Abstract
Outcomes for patients with multiple myeloma (MM) have improved in recent years owing to use of novel agents and high-dose therapy followed by autologous stem cell transplant (ASCT). We analyzed the outcomes of 511 consecutive patients treated with novel therapies at our institution between 2006 and 2014 to determine the impact of relapse within 12 months of initiating treatment. A total of 82 patients (16.0%) experienced early relapse, with median time to relapse of 8.0 months (95% confidence interval (CI); 6.3, 8.9). Median overall survival (OS) was significantly worse for this group at 21.0 months (95% CI; 16.3, 27.2) vs not reached (NR) (95% CI; 96.3, NR) for those with late relapse (P<0.001). Survival outcomes remained poor among early relapse patients irrespective of depth of response to initial therapy. In multivariate analysis, low albumin and high-risk cytogenetics predicted early relapse. Outcomes of early relapse from early ASCT were also considered; median OS from ASCT for those relapsing within 12 months was 23.1 months (95% CI; 15.7, 32.4) vs 122.2 months (95% CI; 111.5, 122.2) for the remaining patients (P<0.001). Early relapse remains a marker of poor prognosis in the current era, and such patients should be targeted for clinical trials.
INTRODUCTION
Outcomes for patients with multiple myeloma (MM) have improved considerably in recent years, in large part owing to widespread adoption of novel agents and high-dose therapy followed by autologous stem cell transplant (ASCT). Still, for patients who survive initial treatment, the natural history of the disease frequently involves relapse, necessitating multiple lines of subsequent therapy. Study of the clinical course of relapsed MM in the era of conventional therapies found decreased durability of response with each successive salvage regimen,1 a finding which has been attributed to acquired drug resistance and the underlying kinetics of the disease.2 Accordingly, time to progression has historically been considered an important prognostic factor.3,4 In fact, the duration of initial response identifies a group of patients with particularly poor outcomes even in the absence of conventional high-risk factors, including high-risk cytogenetics by fluorescent in situ hybridization (FISH). This has been further confirmed in the transplant setting, with reduced overall survival (OS) observed in patients relapsing within 12 months of receiving high-dose therapy and ASCT, both in the era of conventional5 and novel agents.6 A relevant question is whether early relapse following induction or early transplant carries similar prognostic value in the current treatment landscape, in which effective agents are used upfront and a variety of options exist for salvage therapy. In the present study, we evaluated patients treated at our institution after 2006 to assess the impact of early relapse following induction with a novel agent or after an early ASCT, as well as to identify factors that may predispose to early relapse in these two settings.
PATIENTS AND METHODS
Patients
Relevant demographic, clinical and laboratory data of patients treated for MM at the Mayo Clinic (Rochester, MN, USA) are maintained in a continuously updated research database. For those who receive long-term follow-up at outside institutions, updated data are requested from patients and providers on an annual basis. For the purpose of this study, we identified all consecutive patients evaluated for MM at Mayo Clinic within 30 days of diagnosis and initiated treatment between 1 January 2006 and 31 December 2014. Patients with primary refractory disease (that is, failure to achieve at least a partial response (PR) to initial therapy) were excluded, as were patients who had continuing response but <12 months of follow-up from the start of initial therapy. Those who did not receive a novel agent with induction were similarly excluded. Initial and subsequent regimens (when applicable) were noted, along with corresponding start and end dates. Date of first relapse was recorded for all evaluable patients, in accordance with the criteria described below. We additionally gathered data on an expanded, secondary cohort of patients who were seen at Mayo Clinic primarily for transplant over a similar time period. Patients in this cohort were only considered if they had received ASCT within 12 months of diagnosis. Unlike patients in the primary cohort, they did not need to be seen within 30 days of diagnosis, and in many cases, had received initial therapy at outside institutions. Additionally, they did not require induction with a novel agent and primary refractory patients were included. The study was conducted with the approval of the Mayo Clinic Institutional Review Board.
Disease definitions
Novel therapies include immunomodulatory drugs and proteosome inhibitors. Relapse was defined based on recommendations from the International Myeloma Workshop Consensus Panel.7 Specifically, first relapse was defined as progression following induction therapy in patients who did not have primary refractory disease. Depth of response and progression were determined based on criteria defined by the International Myeloma Working Group.8 Relapse was considered early if it occurred within 12 months of starting initial therapy. The late relapse group consisted of patients who either relapsed after this point or who had continuing response with at least 12 months of follow-up. ASCT was considered early if it occurred within 12 months of diagnosis. FISH analysis was performed, as previously described,9 by utilization of the following probes: 3cen (D3Z1), 7cen (D7Z1), 9cen (D9Z1), 15cen (D15Z4), 11q13 (CCND1-XT), 14q32 (IGHXT), 13q14 (RB1), 13q34 (LAMP1), 14q32 (5′IGH, 3′IGH), 17p13.1 (p53), and 17cen (D17Z1). The result from these probes is reported positive when the percentage of cells possessing the evaluated abnormality falls above a prespecified cutoff (indicated below). Specificity was enhanced by immunofluorescent detection of cytoplasmic immunoglobulin light in chain in plasma cells.9 High-risk FISH was defined as the loss of a p53 gene locus (either del17p (>7%) or monosomy 17 (>9%)) or presence of t(4;14) (>3%), t(14;16) (>5%) or t(14;20) (>6%) at the time of diagnosis.
Statistical analysis
JMP 10.0.0 (SAS Institute Inc., Cary, NC, USA) was used to perform all statistical analyses. Kaplan–Meier curves were generated for analyzing OS, and the log-rank test was used to compare survival curves. Nominal variables were compared with chi-square tests and Fisher exact tests, and continuous variables were compared with Mann–Whitney U-test or Kruskal–Wallis test. Multivariate analyses were performed using the Cox proportional hazards model.
RESULTS
Early vs late relapse from diagnosis
Five-hundred and eleven patients with newly diagnosed MM were found to meet study criteria and were included in the analysis. Baseline demographic and laboratory values, separated by relapse group, are presented in Table 1. The median estimated follow-up for the entire cohort was 44.9 months (95% confidence interval (CI); 41.9, 49.3); 344 (67.3%) were alive at the time of analysis. Eighty-two (16.0%) patients relapsed within 12 months of starting treatment, compared with 429 (84.0%) who either relapsed after 1 year or had continuing response at the time of analysis. A total of 34 (41.5%) patients in the early relapse group had received ASCT at some point, compared with 242 (56.4%) late relapse patients (P = 0.01). Of the patients undergoing ASCT, 19 (55.8%) in the early relapse group compared with 209 (86.3%) in the late relapse group underwent ASCT before relapse (P<0.001).
Table 1.
Baseline clinical and laboratory characteristics of primary cohort
| Characteristics | Early relapse (n = 82) | Late relapse (n = 429) | P-value |
|---|---|---|---|
| Male (%) | 69.5 | 55.7 | 0.021 |
| Age (years) | |||
| Median | 65.9 | 65.4 | NS |
| Range | 28.7–91.0 | 31.9–88.8 | |
| Serum M-spike (g/dl) | |||
| Median | 2.9 | 2.5 | NS |
| Range | 0.0–10.3 | 0–10.0 | |
| Albumin (g/dl) | |||
| Median | 3.4 | 3.5 | <0.001 |
| Range | 1.7–4.4 | 2.1–4.8 | |
| <3.5 (%) | 62.2 | 40.4 | <0.001 |
| Calcium (g/dl) | |||
| Median | 9.4 | 9.5 | NS |
| Range | 7.5–15.9 | 4.5–15.3 | |
| Creatinine (g/dl) | |||
| Median | 1.1 | 1 | NS |
| Range | 0.5–6.4 | 0.4–10.0 | |
| At least 2.0 (%) | 18.9 | 12.4 | NS |
| Hemoglobin (g/dl) | |||
| Median | 11 | 11 | NS |
| Range | 6.1–15.7 | 5.7–16.2 | |
| Serum β2M (mg/l) | |||
| Median | 4.4 | 3.8 | 0.028 |
| Range | 1.9–53.6 | 1.2–48.2 | |
| >5.5 (%) | 40.3 | 27.8 | 0.048 |
| LDH (U/l) | |||
| Median | 172 | 161 | 0.039 |
| Range | 80–1083 | 51–683 | |
| At least ULN (%) | 34.9 | 24.1 | NS |
| BMPC (%) | |||
| Median | 59 | 52 | NS |
| Range | 5.0–98.0 | 2.0–100.0 | |
| PCLI (%) | |||
| Median | 1.2 | 0.8 | 0.043 |
| Range | 0.0–13.0 | 0–11.0 | |
| >3 (%) | 22 | 9.6 | 0.027 |
| High-risk FISH (%) | |||
| Yes | 65.7 | 31.2 | <0.001 |
| ISS (%) | |||
| Stage 3 | 42 | 29.9 | NS |
| Light chain type (%) | |||
| Kappa | 54.4 | 66.8 | NS |
| Lambda | 45.6 | 33.2 | 0.049 |
Abbreviations: BMPC, bone marrow plasma cell; β2M, beta-2 microglobulin; FISH, fluorescent in situ hybridization; LDH, lactate dehydrogenase; ISS, international staging system; NS, not significant; PCLI, plasma cell labeling index; ULN, upper limit of normal.
A variety of induction regimens were utilized, each incorporating an immunomodulatory drug, proteosome inhibitor or both. Early relapse was experienced by 42 (15.7%) of the 267 patients receiving lenalidomide and dexamethasone for induction, 18 (22.8%) of the 79 receiving cyclophosphamide, bortezomib and dexamethasone, 7 (15.2%) of the 46 patients receiving bortezomib, lenalidomide and dexamethasone and 8 (20.5%) of the 39 receiving bortezomib and dexamethasone. The remaining patients received various other combinations of these drugs. Of those with early relapse, 10 (12.2%) achieved complete response (CR) as best response to initial treatment, 16 (19.5%) achieved very good partial response (VGPR) and 56 (68.3%) achieved PR. In the late relapse group, best response was CR in 103 (24.0%), VGPR in 102 (23.8%) and PR in 224 (52.2%).
A total of 64 (78.0%) patients with early relapse received subsequent treatment. The remaining 18 (22.0%) did not receive additional chemotherapy, most commonly owing to patient/provider decision or death. Among those receiving subsequent therapy, the common second-line regimens included: lenalidomide and dexamethasone (17; 26.6%), cyclophosphamide, bortezomib and dexamethasone (13; 20.3%), and bortezomib and dexamethasone (7; 10.9%). There were a variety of additional regimens used with less frequency, and only 3 (4.7%) of these did not include an immunomodulatory drug or proteosome inhibitor. Best response for second-line therapy could be assessed in 63 patients and was CR in 7 (11.1%), VGPR in 5 (7.9%), PR in 17 (27.0%), stable disease in 21 (33.3%) and progressive disease in 13 (20.6%). ASCT was a component of subsequent treatment for 15 (18.3%) patients. Overall, early relapse patients were exposed to a median of two lines of therapy (range; 1, 12), while late relapse patients were exposed to a median of 3 (range; 1, 16). More recently approved agents, including pomalidomide, carfilzomib, ixazomib and daratumumab, were available to a subset of patients in both cohorts, often in a clinical trial setting. A total of 13 (15.9%) early relapse patients and 123 (28.7%) late relapse patients were exposed to salvage regimens containing one or more of these newer therapies.
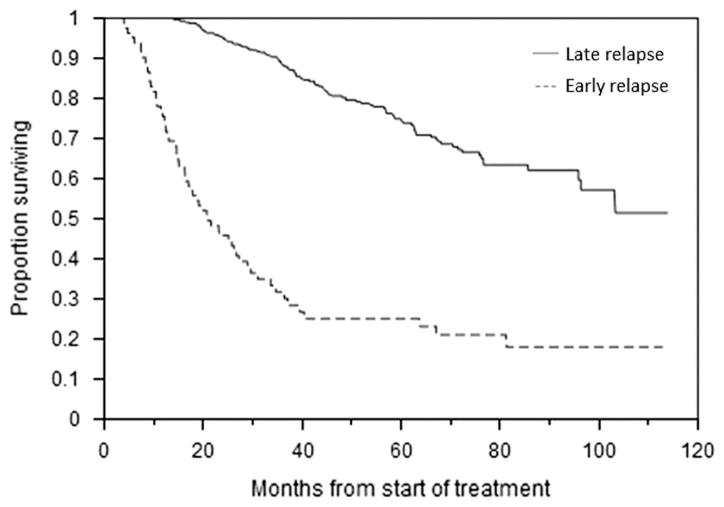
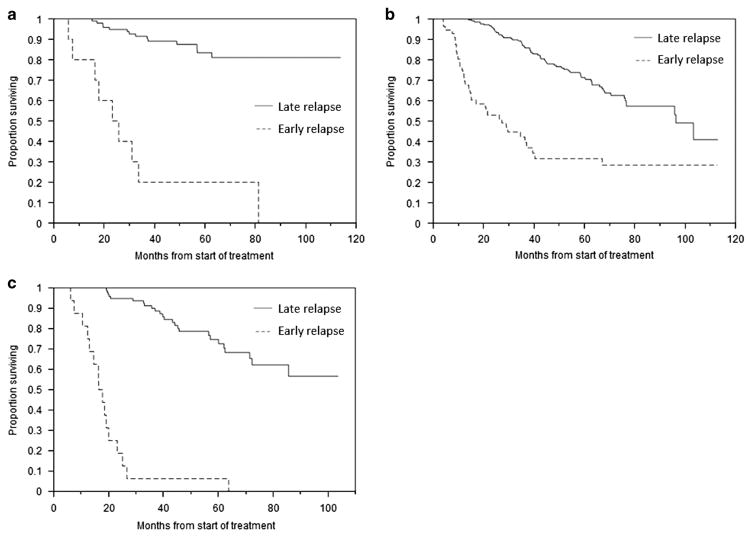
Median OS for the entire cohort was 103.3 months (95% CI; 81.3, not reached (NR)) from the start of treatment. At the time of analysis, a total of 338 (66.1%) patients had experienced first relapse. In the late relapse group, 256 (59.7%) had progressed, while 173 (40.3%) had continuing response. Median time to relapse for the entire cohort was 26.9 months (95% CI; 24.7, 28.4). In the early group, relapse occurred at a median of 8.0 months (95% CI; 6.3, 8.9) compared with 30.2 months (95% CI; 28.4, 31.7) for those in the late group (P<0.001). Twenty-one (25.6%) early relapse patients were alive at the time of analysis compared with 323 (75.3%) late relapse patients (P<0.001). Median OS for those with early relapse was 21.0 months (95% CI; 16.3, 27.2) vs NR (95% CI; 96.3, NR) for the rest (Figure 1; P<0.001). The survival disadvantage persisted even when only those early relapse patients who received subsequent therapies were considered, with a median OS of 26.7 months (95% CI; 19.1, 36.4) vs NR (95% CI; 96.3, NR) for the late relapse patients (P<0.001). Survival outcomes were also considered in the context of best response to initial therapy. Among those attaining CR, median OS was 24.5 months (95% CI; 5.8, 33.6) for early relapse patients vs NR (95% CI; NR, NR) (Figure 2a; P<0.001); for PR, median OS was 27.2 months (95% CI; 14.6, 39.5) vs 96.3 months (95% CI; 76.6, NR) (Figure 2b; P<0.001); and for VGPR, median OS was 17.1 months (95% CI; 12.3, 20.0) vs NR (95% CI; 72.3, NR) (Figure 2c; P<0.001).
Figure 1.
OS from the start of therapy. Kaplan–Meier curve demonstrating difference in OS between early and late relapse patients.
Figure 2.
OS according to depth of initial response. (a) Kaplan–Meier curves demonstrating differences in OS between early and late relapse patients among those achieving complete response to induction therapy. (b) OS of early and late relapse patients among those achieving partial response to induction therapy. (c) OS of early and late relapse patients among those achieving very good partial response to induction therapy.
In a univariate analysis considering baseline characteristics presented in Table 1, male gender, albumin <3.5 g/dl, beta-2 microglobulin (β2M) >5.5 mg/l, lambda light chain type, serum lactate dehydrogenase (LDH), plasma cell labeling index (PCLI) >3% and high-risk FISH were associated with early relapse. When male gender, serum albumin <3.5 g/dl, β2M>5.5 mg/l, lambda light chain type, serum LDH, PCLI>3% and high-risk FISH were considered in a multivariate analysis, only albumin <3.5 g/dl and high-risk FISH retained predictive value (Table 2). There was additionally a trend toward association between PCLI>3% and early relapse, but this did not achieve statistical significance (P = 0.07).
Table 2.
Multivariate analyses evaluating predictors of early relapse
| Prognostic factor | Multivariate analysis | |
|---|---|---|
|
| ||
| HR | P | |
| Male gender | 1.8 | 0.32 |
| Serum albumin <3.5 g/dl | 1.6 | <0.01 |
| Beta-2 microglobulin >5.5 mg/l | 1.5 | 0.93 |
| Lambda light chain type | 1.7 | 0.23 |
| Serum LDH | 1.4 | 0.76 |
| PCLI at least 3% | 1.2 | 0.07 |
| High-risk FISH | 1.7 | <0.001 |
Abbreviations: FISH, fluorescent in situ hybridization; HR, hazard ratio; LDH, lactate dehydrogenase; PCLI, plasma cell labeling index.
Early vs late relapse after early ASCT
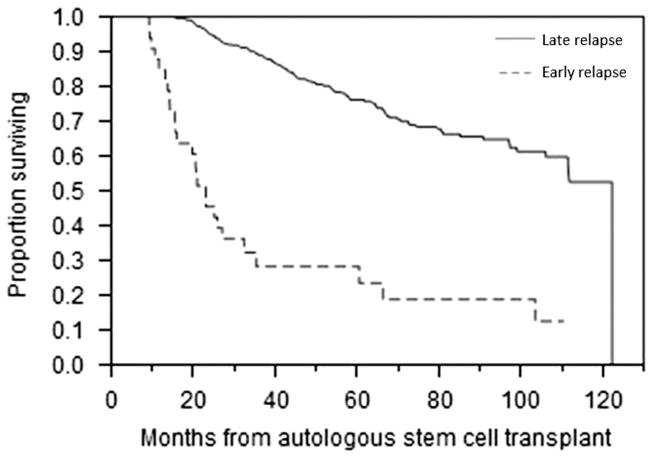
We also considered the impact of early relapse after early ASCT on prognosis. A total of 561 patients met expanded eligibility criteria for inclusion in this secondary cohort. One hundred and seventy-one patients were common between the two groups. Relevant and available baseline characteristics are presented in Table 3. The median estimated follow-up from ASCT for this cohort was 56.4 months (95% CI; 54.0, 61.2); 407 (72.5%) were alive at the time of analysis. Forty-six (8.2%) patients were primary refractory. Among the entire cohort, 33 (5.8%) patients had relapsed within 12 months of the transplant. The median OS from transplant for those relapsing within 12 months was 23.1 months (95% CI; 15.7, 32.4) compared with 122.2 months (95% CI; 111.5, 122.2) for the remaining patients (Figure 3; P<0.001). In the early relapse group, 6 (18.1%) patients received maintenance therapy following ASCT compared with 125 (23.6%) in the late relapse group (P = 0.532). In a univariate analysis considering variables in Table 3, serum β2M, bone marrow plasma cells, bone marrow plasma cells at diagnosis and PCLI>3% were associated with early relapse. Data on high-risk FISH were incomplete for this cohort and therefore were not considered. In multivariate analysis, only PCLI>3% remained predictive (hazard ratio (HR) 1.5, P<0.001).
Table 3.
Baseline clinical and laboratory characteristics of ASCT cohort (at the time of diagnosis, unless otherwise indicated)
| Characteristics | Early relapse (n = 33) | Late relapse (n = 528) | P-value |
|---|---|---|---|
| Male (%) | 57.6 | 61.8 | NS |
| Age (years) | |||
| Median | 59.3 | 60.6 | NS |
| Range | 32.2–72.5 | 24.4–75.3 | |
| Serum M-spike (g/dl) | |||
| Median | 3 | 2.5 | NS |
| Range | 0.0–10.3 | 0–10.5 | |
| Albumin (g/dl) | |||
| Median | 3.4 | 3.7 | NS |
| Range | 2.0–5.4 | 2.0–5.2 | |
| <3.5 (%) | 57.6 | 48.6 | NS |
| Creatinine (g/dl) | |||
| Median | 0.9 | 0.9 | NS |
| Range | 0.5–4.6 | 0.5–8.6 | |
| Serum β2M (mg/l) | |||
| Median | 4.4 | 3.7 | 0.03 |
| Range | 1.9–28.9 | 1.1–100.0 | |
| >5.5 (%) | 6.1 | 5.7 | NS |
| LDH (U/l) | |||
| Median | 198 | 184 | NS |
| Range | 126–616 | 2.6–2244 | |
| At least ULN (%) | 33.3 | 24.5 | NS |
| BMPC (%) | |||
| Median | 60 | 43.8 | 0.014 |
| Range | 2.0–90.0 | 1.0–95.0 | |
| BMPC (%) at ASCT | |||
| Median | 9 | 4 | <0.001 |
| Range | 1.0–74.0 | 0.0–94.0 | |
| PCLI (%) | |||
| Median | 1.1 | 0.8 | <0.001 |
| Range | 0.0–5.2 | 0–10.0 | |
| >3 (%) | 28.1 | 4 | <0.001 |
| ISS (%) | |||
| Stage 3 | 36.3 | 28 | NS |
Abbreviations: ASCT, autologous stem cell transplant; BMPC, bone marrow plasma cell; β2M, beta-2 microglobulin; LDH, lactate dehydrogenase; ISS, international staging system; NS, not significant; PCLI, plasma cell labeling index; ULN, upper limit of normal.
Figure 3.
OS from the start of therapy of early ASCT population. Kaplan–Meier curve demonstrating difference in OS between early and late relapse in early ASCT patients.
DISCUSSION
The improvement in outcomes for patients with MM following introduction of novel therapies has been clearly demonstrated in recent years.10,11 Benefit has been seen across multiple dimensions but has been observed especially in terms of response rates and OS. In the present manuscript, median OS was 103.3 months or approximately 8.6 years from the start of treatment for the entire cohort. Although these numbers select only for those who were sufficiently healthy to receive chemotherapy and excluded patients with primary refractory disease, the overall success of the current therapeutic approach remains considerable, particularly when compared with historical controls. At the same time, relapse is recognized as an inevitable consequence of the disease, and we have identified a subpopulation for whom survival outcomes are both statistically and clinically inferior. Patients who relapse within 12 months of initiating therapy with novel agents, irrespective of transplant status or depth of response, have markedly reduced survival times. Despite the availability of potent therapies that have transformed the management of MM, this group remains at high risk for poor outcomes.
In this study, we additionally evaluated impact of early relapse in patients who had proceeded to ASCT within 12 months of diagnosis. The appropriate timing of ASCT in the management of MM remains subject to debate, with limited data examining the effect of early ASCT on survival.12–14 Still, it is a common approach to proceed to high-dose therapy and ASCT as a means of minimizing exposure to chemotherapeutic agents; therefore, early relapse following early ASCT was regarded as an important prognostic variable in our analysis. We relaxed inclusion criteria in order to capture a larger population of these patients who largely had received induction and subsequent treatment regimens at outside facilities but had been seen at our institution for the purpose of ASCT. The 33 patients from this cohort who experienced relapse early following ASCT experienced poor OS, mirroring the results from the primary cohort. This strengthens the observation that early relapse is likely a reflection of aggressive tumor biology, independent of commonly employed management strategies in the current treatment landscape.
Time to progression has previously been demonstrated as an important prognostic factor in the era of conventional agents.3,4 However, conventional treatment was notoriously poor, both in terms of generating a response and improving survival. It would therefore fit with expectations that patients with aggressive myeloma biology, manifesting as early progression, would experience worse outcomes. This question has additionally been considered in the post-ASCT setting, with findings that relapse within 12 months of ASCT was associated with reduced OS.5 In that study, novel agent exposure was associated with improved median OS following relapse (15.9 vs 4.5 months, P<0.01). Furthermore, early data have suggested that novel therapies may be able to overcome traditional sources of resistance and, consequently, limit the impact of underlying, aggressive tumor biology.15–19 Although this might be the case in certain settings, the present data suggest that time to progression remains an important prognostic variable in the current era, with patients who experience early relapse from induction with a novel agent representing a uniquely high-risk subpopulation.
The findings from our study are related to but distinct from recently published analyses by Jimenez-Zepeda et al.,6 who investigated early relapse post-ASCT in patients treated with novel agents. Median OS was found to be significantly shorter at 20 months for those who relapsed within 12 months of ASCT vs 93 months for those without early relapse (P = 0.001). The present study focused on patients who relapsed within 1 year of initiating therapy, and the majority (76.8%) had not received ASCT at the time of relapse; among these, ASCT was a component of subsequent therapy for 15 (18.3%). However, the results from the secondary cohort of ASCT patients we studied does provide a more direct correlation and confirmation of these results. Jimenez-Zepeda et al.6 found high-risk FISH in only one of 27 early relapse patients; this contrasts with the early relapse group in our study, which was characterized by high-risk FISH in nearly two-thirds of patients. Despite these differences, the findings from the two studies are related by the similar conclusion that the kinetics of the disease should be respected, even in the presence of novel agent alternatives. It is worth noting that our data set was not able to capture clonal evolution of cytogenetic abnormalities from time of diagnosis to relapse, as FISH was not uniformly performed at relapse. This is a data point that might be incorporated into future studies to further enhance understanding of this disease process.
Our group has recently assessed the impact of depth of response to induction therapy on outcomes in the era of novel therapies.20 In that study, patients with primary refractory disease were found to have worse outcomes compared with those who achieved at least a PR to initial treatment. Further, patients who achieved a depth of response that was at least VGPR experienced longer median OS compared with those who only attained PR. The current study places those findings into context, serving as a reminder that response durability must be considered along with response depth when assessing prognosis. In the present report, we evaluated survival outcomes in relation to depth of initial treatment response and found that attaining CR or VGPR conferred no measure of protection. In fact, patients who relapsed from PR actually possessed the longest median OS among the group. Prior reports have identified comparatively rapid relapses and shorter survival times in patients with high-risk cytogenetics, including t(4;14) and del(17)p.21,22 In such cases, rapid decrease in tumor burden may be observed, reflective of high proliferative activity, though this is frequently not sustained.23 A large portion of the early relapse group in our study possessed these cytogenetic abnormalities, which may account for the aggressive disease behavior and poor outcomes.
The retrospective design of this study does limit the scope of its conclusions. However, this is the largest known series reported on this patient population, and the findings are supported by related studies, as discussed above. Most importantly, the data highlight a group of patients for whom outcomes continue to remain unsatisfactory. Subsequent therapies used for early relapse patients were largely similar to those utilized as first-line therapy, and in some cases, patients were rechallenged with the identical regimen used during induction. The number of patients proceeding to high-dose therapy and ASCT or entering clinical trials was comparatively small. Although not all such patients were candidates for aggressive second-line treatment, the poor prognosis conferred by early relapse status should alert providers to the high-risk nature of this population. When available, such patients should be considered for clinical trials, ideally designed to identify agents that can overcome the aggressive plasma cell biology that appears to underlie this manifestation of disease.
Acknowledgments
This work was supported in part by the Mayo Clinic Hematological Malignancies Program, R01 CA 168762-03 (to SKK and SVR), R01 CA 107476-11 (to SKK and SVR) and R01 CA 167511-2 (to SKK).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
NM and SKK were involved in design of concept, data collection, analysis and writing the manuscript. SVR, MQL, FKB, AD, MAG, SRH, DD, PK, LH, JAL, SJR, RSG and RAK were all involved in writing the manuscript.
References
- 1.Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 2.Drewinko B, Alexanian R, Boyer H, Barlogie B, Rubinow SI. The growth fraction of human myeloma cells. Blood. 1981;57:333–338. [PubMed] [Google Scholar]
- 3.Riccardi A, Mora O, Tinelli C, Porta C, Danova M, Brugnatelli S, et al. Response to first-line chemotherapy and long-term survival in patients with multiple myeloma: results of the MM87 prospective randomised protocol. Eur J Cancer. 2003;39:31–37. doi: 10.1016/s0959-8049(02)00529-4. [DOI] [PubMed] [Google Scholar]
- 4.Durie BG, Jacobson J, Barlogie B, Crowley J. Magnitude of response with myeloma frontline therapy does not predict outcome: importance of time to progression in southwest oncology group chemotherapy trials. J Clin Oncol. 2004;22:1857–1863. doi: 10.1200/JCO.2004.05.111. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Mahmood ST, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transplant. 2008;42:413–420. doi: 10.1038/bmt.2008.180. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Zepeda VH, Reece DE, Trudel S, Chen C, Tiedemann R, Kukreti V. Early relapse after single auto-SCT for multiple myeloma is a major predictor of survival in the era of novel agents. Bone Marrow Transplant. 2015;50:204–208. doi: 10.1038/bmt.2014.237. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101:4569–4575. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 10.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16:1600–1603. doi: 10.1634/theoncologist.2011-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 13.Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–1592. doi: 10.1002/cncr.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous Transplantation and Maintenance Therapy in Multiple Myeloma. N Engl J Med. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 15.Jagannath S, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21:151–157. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- 16.Barlogie B, Pineda-Roman M, van Rhee F, Haessler J, Anaissie E, Hollmig K, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112:3115–3121. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 18.Reece D, Song KW, Fu T, Roland B, Chang H, Horsman DE, et al. Influence of cytogenetics in patients with relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone: adverse effect of deletion 17p13. Blood. 2009;114:522–525. doi: 10.1182/blood-2008-12-193458. [DOI] [PubMed] [Google Scholar]
- 19.Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t (4;14) myeloma but not outcome of patients with del(17p) J Clin Oncol. 2010;28:4630–4634. doi: 10.1200/JCO.2010.28.3945. [DOI] [PubMed] [Google Scholar]
- 20.Majithia N, Vincent Rajkumar S, Lacy MQ, Buadi FK, Dispenzieri A, Gertz MA, et al. Outcomes of primary refractory multiple myeloma and the impact of novel therapies. Am J Hematol. 2015;90:981–985. doi: 10.1002/ajh.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaksic W, Trudel S, Chang H, Trieu Y, Qi X, Mikhael J, et al. Clinical outcomes in t(4;14) multiple myeloma: a chemotherapy-sensitive disease characterized by rapid relapse and alkylating agent resistance. J Clin Oncol. 2005;23:7069–7073. doi: 10.1200/JCO.2005.17.129. [DOI] [PubMed] [Google Scholar]
- 22.Cavo M, Terragna C, Renzulli M, Zamagni E, Tosi P, Testoni N, et al. Poor outcome with front-line autologous transplantation in t(4;14) multiple myeloma: low complete remission rate and short duration of remission. J Clin Oncol. 2006;24:e4–e5. doi: 10.1200/JCO.2005.04.7506. [DOI] [PubMed] [Google Scholar]
- 23.Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]