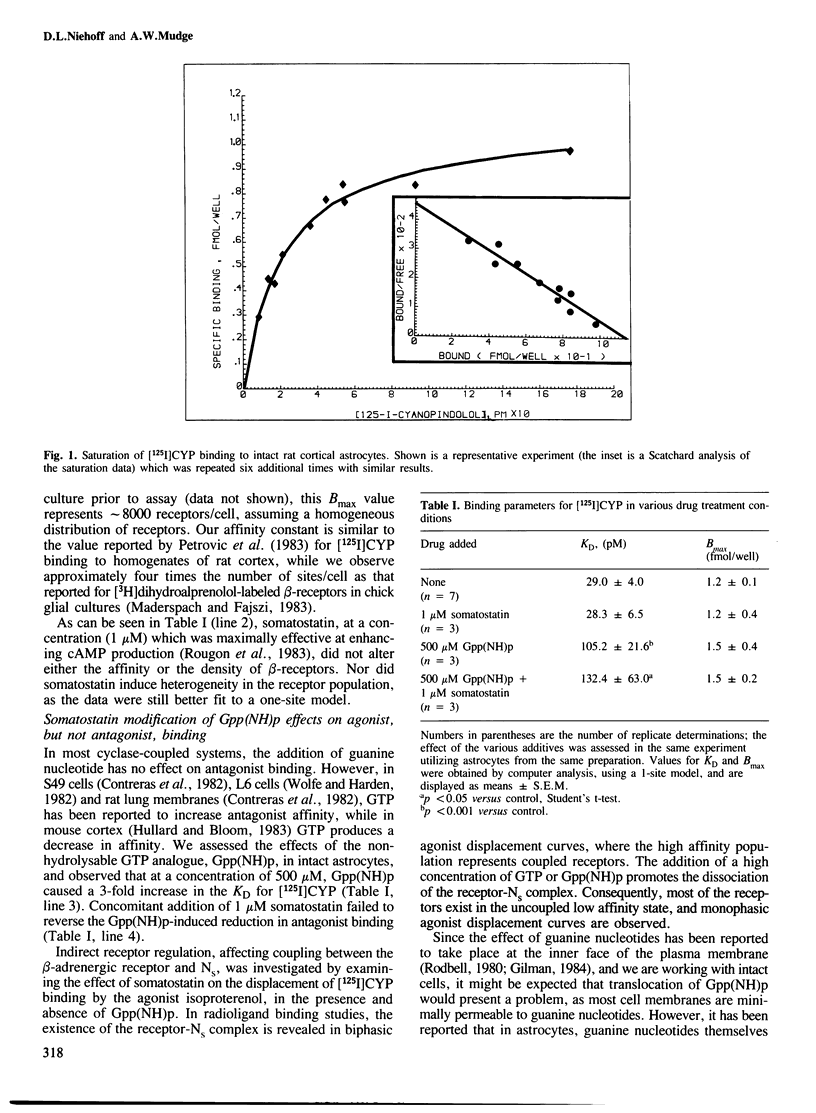
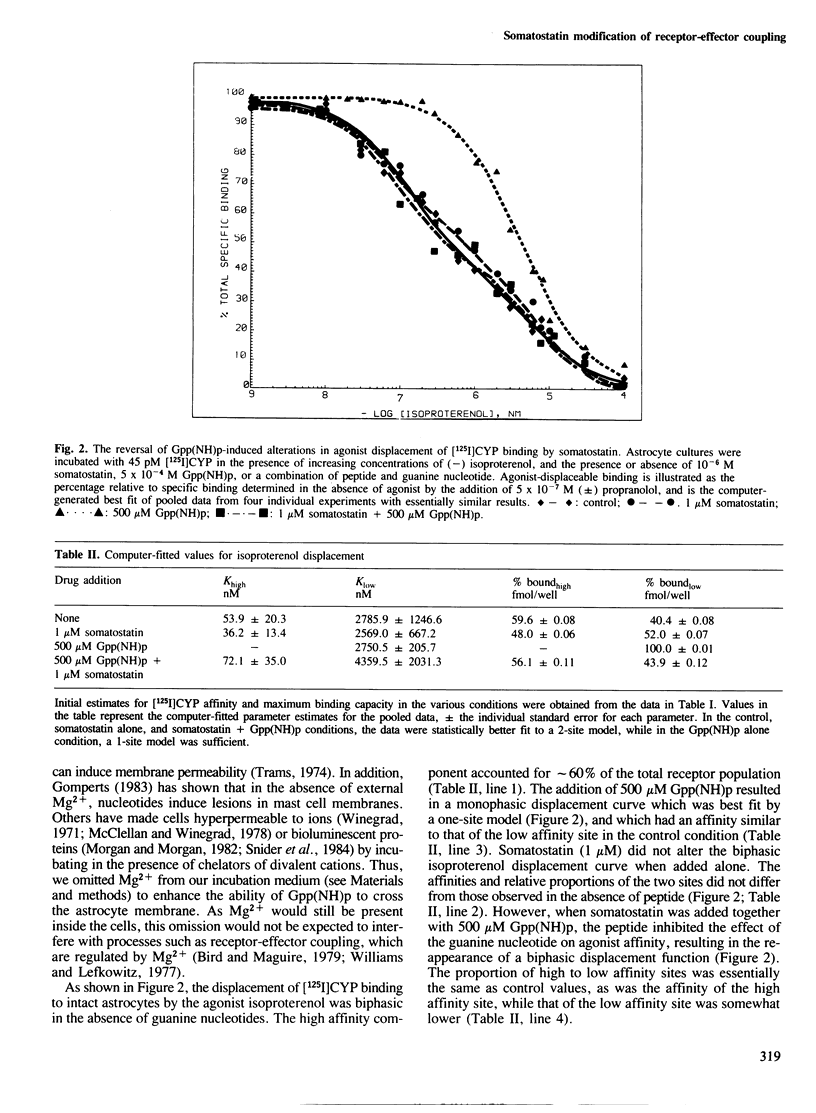
Abstract
The neuropeptide somatostatin potentiates beta-adrenergic receptor-mediated cAMP formation in astrocytes derived from neonatal rat cortex but does not affect cAMP levels by itself. beta-Adrenergic receptors in these cells can be specifically labeled with the high affinity antagonist [125I] cyanopindolol ([125I]CYP). In addition, astrocytes display both high and low affinity binding sites for the agonist isoproterenol, which are thought to represent receptors which are coupled or uncoupled, respectively, to the guanine nucleotide regulatory protein. We find that somatostatin does not modify beta-receptor density, nor receptor affinity for either the antagonist ([125I]CYP) or for the agonist isoproterenol. In the presence of the guanine nucleotide analogue, Gpp(NH)p, only low affinity (uncoupled) displacement of [125I]CYP binding by isoproterenol is observed. However, somatostatin (1 microM), when added to the cells together with Gpp(NH)p, prevents the nucleotide-induced loss of the high affinity (coupled) component of agonist displacement. This result suggests that somatostatin increases noradrenaline-induced cAMP production by enhancing coupling between the beta-receptor and the stimulatory guanine nucleotide regulatory protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird S. J., Maguire M. E. The agonist-specific effect of magnesium ion on binding by beta-adrenergic receptors in S49 lymphoma cells. Interaction of GTP and magnesium in adenylate cyclase activation. J Biol Chem. 1978 Dec 25;253(24):8826–8834. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang D. M., Costa E. Evidence for internalization of the recognition site of beta-adrenergic receptors during receptor subsensitivity induced by (-)-isoproterenol. Proc Natl Acad Sci U S A. 1979 Jun;76(6):3024–3028. doi: 10.1073/pnas.76.6.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins C. J., Lust W. D., Passonneau J. V. Regulation of glycogen metabolism in primary and transformed astrocytes in vitro. J Neurochem. 1983 Jan;40(1):128–136. doi: 10.1111/j.1471-4159.1983.tb12662.x. [DOI] [PubMed] [Google Scholar]
- Engel G., Hoyer D., Berthold R., Wagner H. (+/-)[125Iodo] cyanopindolol, a new ligand for beta-adrenoceptors: identification and quantitation of subclasses of beta-adrenoceptors in guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1981;317(4):277–285. doi: 10.1007/BF00501307. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Agnati L. F., Benfenati F., Cimmino M., Algeri S., Hökfelt T., Mutt V. Modulation by cholecystokinins of 3H-spiroperidol binding in rat striatum: evidence for increased affinity and reduction in the number of binding sites. Acta Physiol Scand. 1981 Dec;113(4):567–569. doi: 10.1111/j.1748-1716.1981.tb06942.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Harden T. K., Cotton C. U., Waldo G. L., Lutton J. K., Perkins J. P. Catecholamine-induced alteration in sedimentation behavior of membrane bound beta-adrenergic receptors. Science. 1980 Oct;210(4468):441–443. doi: 10.1126/science.6254143. [DOI] [PubMed] [Google Scholar]
- Hoffman B. B., Lefkowitz R. J. Radioligand binding studies of adrenergic receptors: new insights into molecular and physiological regulation. Annu Rev Pharmacol Toxicol. 1980;20:581–608. doi: 10.1146/annurev.pa.20.040180.003053. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Reynolds E. E., Molinoff P. B. Agonist-induced changes in the properties of beta-adrenergic receptors on intact S49 lymphoma cells. Time-dependent changes in the affinity of the receptor for agonists. Mol Pharmacol. 1984 Mar;25(2):209–218. [PubMed] [Google Scholar]
- Insel P. A., Sanda M. Temperature-dependent changes in binding to beta-adrenergic receptors of intact S49 lymphoma cells. Implications for the state of the receptor that activates adenylate cyclase under physiological conditions. J Biol Chem. 1979 Jul 25;254(14):6554–6559. [PubMed] [Google Scholar]
- Karobath M., Sperk G. Stimulation of benzodiazepine receptor binding by gamma-aminobutyric acid. Proc Natl Acad Sci U S A. 1979 Feb;76(2):1004–1006. doi: 10.1073/pnas.76.2.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Lundberg J. M., Hedlund B., Bartfai T. Vasoactive intestinal polypeptide enhances muscarinic ligand binding in cat submandibular salivary gland. Nature. 1982 Jan 14;295(5845):147–149. doi: 10.1038/295147a0. [DOI] [PubMed] [Google Scholar]
- Maderspach K., Fajszi C. Development of beta-adrenergic receptors and their function in glia-neuron communication in cultured chick brain. Brain Res. 1983 Feb;282(3):251–257. doi: 10.1016/0165-3806(83)90064-0. [DOI] [PubMed] [Google Scholar]
- Martin I. L., Candy J. M. Facilitation of benzodiazepine binding by sodium chloride and GABA. Neuropharmacology. 1978 Nov;17(11):993–998. doi: 10.1016/0028-3908(78)90145-4. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D. An autoradiographic analysis of beta adrenergic receptors on immunocytochemically defined astroglia. J Pharmacol Exp Ther. 1983 Jul;226(1):282–290. [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan G. B., Winegrad S. The regulation of the calcium sensitivity of the contractile system in mammalian cardiac muscle. J Gen Physiol. 1978 Dec;72(6):737–764. doi: 10.1085/jgp.72.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobe F., Kozousek V., Dean D. M., Livett B. G. Pharmacological characterization of adrenal paraneurons: substance P and somatostatin as inhibitory modulators of the nicotinic response. Brain Res. 1979 Dec 14;178(2-3):555–566. doi: 10.1016/0006-8993(79)90714-5. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Narumi S., Kimelberg H. K., Bourke R. S. Effects of norepinephrine on the morphology and some enzyme activities of primary monolayer cultures from rat brain. J Neurochem. 1978 Dec;31(6):1479–1490. doi: 10.1111/j.1471-4159.1978.tb06575.x. [DOI] [PubMed] [Google Scholar]
- Pelton E. W., 2nd, Kimelberg H. K., Shipherd S. V., Bourke R. S. Dopamine and norepinephrine uptake and metabolism by astroglial cells in culture. Life Sci. 1981 Apr 6;28(14):1655–1663. doi: 10.1016/0024-3205(81)90322-2. [DOI] [PubMed] [Google Scholar]
- Petrovic S. L., McDonald J. K., Snyder G. D., McCann S. M. Characterization of beta-adrenergic receptors in rat brain and pituitary using a new high-affinity ligand, [125I]iodocyanopindolol. Brain Res. 1983 Feb 21;261(2):249–259. doi: 10.1016/0006-8993(83)90628-5. [DOI] [PubMed] [Google Scholar]
- Pittman R. N., Molinoff P. B. Interactions of agonists and antagonists with beta-adrenergic receptors on intact L6 muscle cells. J Cyclic Nucleotide Res. 1980;6(6):421–435. [PubMed] [Google Scholar]
- Porzig H., Becker C., Reuter H. Competitive and non-competitive interactions between specific ligands and beta-adrenoceptors in living cardiac cells. Naunyn Schmiedebergs Arch Pharmacol. 1982 Nov;321(2):89–99. doi: 10.1007/BF00518474. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Role L. W., Leeman S. E., Perlman R. L. Somatostatin and substance P inhibit catecholamine secretion from isolated cells of guinea-pig adrenal medulla. Neuroscience. 1981;6(9):1813–1821. doi: 10.1016/0306-4522(81)90215-3. [DOI] [PubMed] [Google Scholar]
- Rougon G., Noble M., Mudge A. W. Neuropeptides modulate the beta-adrenergic response of purified astrocytes in vitro. Nature. 1983 Oct 20;305(5936):715–717. doi: 10.1038/305715a0. [DOI] [PubMed] [Google Scholar]
- Snider R. M., McKinney M., Forray C., Richelson E. Neurotransmitter receptors mediate cyclic GMP formation by involvement of arachidonic acid and lipoxygenase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3905–3909. doi: 10.1073/pnas.81.12.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin M., Simons P., Jaeggi K., Wigger N. CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem. 1983 Mar 25;258(6):3496–3502. [PubMed] [Google Scholar]
- Staehelin M., Simons P. Rapid and reversible disappearance of beta-adrenergic cell surface receptors. EMBO J. 1982;1(2):187–190. doi: 10.1002/j.1460-2075.1982.tb01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddith R. L., Hutchison H. T., Haber B. Uptake of biogenic amines by glial cells in culture I. A neuronal-like transport system for serotonin. Life Sci. 1978 Jun 26;22(24):2179–2187. doi: 10.1016/0024-3205(78)90569-6. [DOI] [PubMed] [Google Scholar]
- Terasaki W. L., Brooker G. [125I]Iodohydroxybenzylpindolol binding sites on intact rat glioma cells. Evidence for beta-adrenergic receptors of high coupling efficiency. J Biol Chem. 1978 Aug 10;253(15):5418–5425. [PubMed] [Google Scholar]
- Toews M. L., Harden T. K., Perkins J. P. High-affinity binding of agonists to beta-adrenergic receptors on intact cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3553–3557. doi: 10.1073/pnas.80.12.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G. Evidence for ATP action on the cell surface. Nature. 1974 Dec 6;252(5483):480–482. doi: 10.1038/252480a0. [DOI] [PubMed] [Google Scholar]
- Wastek G. J., Speth R. C., Reisine T. D., Yamamura H. I. The effect of gamma-aminobutyric acid on 3H-flunitrazepam binding in rat brain. Eur J Pharmacol. 1978 Aug 15;50(4):445–447. doi: 10.1016/0014-2999(78)90152-8. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., McConnaughey M. M., Strawbridge R. A., Fleming J. W., Jones L. R., Besch H. R., Jr Muscarinic cholinergic receptor modulation of beta-adrenergic receptor affinity for catecholamines. J Biol Chem. 1978 Jul 25;253(14):4833–4836. [PubMed] [Google Scholar]
- Whitaker P. M., Vint C. K., Morin R. [3H]imipramine labels sites on brain astroglial cells not related to serotonin uptake. J Neurochem. 1983 Nov;41(5):1319–1323. doi: 10.1111/j.1471-4159.1983.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]
- Winegrad S. Studies of cardiac muscle with a high permeability to calcium produced by treatment with ethylenediaminetetraacetic acid. J Gen Physiol. 1971 Jul;58(1):71–93. doi: 10.1085/jgp.58.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. B., Harden T. K. Guanine nucleotides modulate the affinity of antagonists at beta-adrenergic receptors. J Cyclic Nucleotide Res. 1981;7(5):303–312. [PubMed] [Google Scholar]
- Yamada S., Yamamura H. I., Roeske W. R. The regulation of cardiac alpha 1-adrenergic receptors by guanine nucleotides and by muscarinic cholinergic agonists. Eur J Pharmacol. 1980 May 2;63(2-3):239–241. doi: 10.1016/0014-2999(80)90455-0. [DOI] [PubMed] [Google Scholar]


