Abstract
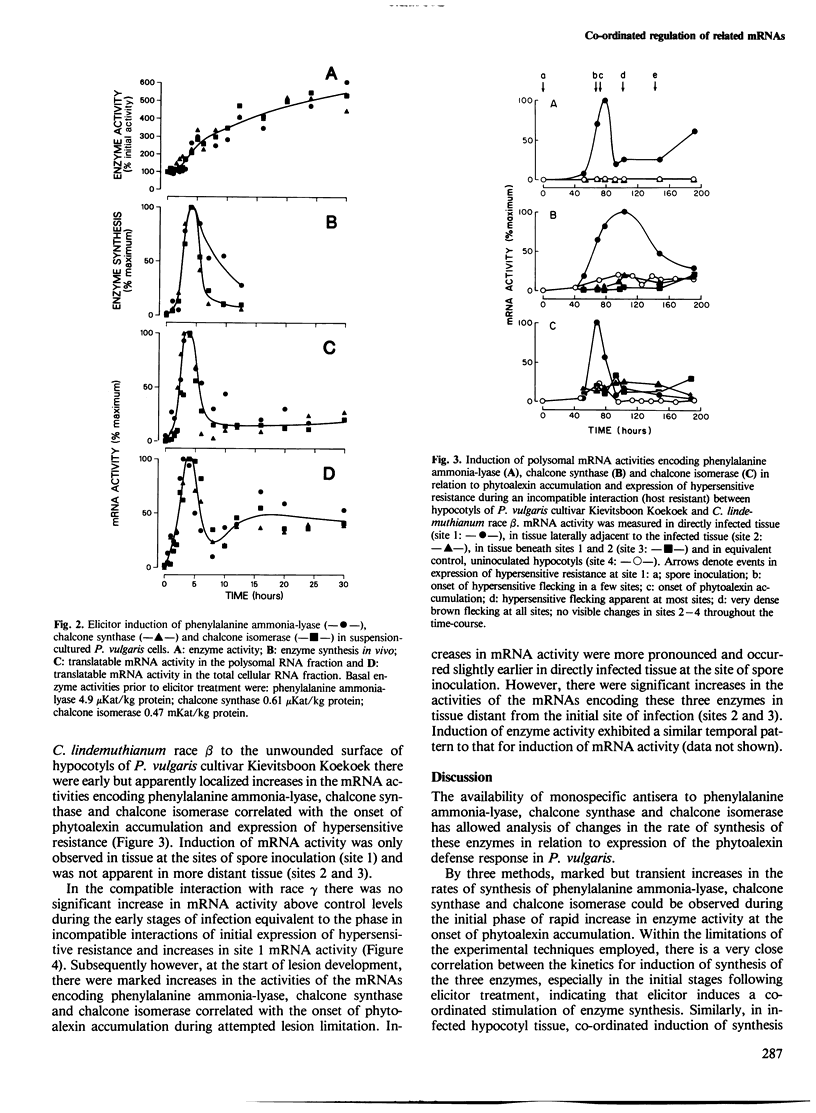
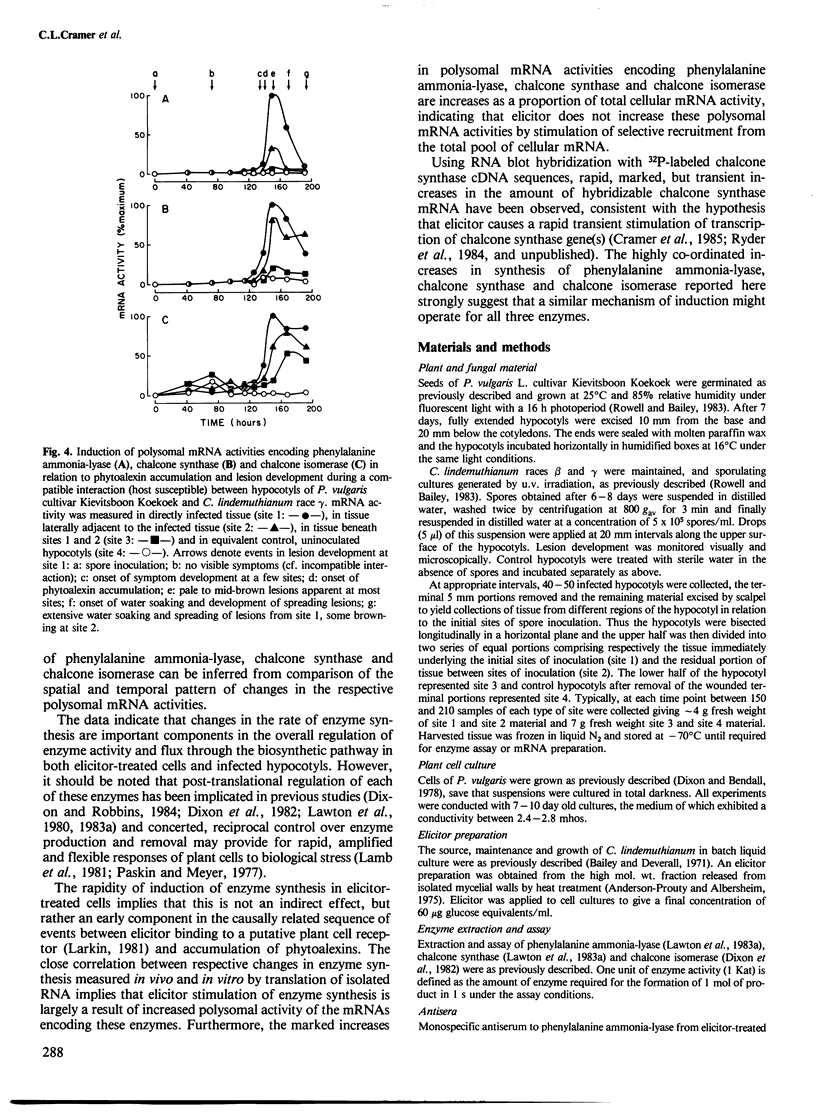
Changes in the rates of synthesis of three enzymes of phenyl-propanoid biosynthesis in Phaseolus vulgaris L. (dwarf French bean) have been investigated by immunoprecipitation of [35S]methionine-labeled enzyme subunits with mono-specific antisera. Elicitor causes marked, rapid but transient co-ordinated increases in the rate of synthesis of phenyl-alanine ammonia-lyase, chalcone synthase and chalcone isomerase concomitant with the phase of rapid increase in enzyme activity at the onset of accumulation of phenyl-propanoid-derived phytoalexin antibiotics in suspension cultures of P. vulgaris. Co-ordinate induction of enzyme synthesis is also observed in hypocotyl tissue during race:cultivar-specific interactions with Colletotrichum lindemuthianum, causal agent of anthracnose. In an incompatible interaction (host resistant) there are early increases apparently localized to the initial site of infection prior to the onset of phytoalexin accumulation and expression of hypersensitive resistance. In contrast, in a compatible interaction (host susceptible) there is no induction of synthesis in the early stages of infection, but a delayed widespread response at the onset of lesion formation associated with attempted lesion limitation. It is concluded that expression of the phytoalexin defense response in biologically stressed cells of P. vulgaris characteristically involves co-ordinate induction of synthesis of phytoalexin biosynthetic enzymes.
Keywords: chalcone isomerase, chalcone synthase, enzyme synthesis, phenylalanine ammonia-lyase, plant disease resistance
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson-Prouty A. J., Albersheim P. Host-Pathogen Interactions: VIII. Isolation of a Pathogen-synthesized Fraction Rich in Glucan That Elicits a Defense Response in the Pathogen's Host. Plant Physiol. 1975 Aug;56(2):286–291. doi: 10.1104/pp.56.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. N., Dixon R. A., Bailey J. A., Rowell P. M., Lamb C. J. Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3384–3388. doi: 10.1073/pnas.81.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lamb C. J. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983;55:1–136. doi: 10.1002/9780470123010.ch1. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Haffner M. H., Chin M. B., Lane B. G. Wheat embryo ribonucleates. XII. Formal characterization of terminal and penultimate nucleoside residues at the 5'-ends of "capped" RNA from imbibing wheat embryos. Can J Biochem. 1978 Jul;56(7):729–733. doi: 10.1139/o78-109. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Merritt T. K., Butt V. S. Synthesis and removal of phenylalanine ammonia-lyase activity in illuminated discs of potato tuber parenchyme. Biochim Biophys Acta. 1979 Jan 18;582(2):196–212. doi: 10.1016/0304-4165(79)90384-2. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Rubery P. H. Interpretation of the rate of density labelling of enzymes with 2H2O. Possible implications for the mode of action of phytochrome. Biochim Biophys Acta. 1976 Feb 24;421(2):308–318. doi: 10.1016/0304-4165(76)90297-x. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. J. Elicitor induction of mRNA activity. Rapid effects of elicitor on phenylalanine ammonia-lyase and chalcone synthase mRNA activities in bean cells. Eur J Biochem. 1983 Jan 17;130(1):131–139. [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. Rapid induction of the synthesis of phenylalanine ammonia-lyase and of chalcone synthase in elicitor-treated plant cells. Eur J Biochem. 1983 Jan 1;129(3):593–601. doi: 10.1111/j.1432-1033.1983.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Lamb C. J. Elicitor modulation of the turnover of L-phenylalanine ammonia-lyase in French bean cell suspension cultures. Biochim Biophys Acta. 1980 Dec 1;633(2):162–175. doi: 10.1016/0304-4165(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Paskin N., Mayer R. J. The role of enzyme degradation in enzyme turnover during tissue differentiation. Biochim Biophys Acta. 1977 Jan 3;474(1):1–10. doi: 10.1016/0005-2787(77)90208-8. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Methods for analysis of enzyme synthesis and degradation in animal tissues. Methods Enzymol. 1975;40:241–266. doi: 10.1016/s0076-6879(75)40020-9. [DOI] [PubMed] [Google Scholar]
- Schröder J., Betz B., Hahlbrock K. Light-induced enzyme synthesis in cell suspension cultures of Petroselinum hortense. Demonstration in a heterologous cell-free system of rapid changes in the rate of phenylalanine ammonia-lyase synthesis. Eur J Biochem. 1976 Aug 16;67(2):527–541. doi: 10.1111/j.1432-1033.1976.tb10719.x. [DOI] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]




