deflates 17% in width and 9% in length, indicating that turgor pressure normally inflates the cell wall by >50% by volume (Figure 3, A and B). The finding that cell wall stretches twofold more in width than in length is also significant. For a cylindrical thin shell, the stress (force per unit area) around the cell circumference is two times greater than the stress along the longitudinal axis; this twofold ratio in expansion thus suggests that the cell wall is an isotropic material that stretches uniformly in all directions (for a fuller discussion, see Chang and Huang, 2014). We also measured how much cells shrink in response to a range of sorbitol concentrations. Dose–response curves indicated that at 1.5 M external molarity, cells shrink to the dimensions of the relaxed state of the cell walls. These results, coupled with theoretical modeling, led to an estimate of internal pressure of ∼1.5 MPa, which yields a Young’s elastic modulus of ∼50 MPa.
FIGURE 3:
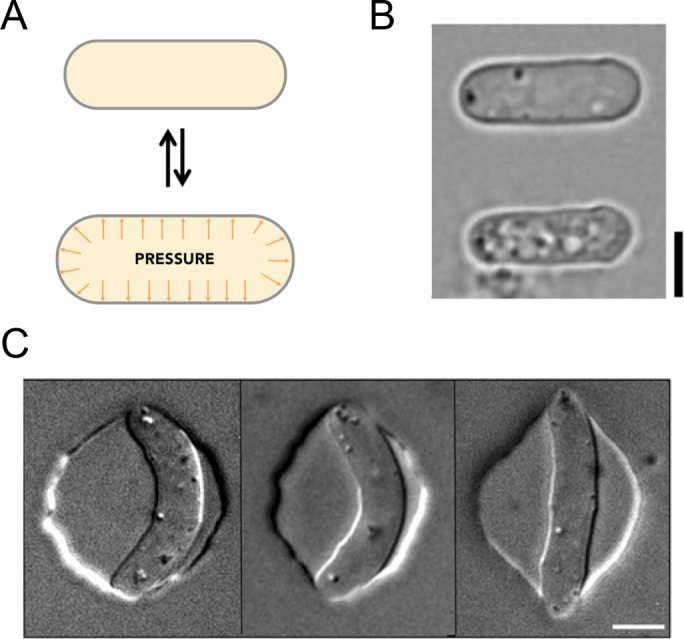
Measuring mechanical properties of fission yeast cells. (A) Modeling the cell wall as an elastic shell that is inflated by turgor pressure. (B) The cell shrinks upon loss of turgor. Bright-field images of a fission yeast cell before (top) and after (bottom) turgor pressure has been released via a laser cut to the cell surface. Reproduced with permission from Atilgan et al. (2015). (C) Images of fission yeast cells inserted into microfabricated chambers of varying stiffness (left to right: hard to soft) to measure cellular mechanical properties. Reproduced with permission from Minc et al. (2009). Scale bars, 5 μm.
In a second set of experiments, we inserted yeast cells into microfabricated circular wells made of polydimethylsiloxane (PDMS) of varying elasticity (Minc et al., 2009). Cells distorted chambers fashioned from soft PDMS, whereas chambers with stiff PDMS bent cells like a fortune cookie (Figure 3C). We estimated the stiffness of the cell by modeling the cell as a beam that buckles under compressive forces. Despite different approaches, these studies gave estimated parameters similar to the analyses in the preceding paragraph. Further, analyses of cells growing and distorting PDMS wells generated force–velocity curves for cell growth.
These estimated parameters revealed that fission yeast are strikingly tough and strong (Minc et al., 2009; Atilgan et al., 2015). Turgor pressure of 1.5 MPa (15 atm) is similar to the pressure found inside a steam engine or within a racing bike tire. The elasticity of the cell wall is similar to that of tough rubber. The stall force of the growing tip is estimated at 1 μN/μm2, which is similar to a human hand supporting hundreds of kilograms—a force that is sufficient for the yeast cell to pierce skin. These forces are orders of magnitude higher than those present when animal cells interact with substrates (du Roure et al., 2005; Style et al., 2014).
Why might a yeast cell need to have such high turgor pressure? One likely answer is that turgor pressure is suited for the lifestyle of fungal cells in their natural environment. Although we understand little about the natural habitat of fission yeast, these cells may live in soil with variable osmotic conditions, and thus high turgor pressure could facilitate their growth in confined spaces and provide sufficient force to pierce objects in search of food. These measurements raise interesting questions about how cells set and regulate their turgor pressure and how these mechanical properties affect cell fitness, growth, and other cellular processes.
THE ROLE OF TURGOR PRESSURE IN SHAPING THE CELL
One function of turgor pressure is to inflate the cell wall to shape the cell and drive cell growth. We began our studies of cell-shape mechanics by investigating a cell-shape transition during “new-end” formation (Atilgan et al., 2015). On separation of two daughter cells at the end of cytokinesis, the flat septum cell wall adopts a rounded shape at the new end (Figure 2). This change in shape could in principle arise from active cell growth and wall remodeling, or, alternatively, it could arise as a simple bulging of the cell wall in response to turgor pressure, without additional cell-wall growth. We tested these possibilities through a series of experiments and modeling. One key experiment showed that in a septated cell, lysis of one of the sister cells causes the septum to rapidly bulge out into the lysed cell, presumably driven by turgor pressure in the intact cell. In other experiments, we discovered that cells treated with the actin inhibitor latrunculin A are still able to complete cytokinesis and form the rounded new ends, suggesting that active growth is not needed for this shape change. Decreasing turgor pressure led to flatter cell ends. Finite-element modeling demonstrated that the effect of turgor pressure on the cell wall is sufficient to explain the rounded shape of the new end. In addition, modeling suggested that the cell wall at the end is not only thinner but also two times softer than the mature lateral cell wall. Thus shaping the new end is one of the simplest examples of how turgor pressure alone can shape the cell wall.
Turgor pressure is also predicted to drive the much more complex process of tip growth (Chang and Huang, 2014). Physical models of tip growth posit that new cell wall is deposited at the growing cell tip, and turgor pressure drives the mechanical expansion of the viscoelastic wall (Lockhart, 1965; Boudaoud, 2003; Dumais et al., 2006; Campas and Mahadevan, 2009). After the cell wall is inserted in a specified region at the cell tip, it may flow like a viscous material and stretch like an elastic material as it moves from the tip to become the lateral cell wall. A maturation process may lead to stiffening of the cell wall as it travels from cell tip to the lateral side of the cell.
Modulation of cell-wall insertion and mechanical properties in space and time dictate the shape and size of the growing tip (Chang and Huang, 2014). Models of these processes (Figure 1), which were developed for plant cells, have recently been applied to cell-tip growth in fission yeast (Drake and Vavylonis, 2013; Abenza et al., 2015). The intracellular machinery of polarity factors, membrane trafficking, and cytoskeleton that regulates cell-wall synthases, modifying enzymes, and mechanical inputs may all modulate the properties of the cell wall to produce a cell tip with a particular shape. Recent studies suggest that this interplay may be quite complex; for instance, there may be mechanisms in which the mechanical properties of the cell wall feed back on the regulation of the polarity machinery (Bonazzi et al., 2014). In general, there is still much to do to test and further develop these models to integrate the mechanical and molecular data. Important questions to address include where cell wall is synthesized at the cell tips, how polarity factors modulate the assembly and mechanical properties of the cell wall, and how mechanics and geometry feed back on polarized growth. Analyses of mutants with altered shapes and/or mechanical properties will provide important tests of the models (Chang and Huang, 2014).
MECHANICS OF CYTOKINESIS
Turgor pressure pushes outward on the plasma membrane against the wall and thus is predicted to oppose the inward movement of the plasma membrane. Our measurements of high turgor pressure suggest that quite significant forces at the cytokinetic furrow and endocytic pit may be needed to move the plasma membrane inward in opposition to this pressure. However, it is also possible that the cell has mechanisms that somehow compensate for these high pressures. Thus we sought to test experimentally whether turgor pressure affects the mechanics of these processes.
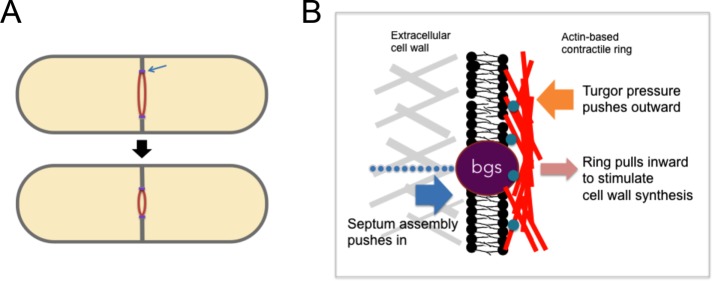
Cytokinesis in fission yeast requires both an actomyosin-based contractile ring and assembly of the cell-wall septum (Figure 4A; Pollard and Wu, 2010). The ring assembles in the medial region of the cell during mitosis. After the chromosomes are segregated, the ring gradually constricts as the septum is formed. During this process, much of the machinery that produces wall at the cell tips is relocated to the medial portion of the cell to build the septum. To move the plasma membrane into the cell for furrow ingression, the actin ring, which is attached to the membrane inside the cell, pull inward on the membrane; in addition, the growth of the cell wall at the septum outside the membrane may push the membrane inward. Based on analogy to the contractile ring in animal cells, the common assumption was that ring constriction provides the primary force.
FIGURE 4:
Mechanics of cytokinesis in fission yeast. (A) Ingression of the cleavage furrow during cytokinesis involves assembly of the septum and constriction of the contractile ring (red). (B) Model of forces involved in furrow ingression at the leading edge of the furrow (blue arrow in A). Septum assembly provides the major force by pushing the membrane inward, opposing turgor pressure. The actin-based contractile ring (red), which is attached to the plasma membrane via membrane proteins (turquoise), provides relatively small pulling forces on the membrane, which may shape the septum by stimulating the activity of glucan synthases (bgs proteins, purple). Adapted from Proctor et al. (2012) and Zhou et al. (2015b).
To test the contribution of turgor pressure, we added a low concentration of sorbitol to cells to lower turgor pressure and found that the furrow ingresses faster, providing initial evidence that turgor pressure is relevant to ingression (Proctor et al., 2012). We then asked, what provides the force for ingression, the constriction of the actin ring, or septum formation? Back-of-the-envelope estimates suggested that the collective forces from the ring are insufficient by orders of magnitude to work against this pressure. When the cell wall is removed, the ring is unable to pinch the cell membrane but instead slides itself in the plane of the membrane; analysis of this process provided an estimate of ring-constriction force that is much smaller than turgor pressure (Stachowiak et al., 2014). Instead of relying on the ring to divide, it is theoretically possible that the assembly of glucan strands by cell-wall synthases at the plasma membrane provides sufficient force for ingression (Proctor et al., 2012). Consistent with this hypothesis, we discovered that, remarkably, ingression of the furrow continued even upon loss of the actin ring (Proctor et al., 2012). These findings suggest that cell-wall assembly, and not the contractile ring, provides the primary force for furrow ingression in fission yeast.
Then what is the function of the contractile ring? One function is to set the position of the machinery that builds the septum. Another function may be to spatially control septum formation as the furrow ingresses. When the ring is disrupted during ingression, the septum continues to grow inward but often in a disorganized manner so that the furrow is no longer circular (Zhou et al., 2015b). As shown in cells manipulated into different shapes, the ring appears to promote cell-wall growth in a curvature-dependent manner; curved parts of the septum grow significantly faster than flat portions. This property suggests a model in which pulling forces from the actin ring, which are predicted to be curvature dependent, locally stimulate the cell-wall synthesis machinery through a mechanosensitive mechanism (Figure 4B). This mechanism may ensure that the septum maintains circularity as it grows inward in order to make cytokinesis more robust (Thiyagarajan et al., 2015; Zhou et al., 2015b).
At the end of cytokinesis, the two sister cells split apart when the completed septum that holds them together is digested away. Turgor pressure may drive this splitting, helping to tear apart the septum and rapidly push the sister cells away from each other as the septum bulges out into the new end (Atilgan et al., 2015; Zhou et al., 2015a). This splitting may function to organize cells in the microcolony and enable cells to grow from the new end. These studies on cytokinesis illustrate how the measurement of a particular parameter (in this case, of turgor pressure) can lead to new insights into cellular mechanism.
MECHANICS OF ENDOCYTOSIS
During endocytosis, the plasma membrane moves inward to form the endocytic pit. As with cytokinesis, we asked whether turgor pressure affects the endocytic process. Clathrin-mediated endocytosis in yeast requires a branched network of actin that assembles around the endocytic pit (Sirotkin et al., 2010). These actin filaments, coupled with myosin type I motor proteins, may provide the force necessary to oppose turgor pressure. We found that reducing turgor pressure with sorbitol significantly accelerates an early step of endocytosis by up to 40% (Basu et al., 2014; see also (Aghamohammadzadeh and Ayscough, 2009). Addition of sorbitol also suppresses endocytic defects caused by partial loss of actin or in certain endocytosis mutants such as WASp mutants, further supporting the idea that actin-dependent forces oppose turgor pressure. Similar actin-dependent mechanisms may oppose membrane tension for endocytosis in animal cells (Boulant et al., 2011). These studies highlight the mechanical requirements of endocytosis and have inspired recent models of endocytosis that examine how actin filaments generate significant local forces to oppose turgor pressure (Carlsson and Bayly, 2014; Dmitrieff and Nedelec, 2015; Dmitrieff and Nedelec, 2016).
PRESSURE IN ANIMAL CELLS
The study of cell mechanics in yeast may establish a framework for studying mechanics in other cell types. Although mammalian cells have long been believed to have negligible internal pressure, recent studies have shown that they do have pressure, which drives significant changes in cell shape. Of interest, pressure is regulated in the cell cycle: during mitosis, cells swell due in part to an increase in osmotic pressure (Son et al., 2015; Zlotek-Zlotkiewicz et al., 2015). Current estimates of intracellular pressure are in the range of 100 kPa at mitosis, ∼10-fold lower than in fission yeast (Zlotek-Zlotkiewicz et al., 2015). As in yeast, internal pressures in animal cells are likely to affect processes such as the generation of cell shape, endocytosis, cell migration, and division (Charras et al., 2005; Boulant et al., 2011; Sedzinski et al., 2011; Houk et al., 2012; Salbreux et al., 2012). In a recent example, internal pressure was shown to affect cytokinesis in an optogenetically controlled Rho system (Wagner and Glotzer, 2016). Instead of a thick cell wall, in mammalian cells, the actin-rich cortex and the extracellular matrix may contribute to the mechanical properties of the cell surface. Thus, although I had initially believed that these studies on turgor pressure in yeast would not be so applicable to animal cells, it is becoming apparent that the concepts and approaches developed in yeast and other walled cells are quite relevant for understanding aspects of the mechanobiology of animal cells.
CONCLUSION
In this Perspective, I have described our scientific journey in studying cell mechanics in fission yeast. We have been challenged to develop new approaches and learn new concepts, and we have been rewarded with exciting new views of cell morphogenesis and division. These initial studies now raise a plethora of fascinating new questions to investigate. For instance, how are mechanical parameters set and maintained by the cell, and how do mechanical signals regulate cellular processes such as cell-wall synthesis and cell polarity mechanisms in order to shape the cell? Why do cells ”care” to adopt a particular shape (Chang and Huang, 2014)? The many experimental advantages in yeast will allow relatively rapid progress in studying how molecules and mechanics affect each other and will lead to more comprehensive models that incorporate both molecular and physical features.
These are still early days of studying the quantitative bases of cell morphogenesis. Using a simple and experimentally tractable model such as yeast enables us to define universal concepts and elucidate conserved molecular and physical features underlying cell morphogenesis. Just as yeast is well appreciated as a model to elucidate molecular mechanisms, it may also serve as a powerful model system for studying cellular mechanics and the basic design principles of cellular form across the kingdoms of life.
Acknowledgments
I am grateful to Nicolas Minc and Areski Boudaoud for providing the initial impetus to study cellular mechanics and to K. C. Huang, Benjamin Knapp, Erdinc Atilgan, Stephen Proctor, Zhou Zhou, Roshni Basu, Otger Campas, Enrique Rojas, and Orna Cohen-Fix for fruitful discussions and comments. I apologize for not citing the work of others more fully, as this was meant to focus on my personal perspective. This work was supported by National Institutes of Health Grant GM056836.
Abbreviation used:
- PDMS
polydimethylsiloxane.
Footnotes
REFERENCES
- Abenza JF, Couturier E, Dodgson J, Dickmann J, Chessel A, Dumais J, Carazo Salas RE. Wall mechanics and exocytosis define the shape of growth domains in fission yeast. Nat Commun. 2015;6:8400. doi: 10.1038/ncomms9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghamohammadzadeh S, Ayscough KR. Differential requirements for actin during yeast and mammalian endocytosis. Nat Cell Biol. 2009;11:1039–1042. doi: 10.1038/ncb1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atilgan E, Magidson V, Khodjakov A, Chang F. Morphogenesis of the fission yeast cell through cell wall expansion. Curr Biol. 2015;25:2150–2157. doi: 10.1016/j.cub.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Munteanu EL, Chang F. Role of turgor pressure in endocytosis in fission yeast. Mol Biol Cell. 2014;25:679–687. doi: 10.1091/mbc.E13-10-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi D, Julien JD, Romao M, Seddiki R, Piel M, Boudaoud A, Minc N. Symmetry breaking in spore germination relies on an interplay between polar cap stability and spore wall mechanics. Dev Cell. 2014;28:534–546. doi: 10.1016/j.devcel.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Boudaoud A. Growth of walled cells: from shells to vesicles. Phys Rev Lett. 2003;91:018104. doi: 10.1103/PhysRevLett.91.018104. [DOI] [PubMed] [Google Scholar]
- Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13:1124–1131. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campas O, Mahadevan L. Shape and dynamics of tip-growing cells. Curr Biol. 2009;19:2102–2107. doi: 10.1016/j.cub.2009.10.075. [DOI] [PubMed] [Google Scholar]
- Carlsson AE, Bayly PV. Force generation by endocytic actin patches in budding yeast. Biophys J. 2014;106:1596–1606. doi: 10.1016/j.bpj.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. Establishment of a cellular axis in fission yeast. Trends Genet. 2001;17:273–278. doi: 10.1016/s0168-9525(01)02279-x. [DOI] [PubMed] [Google Scholar]
- Chang F, Huang K. How and why cells grow as rods. BMC Biol. 2014;12:54. doi: 10.1186/s12915-014-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Martin SG. Shaping fission yeast with microtubules. Cold Spring Harb Perspect Biol. 2009;1:a001347. doi: 10.1101/cshperspect.a001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga RR, Chang F. Dynamic positioning of the fission yeast cell division plane. Proc Natl Acad Sci USA. 2005;102:8228–8232. doi: 10.1073/pnas.0409021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Drake T, Wiley DJ, Buchwald P, Vavylonis D, Verde F. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science. 2012;337:239–243. doi: 10.1126/science.1218377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieff S, Nedelec F. Membrane mechanics of endocytosis in cells with turgor. PLoS Comput Biol. 2015;11:e1004538. doi: 10.1371/journal.pcbi.1004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieff S, Nedelec F. Amplification of actin polymerization forces. J Cell Biol. 2016;212:763–766. doi: 10.1083/jcb.201512019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T, Vavylonis D. Model of fission yeast cell shape driven by membrane-bound growth factors and the cytoskeleton. PLoS Comput Biol. 2013;9:e1003287. doi: 10.1371/journal.pcbi.1003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Silberzan P, Ladoux B. Force mapping in epithelial cell migration. Proc Natl Acad Sci USA. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais J, Shaw SL, Steele CR, Long SR, Ray PM. An anisotropic-viscoplastic model of plant cell morphogenesis by tip growth. Int J Dev Biol. 2006;50:209–222. doi: 10.1387/ijdb.052066jd. [DOI] [PubMed] [Google Scholar]
- Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, Altschuler SJ, Wu LF, Weiner OD. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart JA. An analysis of irreversible plant cell elongation. J Theor Biol. 1965;8:264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Marshall W. 2014. Commonality and what is the hydrogen atom equivalent for cell shape. Available at https://blogs.biomedcentral.com/on-biology/2014/08/04/commonality-and-what-is-the-hydrogen-atom-equivalent-for-cell-shape/ (accessed 4 August 2014) [Google Scholar]
- Martin SG, Arkowitz RA. Cell polarization in budding and fission yeasts. FEMS Microbiol Rev. 2014;38:228–253. doi: 10.1111/1574-6976.12055. [DOI] [PubMed] [Google Scholar]
- Martin SG, McDonald WH, Yates JR, 3rd, Chang F. Tea4p links microtubule plus ends with the formin for3p in the establishment of cell polarity. Dev Cell. 2005;8:479–491. doi: 10.1016/j.devcel.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Milani P, Braybrook SA, Boudaoud A. Shrinking the hammer: micromechanical approaches to morphogenesis. J Exp Bot. 2013;64:4651–4662. doi: 10.1093/jxb/ert169. [DOI] [PubMed] [Google Scholar]
- Minc N, Boudaoud A, Chang F. Mechanical forces of fission yeast growth. Curr Biol. 2009;19:1096–1101. doi: 10.1016/j.cub.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Hirata A, Iida H. Mechanosensitive channels Msy1 and Msy2 are required for maintaining organelle integrity upon hypoosmotic shock in Schizosaccharomyces pombe. FEMS Yeast Res. 2014;14:992–994. doi: 10.1111/1567-1364.12181. [DOI] [PubMed] [Google Scholar]
- Osumi M. The ultrastructure of yeast: cell wall structure and formation. Micron. 1998;29:207–233. doi: 10.1016/s0968-4328(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Perez P, Ribas JC. Cell wall analysis. Methods. 2004;33:245–251. doi: 10.1016/j.ymeth.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Piel M, Tran PT. Cell shape and cell division in fission yeast. Curr Biol. 2009;19:R823–R827. doi: 10.1016/j.cub.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Wu JQ. Understanding cytokinesis: lessons from fission yeast. Nat Rev Mol Cell Biol. 2010;11:149–155. doi: 10.1038/nrm2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SA, Minc N, Boudaoud A, Chang F. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr Biol. 2012;22:1601–1608. doi: 10.1016/j.cub.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbreux G, Charras G, Paluch E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012;22:536–545. doi: 10.1016/j.tcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Sedzinski J, Biro M, Oswald A, Tinevez JY, Salbreux G, Paluch E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature. 2011;476:462–466. doi: 10.1038/nature10286. [DOI] [PubMed] [Google Scholar]
- Sirotkin V, Berro J, Macmillan K, Zhao L, Pollard TD. Quantitative analysis of the mechanism of endocytic actin patch assembly and disassembly in fission yeast. Mol Biol Cell. 2010;21:2894–2904. doi: 10.1091/mbc.E10-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S, Kang JH, Oh S, Kirschner MW, Mitchison TJ, Manalis S. Resonant microchannel volume and mass measurements show that suspended cells swell during mitosis. J Cell Biol. 2015;211:757–763. doi: 10.1083/jcb.201505058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak MR, Laplante C, Chin HF, Guirao B, Karatekin E, Pollard TD, O’Shaughnessy B. Mechanism of cytokinetic contractile ring constriction in fission yeast. Dev Cell. 2014;29:547–561. doi: 10.1016/j.devcel.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Style RW, Boltyanskiy R, German GK, Hyland C, MacMinn CW, Mertz AF, Wilen LA, Xu Y, Dufresne ER. Traction force microscopy in physics and biology. Soft Matter. 2014;10:4047–4055. doi: 10.1039/c4sm00264d. [DOI] [PubMed] [Google Scholar]
- Terenna CR, Makushok T, Velve-Casquillas G, Baigl D, Chen Y, Bornens M, Paoletti A, Piel M, Tran PT. Physical mechanisms redirecting cell polarity and cell shape in fission yeast. Curr Biol. 2008;18:1748–1753. doi: 10.1016/j.cub.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiyagarajan S, Munteanu EL, Arasada R, Pollard TD, O’Shaughnessy B. The fission yeast cytokinetic contractile ring regulates septum shape and closure. J Cell Sci. 2015;128:3672–3681. doi: 10.1242/jcs.166926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PT, Marsh L, Doye V, Inoue S, Chang F. A mechanism for nuclear positioning in fission yeast based upon microtubule pushing. J Cell Biol. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Mata J, Nurse P. Fission yeast cell morphogenesis: identification of new genes and analysis of their role during the cell cycle. J Cell Biol. 1995;131:1529–1538. doi: 10.1083/jcb.131.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E, Glotzer M. Local RhoA activation induces cytokinetic furrows independent of spindle position and cell cycle stage. J Cell Biol. 2016;213:641–649. doi: 10.1083/jcb.201603025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Halladin DK, Rojas ER, Koslover EF, Lee TK, Huang KC, Theriot JA. Bacterial division. Mechanical crack propagation drives millisecond daughter cell separation in Staphylococcus aureus. Science. 2015a;348:574–578. doi: 10.1126/science.aaa1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Munteanu EL, He J, Ursell T, Bathe M, Huang KC, Chang F. The contractile ring coordinates curvature-dependent septum assembly during fission yeast cytokinesis. Mol Biol Cell. 2015b;26:78–90. doi: 10.1091/mbc.E14-10-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotek-Zlotkiewicz E, Monnier S, Cappello G, Le Berre M, Piel M. Optical volume and mass measurements show that mammalian cells swell during mitosis. J Cell Biol. 2015;211:765–774. doi: 10.1083/jcb.201505056. [DOI] [PMC free article] [PubMed] [Google Scholar]