p.V617F JAK2 mutation is the most frequent mutation in myeloproliferative neoplasms (MPNs), present in almost all patients with polycythaemia vera (PV), 50% of essential thrombocythemia (ET) and myelofibrosis (MF), and in some cases of atypical MPNs, myelodysplasia and AML. This mutation is located in exon 14 and affects the pseudokinase domain, resulting in the constitutive activation of kinase function.1 Other mutations have been found in this domain in PV, such as p.C616Y, p.C618R and p.D620E, or the somatic deletion of five amino acids (ΔIREED) that defines a distinct acute lymphoblastic leukemia (ALL) subgroup associated with trisomy 21. A region linking SH2 and JH2 domains (exon 12, residues 537 to 543) can be also mutated in some PV patients.2
During a comprehensive mutational screening of several tyrosine kinase genes, our group identified a new point mutation in JAK2 exon 8 (p.R340Q) in a MF patient.3 Subsequent analyses in additional samples from 443 MPN patients (174 p.V617F JAK2 negative and 269 p.V617F JAK2 positive), as well as 355 control samples without leukemia, led us to the identification of additional changes (p.Y317H and p.N337D) in the same exon. All of them were found in p.V617F JAK2 negative and MPL non-mutated MF cases, but none was recurrent. They affect the FERM domain of the protein, and they have not been described as SNPs in sequence databases except for p.N337D which was described as SNP (rs149683525) in 2014. In silico analysis with PolyPhen-2 showed that p.R340Q and p.Y317H are probably damaging, and in depth molecular analyses showed that both could be somatic changes.4
It has been shown that artificial mutations in highly conserved hydrophobic residues of the JAK1 FERM domain can abrogate protein binding to the cytokine receptor altering its regulatory function.5 However, only one JAK1 FERM domain mutation (p.K204M) with unknown functional significance has been described in an ALL patient.6 In the JAK3 FERM domain, three missense mutations (p.L156P, p.R172Q, and p.E183G) detected in T-ALL patients create a more active and stable JAK3 kinase,7 and p.P132T/A has oncogenic properties in AML.8
Several pieces of evidence suggest that the FERM domain is not only a critical site of interaction with cytokine receptors, but it also plays a dual role on JAK2 activity at different activation states: it maintains wild-type protein in an inactive basal state, and constitutive activation by p.V617F mutation depends on the integrity of this domain.9 On the other hand, p.G341E mutation in hop gene of D. melanogaster causes a leukemia-like disease.10 This gene encodes a non-receptor tyrosine kinase similar to mammalian JAK proteins, and this mutation is located in the FERM domain. As both p.R340Q and p.Y317H are located in a conserved region close to p.G341E in hop, their effects could be similar. All these facts led us to hypothesize that p.R340Q and p.Y317H could also play a role in malignant transformation.
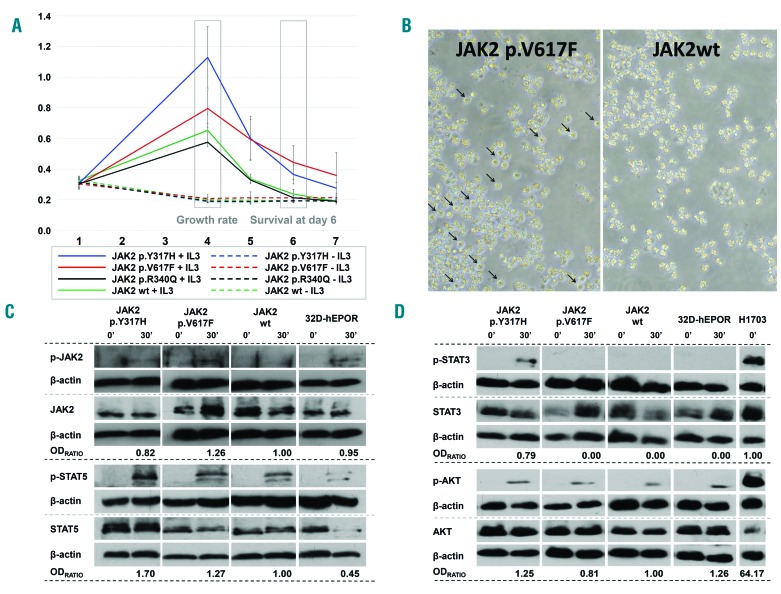
In the present work, 32D-hEPOR cells stably expressing p.V617F JAK2 mutation have been used as a reference model of malignant transformation (Online Supplementary Material & Methods). The expression of p.V617F construct promoted hypersensitivity to IL-3 (P = 0.043), and the survival of these cells at day 6 was significantly higher than those expressing wild-type human JAK2 (P < 0.05). Although we could not detect cytokine-independent growth by the MTS assay (Figure 1A), we observed some p.V617F cell survival by optic microscopy, whereas cells expressing wild-type JAK2 showed very rapid cell death under the same conditions (Figure 1B). By contrast, cells expressing p.R340Q (Online Supplementary Material & Methods) did not show differences with wild-type JAK2 cells (P = 0.427), indicating that it does not result in a proliferative advantage (Figure 1A). Notably, cells expressing p.Y317H (Online Supplementary Material & Methods) showed higher growth rate and enhanced cell survival at day 6 compared to wild-type JAK2 cells (P < 0.001) (Figure 1A) and higher growth rate compared to p.V617F cells (P < 0.001), although they were not able to grow without cytokine stimulation. These results agree with a previous report showing that artificial mutations of this tyrosine residue enhance JAK2 activity.11 As Tyr317 is not conserved in other JAKs, the mechanism by which phosphorylation of Tyr317 affects protein function may be JAK2 specific.11
Figure 1.
Results from 32D-hEPOR cells expressing p.V617F and p.Y317H JAK2 mutations. (A) MTS assays. Graph shows the relative proliferation of p.V617F, p.Y317H and p.R340Q mutants compared to wild-type JAK2 expressing cells in two different conditions (with or without WEHI medium as a source of murine IL-3). The relative absorbance at 492 nm (Y-axis) which is directly proportional to the number of cells, was quantified daily for 7 days (X-axis). The growth rate was calculated as the difference between the maximum absorbance achieved and the absorbance at day 1. Day 6 cell survival was calculated as the difference between the absorbance at day 6 and 1. None of the mutations were able to promote cytokine-independent growth (including p.V617F JAK2 mutation). The p.V617F mutation caused hypersensitivity to IL-3 (P = 0.043) and a significantly higher survival at day 6 (P < 0.05). Cells with p.Y317H showed a higher growth rate and an enhanced cell survival at day 6 compared to wild-type JAK2 cells (P < 0.001) and a higher growth rate compared to p.V617F cells (P < 0.001). Cells with the p.R340Q mutation did not show differences with wild-type JAK2 cells (P = 0.427). (B) Viability of cells (32D-hEPOR-V617FJAK2 vs. 32D-hEPOR-wtJAK2) cultured without IL-3 at day 6. Survival of cells expressing p.V617F mutants (live cells marked with arrows) could be observed under optic microscope; by contrast, cells expressing wild-type JAK2 underwent a rapid cell death. (C,D) Western blot analyses showing JAK2, STAT5, STAT3 and AKT phosphorylation before and after stimulation with IL-3 and hEPO. Cells were starved for 6 h and then stimulated for 30 min with hEPO (5 U/mL) and 20% of undiluted IL-3 conditioned WEHI-3B medium. Intensities of specific bands corresponding to the proteins of interest were measured by relative densitometry (optic density (OD) × mm) to β-actin. The activation of each protein was obtained dividing the relative OD of the phosphorylated protein by the relative OD of the non-phosphorylated form. OD ratio shows the level of activation of the mutated form divided by the level of activation of the wild type. In the case of STAT3, this ratio was obtained dividing the level of activation of the mutated form by the level of activation in the positive control (H1703) because STAT3 activation was not detected in JAK2 wild-type. Results show that p.Y317H mutation was less active than p.V617F on JAK2 phosphorylation, but it has a greater effect on STAT5 phosphorylation (~1.70 vs. ~1.27-fold higher) as well as on AKT phosphorylation (~1.25 vs. ~0.81-fold higher) upon cytokine stimulation. It also promoted STAT3 phosphorylation, whereas p.V617F did not. hEPOR: human erythropoietin receptor; 32D-hEPOR-V617FJAK2: 32D cells stably expressing hEPOR and p.V617F; 32D-hEPOR-wtJAK2: 32D cell stably expressing hEPOR and wild-type JAK2; IL-3: interleukin 3; hEPO: human erythropoietin; OD: optic density.
Cells from MPN patients harbouring p.V617F JAK2 mutation are characterized by abnormal activation of JAK/STAT signalling pathway, as well as RAS/MAPK and PI3K/AKT pathways.1 For this reason, we compared the phosphorylation (activated) state of JAK2, STAT5, STAT3 and AKT in the mutant clones vs. wild-type JAK2 cells (Online Supplementary Material & Methods). As expected, our results showed higher activation of JAK2 in p.V617F cells (~1.26-fold higher) as well as increased STAT5 phosphorylation (~1.27-fold higher) after cytokine stimulation. By contrast, only background levels of STAT3 and AKT phosphorylation could be detected (Figures 1C and 1D). Similarly, although we found that the cytokine-dependent activation of JAK2 was weaker in p.Y317H than in p.V617F cells (Figure 1C), p.Y317H cells showed higher STAT5 phosphorylation (~1.70 vs. ~1.27-fold higher) and AKT phosphorylation (~1.25 vs. ~0.81-fold higher). In addition, this variant activates STAT3, again after stimulation, whereas p.V617F does not (Figure 1D). These results show that, despite activating several signal pathways,1 the major target of p.V617F is STAT5.12 Our model shows features of malignant transformation (i.e., higher cytokine-dependent growth rate, higher cell survival and higher cytokine-dependent cell signalling) in cells with p.Y317H compared to wild type JAK2. Besides, the mechanism by which p.Y317H causes cell proliferation differs from the effect induced by the p.V617F mutation as p.Y317H mediates downstream signalling not only through STAT5 but also by STAT3 and AKT.
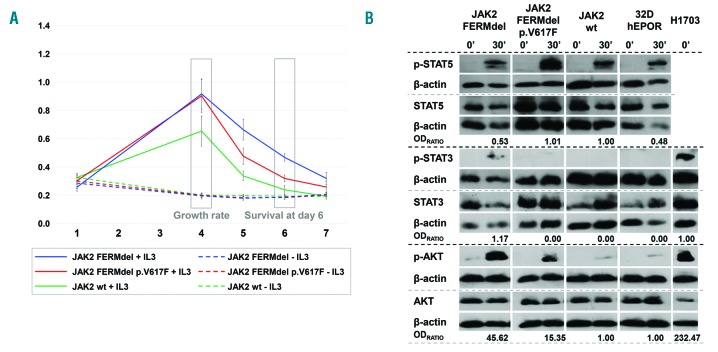
Since p.Y317H is located in the FERM domain, we wanted to test the role of this domain in cellular transformation and signalling. We created 32D-hEPOR cell clones stably expressing either wild-type or p.V617F JAK2 together with the deletion of the whole FERM domain (FERMdel) (Online Supplementary Material & Methods). Both clones were then cultured in parallel with wild-type JAK2 cells in the presence or absence of IL-3. None of the FERMdel mutants were able to grow in absence of IL-3, but they showed a higher growth rate and an enhanced cell survival at day 6 (P < 0.001) (Figure 2A) upon cytokine stimulation. Remarkably, the deletion of the FERM domain decreased the effect of p.V617F mutation on cell transformation.
Figure 2.
Results from 32D-hEPOR cells expressing FERM deleted JAK2 with and without p.V617F mutation. (A). MTS assay. The graph shows the relative proliferation of 32D-hEPOR cell clones expressing wild-type JAK2 and p.V617F mutation with the deletion of the whole FERM domain (FERMdel) compared with 32D-hEPOR-wtJAK2 cells in two different conditions (with or without WEHI medium as a source of murine IL-3). The relative absorbance at 492 nm (Y-axis), which is directly proportional to the number of cells, was quantified daily for 7 days (X-axis). The growth rate was calculated as the difference between the maximum absorbance achieved and the absorbance at day 1. Day 4 cell survival was calculated as the difference between the absorbance at day 6 and 1. Results show that both FERMdel mutants (with and without the p.V617D mutation) were unable to promote cytokine-independent growth, but they showed a higher growth rate and an enhanced cell survival at day 6 (P < 0.001). Cell viability of the FERMdel p.V617F mutants was markedly decreased after cytokine stimulation. (B) Western-blot analyses showing STAT5, STAT3 and AKT phosphorylation in response to IL-3 and hEPO. Cells were starved for 6 h and then stimulated for 30 min with both hEPO (5 U/mL) and 20% of undiluted IL-3 conditioned WEHI-3B medium. Intensities of specific bands corresponding to the proteins of interest were measured by relative densitometry (optic density (OD) × mm) to β-actin. The level of activation for each protein was calculated dividing the relative OD of the phosphorylated protein by the relative OD of the non-phosphorylated form. OD ratio shows the level of activation of the mutated form divided by the level of activation of the wild type. In the case of STAT3, this ratio was obtained dividing the level of activation of the mutated form by the level of activation in the positive control (H1703) because STAT3 activation was not detected in JAK2 wild-type. Results show that deletion of the FERM domain causes a reduced STAT5 phosphorylation in wtJAK2 cells (~1.88-fold lower, 1/0.53) after cytokine stimulation. This phosphorylation is maintained in FERMdel p.V617F cells but dramatically reduced when compared to p.V617F with an intact FERM domain. STAT3 phosphorylation could only be detected in FERMdel wtJAK2 cells. Cytokine-dependent AKT phosphorylation is also highly increased, ~15.35-fold in FERMdel p.V617F cells but ~45.62-fold in FERMdel wtJAK2 cells. hEPOR: human erythropoietin receptor; 32D-hEPOR: 32D cells stably expressing hEPOR; FERMdel: deletion of the FERM domain; 32D-hEPOR-wtJAK2: 32D cell stably expressing hEPOR and wild-type JAK2. IL-3: interleukin 3; hEPO: human erythropoietin; OD: optic density.
After cytokine stimulation, the deletion of the FERM domain led to a reduced STAT5 phosphorylation (~1.88-fold lower, 1/0.53) compared with wild type-JAK2. Similarly, although STAT5 phosphorylation was maintained in FERMdel p.V617F cells, it was dramatically reduced when compared with p.V617F cells with intact FERM domain. By contrast, STAT3 phosphorylation was detected in FERMdel wtJAK2 cells but not in FERMdel p.V617F cells. Finally, AKT phosphorylation was increased ~15.35-fold in FERMdel p.V617FJAK2 cells and ~45.62-fold in FERMdel wtJAK2 cells, compared with wild type-JAK2 (Figure 2B). Thus, the deletion of the FERM domain per se causes hypersensitivity to IL-3 and enhanced cell viability. This deletion promotes increased STAT3 and AKT phosphorylation after cytokine stimulation but fails to activate STAT5, confirming that this domain is essential to STAT5 activation by JAK2.9 Therefore, p.Y317H does not completely inactivate the FERM domain (STAT5 activation is retained), but it could alter its conformation state promoting the activation of STAT3 and AKT (similar to the deletion of FERM). This suggests that p.Y317H behaves as a gain-of-function mutation that could act as a driver in the oncogenic process.
When mutations in CALR were described in MPNs, we observed that the MF patient with p.Y317H also harboured the CALR type 28 mutation (E378fs*45). It has been reported that some ET patients with type 2-like CALR mutations progress to myelofibrosis due to the presence of additional mutations.13 p.Y317H could be one of these mutations that cooperate to the progression of the disease or the development of a more aggressive state, although we could not test if both mutations affect the same cellular clone.
Coexistence of mutations in both CALR and JAK2 are being reported in ET and MF with frequencies from 1% to 6.8 %14 breaking the widespread belief that both are mutually exclusive events.15 It has been proposed that cases with both genes mutated could correspond to a specific subgroup of patients with special follow up and management.15 However, although several JAK2 mutations have been published to date, only p.V617F and exon 12 mutations in PV are routinely studied.
Here, we describe that the JAK2 FERM domain can be mutated in rare cases of MPNs, playing a role in the regulation of the activity of this protein with specific effects on signalling. In addition, these changes can coexist with functional mutations in other relevant genes (like CALR) in the same patients, although further research is required to demonstrate whether both events cooperate or drive the disease independently. This finding could be relevant for the diagnosis of a minor proportion of patients and for the design of new therapeutic approaches.
Supplementary Material
Acknowledgments
The authors also thank Diego Calavia for statistical advice and Beatriz Vera for technical assistance.
Footnotes
Funding: this work has been funded with the help of the PIUNA Program of the University of Navarra. L.E.-A. and D.N.-H. have PhD Studentships granted by the Ministry of Education, Culture and Sports of Spain (FPU14/03669 and FPU13/00424 respectively) within the University Faculty Training (FPU) program.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol. 2016;98:375–389. [DOI] [PubMed] [Google Scholar]
- 2.Karow A, Waller C, Reimann C, Niemeyer CM, Kratz CP. JAK2 mutations other than V617F: a novel mutation and mini review. Leuk Res. 2008;32(2):365–366. [DOI] [PubMed] [Google Scholar]
- 3.Aranaz P, Ormazábal C, Hurtado C, et al. A new potential oncogenic mutation in the FERM domain of JAK2 in BCR/ABL1-negative and V617F-negative chronic myeloproliferative neoplasms revealed by a comprehensive screening of 17 tyrosine kinase coding genes. Cancer Genet Cytogenet. 2010;199(1):1–8. [DOI] [PubMed] [Google Scholar]
- 4.Erquiaga I, Hurtado C, Aranaz P, Novo FJ, Vizmanos JL. A simple approach for classifying new mutations as somatic or germinal in DNA samples lacking paired tissue. Biotechniques. 2014;56(6):327–329. [DOI] [PubMed] [Google Scholar]
- 5.Haan S, Margue C, Engrand A, et al. Dual role of the Jak1 FERM and kinase domains in cytokine receptor binding and in stimulation-dependent Jak activation. J Immunol. 2008;180(2):998–1007. [DOI] [PubMed] [Google Scholar]
- 6.Flex E, Petrangeli V, Stella L, et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205(4):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott NE, Cleveland SM, Grann V, Janik J, Waldmann TA, Davé UP. FERM domain mutations induce gain of function in JAK3 in adult T-cell leukemia/lymphoma. Blood. 2011;118(14):3911–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riera L, Lasorsa E, Bonello L, et al. Description of a novel Janus kinase 3 P132A mutation in acute megakaryoblastic leukemia and demonstration of previously reported Janus kinase 3 mutations in normal subjects. Leuk Lymphoma. 2011;52(9):1742–1750. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Ma Y, Seemann J, Huang LJ. A regulating role of the JAK2 FERM domain in hyperactivation of JAK2(V617F). Biochem J. 2010;426(1):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison D, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14(12):2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson SA, Koleva RI, Argetsinger LS, et al. Regulation of Jak2 function by phosphorylation of Tyr317 and Tyr637 during cytokine signaling. Mol Cell Bio. 2009;29(12):3367–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vainchenker W, Constantinescu S. JAK/STAT signaling in hematological malignancies. Oncogene. 2012;32(21):2601–2613. [DOI] [PubMed] [Google Scholar]
- 13.Tefferi A, Lasho TL, Finke C, et al. Type 1 vs type 2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia. 2014;28(7):1568–1570. [DOI] [PubMed] [Google Scholar]
- 14.McGaffin G, Harper K, Stirling D, McLintock L. JAK2 V617F and CALR mutations are not mutually exclusive; findings from retrospective analysis of a small patient cohort. Br J Haematol. 2014;167(2):276–278. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed RZ, Rashid M, Ahmed N, Nadeem M, Shamsi TS. Coexisting JAK2V617F and CALR exon 9 mutations in Myeloproliferative Neoplasms - Do they designate a new subtype? Asian Pac J Cancer Prev. 2016;17(3):923–926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.