Abstract
OBJECTIVE
Identifying children ready for extubation is desirable to minimize morbidity and mortality associated with prolonged mechanical ventilation and extubation failure. We determined the accuracy of an extubation readiness test (RESTORE ERT) in predicting successful extubation in children with acute respiratory failure from lower respiratory tract disease.
DESIGN
Secondary analysis of data from the RESTORE clinical trial, a pediatric multicenter cluster randomized trial of sedation.
SETTING
17 pediatric intensive care units in the intervention arm.
PATIENTS
Children 2 weeks to 17 years receiving invasive mechanical ventilation for lower respiratory tract disease.
INTERVENTION
ERT in which spontaneously breathing children with oxygenation index ≤6 were placed on FiO2 of 0.50, positive end expiratory pressure of 5 cm H2O, and pressure support.
MEASUREMENTS AND MAIN RESULTS
Of 1,042 children, 444 (43%) passed their first ERT. Of these, 295 (66%) were extubated within 10 hours of starting the ERT, including 272 who were successfully extubated, for a positive predictive value of 92%. Among 861 children who were extubated for the first time within 10 hours of performing an ERT, 788 passed their ERT and 736 were successfully extubated for a positive predictive value of 93%. The median time of day for extubation with an ERT was 12:15H compared with 14:54H for extubation without an ERT within 10 hours (p<0.001).
CONCLUSIONS
In children with acute respiratory failure from lower respiratory tract disease, an ERT, as described, should be considered at least daily if the oxygenation index is ≤6. If the child passes the ERT, there is a high likelihood of successful extubation.
Keywords: critical care, endotracheal intubation, endotracheal extubation, mechanical ventilation, pediatrics, predictive value of tests, work of breathing
BACKGROUND
Identifying intubated children who are ready for endotracheal extubation is desirable to minimize the morbidity and mortality associated with prolonged mechanical ventilation and extubation failure. Prolonged mechanical ventilation and extubation failure are associated with biopsychosocial risks, increased hospital costs, and prolonged pediatric intensive care unit (PICU) stay.(1)
Extubation readiness tests (ERTs) aid in identifying who may be successfully extubated. In most ERTs, the child is placed on minimal ventilator settings and is observed for signs of distress or impaired gas exchange.(2, 3) For example, Randolph et al used an ERT in which children who were relatively awake and spontaneously breathing on stable minimal ventilator settings were placed on pressure support ventilation with FiO2 of 0.50 and positive end expiratory pressure (PEEP) of 5 cm H2O for 2 hours.(4) This ERT had a positive predictive value (PPV) of 87% for predicting successful extubation.(4)
We recently completed RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure), a multicenter cluster randomized trial of sedation in children.(5) The sedation protocol for the intervention arm included an ERT, modified from the ERT by Randolph et al and used in the pediatric prone positioning trial in order to apply to children with lower respiratory tract dysfunction.(4, 6) In this secondary analysis of data from RESTORE, we determined the accuracy of this modified ERT in predicting successful extubation in children with acute respiratory failure from lower respiratory tract disease.
METHODS
The primary aim of RESTORE was to determine whether critically ill children, managed with a nurse-implemented, goal-directed sedation protocol, would experience fewer days of mechanical ventilation than children receiving usual care.(5) The trial was conducted from June 2009 to December 2013. Of the 31 United States PICUs that participated, 17 were randomized to the intervention arm and comprised the children used in this secondary analysis. RESTORE was approved by the institutional review board at each participating site and parental permission was obtained for all subjects.
Subjects
RESTORE enrolled children aged 2 weeks to 17 years receiving invasive mechanical ventilation for acute airways and/or parenchymal lung disease and excluded those whose length of mechanical ventilation was unlikely to be altered by sedation management, such as mechanical ventilation only for postoperative care. In all analyses, we excluded children admitted with tracheostomies or those with new tracheostomies.
Extubation Readiness Test
The RESTORE ERT was a core element of the research intervention (Table 1).(6) All children were screened daily for ERT eligibility, which included presence of spontaneous breathing and an oxygenation index (OI) or oxygen saturation index (OSI) ≤6.(7) This modification of Randolph et al provided an assessment of lower respiratory tract function.(4, 6, 7) Children were placed sequentially on FiO2 of 0.50, PEEP of 5 cm H2O, and a ventilator mode of continuous positive airway pressure and pressure support. The level of pressure support was based on endotracheal tube size and was applied above PEEP. Children were monitored for up to 2 hours for passing physiologic parameters, that is, oxygen saturations ≥95%, exhaled tidal volumes ≥5 mL/kg ideal body weight, and acceptable respiratory rates for age. Clinicians used their judgment when evaluating parameters in a child with a large air leak. The bedside team worked to optimize the child’s capacity to pass the ERT. Specifically, the team assured that the child’s endotracheal tube was clear of secretions and that routine cares were completed before the ERT.
Table 1.
Daily Test for Patient Readiness for Extubation
Every morning at 06:00 ± 2 hours the child is assessed for the following:
|
| If the child meets these ERT criteria, then the child is tested for extubation readiness. |
| RESTORE Extubation Readiness Test (ERT): |
| 1. Temporarily stop enteral feedings |
| 2. If FiO2 not at 0.50, then titrate FiO2 to 0.50 as tolerated and assess; if tolerated, then |
| 3. If PEEP not at 5 cm H2O, then titrate PEEP to 5 cm H2O as tolerated and reassess |
4. Evaluate SpO2 after each ventilator change
|
The child is ready for extubation (from a pulmonary perspective) if all 3 of the following are present for ≥2 hours:
|
| If the child does not pass the ERT, s/he is returned to their pre-test ventilator settings and re-tested the next morning. If the clinical team judges that the child failed the ERT because of sedation-related hypoventilation, then a modified daily arousal assessment is completed and an ERT is repeated before 16:00 as per RESTORE study protocol. If the child does not meet the criteria at 16:00, s/he is returned to their pre-test ventilator settings and re-tested the following morning. |
| If the child passes the ERT, s/he is placed on comfortable ventilator settings and the medical team is notified that the child is ready (from a pulmonary perspective) for unassisted breathing. Extubation may be delayed for non-pulmonary reasons. |
Reprinted from J Crit Care, Vol. 21, No. 1, Curley MA, Arnold JH, Thompson JE, et al, Clinical trial design–effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome, 23–32, 2006, with permission from Elsevier.
Per protocol, the child’s phase of illness based on the clinical team’s determination of required respiratory support guided prescribed levels of sedation.(5) Children passing the daily ERT screen began ERT testing anytime before multidisciplinary walk rounds (typically between 04:00H to 08:00H). If the child passed the ERT, extubation was recommended within 6 hours of completing the test. If the child failed the ERT, the child was returned to pre-ERT ventilator settings and re-tested the next morning provided that the child met criteria for the ERT. If excessive sedation precipitated ERT failure, the team was instructed to wean sedation and repeat the ERT later during the day. Management of the child post-extubation, particularly with the use of non-invasive ventilation (NIV), was left to the care team’s discretion.
Outcome Measures and Statistical Analysis
Our primary cohort included children who had a planned extubation within 10 hours of starting their first ERT. For our secondary cohort, we used the first planned extubation and the ERT performed prior to this extubation if done within 10 hours of the extubation. The 10-hour limit covered the day shift in participating PICUs.
Our primary outcome was successful extubation defined as discontinuation of invasive mechanical ventilation for at least 24 hours. Our secondary outcome was successful extubation without the use of NIV, i.e., biphasic positive airway pressure or continuous positive airway pressure ≥5 cm H2O, immediately post-extubation. For both cohorts and outcomes, we calculated the diagnostic accuracy of the ERT, i.e., PPV, negative predictive value, sensitivity, and specificity, in predicting successful extubation.
For both cohorts, we compared characteristics of children who passed vs failed the ERT, and children successfully extubated vs reintubated within 24 hours. We also compared children extubated within 10 hours of passing their first ERT vs not extubated for the primary cohort, and children who were extubated with an ERT vs extubated without an ERT for the secondary cohort. Characteristics included demographic variables, baseline Pediatric Cerebral Performance Category (PCPC) and Pediatric Overall Performance Category (POPC) scores(8), Pediatric Risk of Mortality (PRISM) III-12 score(9), type of lung disease, early neuromuscular blockade use, length of mechanical ventilation, sedative exposure, severity of lung disease(10), and State Behavioral Scale.(11) For those extubated, we also compared the use of NIV post-extubation.
Groups were compared using linear, logistic, and cumulative logit regression accounting for PICU as a cluster variable using generalized estimating equations for log-transformed continuous, binary, and ordinal variables, respectively. Stepwise multivariable logistic regression was performed to predict the probability of ERT failure and extubation failure (reintubation within 24 hours) in both cohorts, using the characteristics above as potential covariates. A p-value of <0.05 was required for a covariate to enter and stay in the model. For the secondary cohort, the times of day for extubation for children with and without ERTs were compared using the Wilcoxon rank sum test for location shift and the Siegel-Tukey test for variability shift. All tests were performed using SAS, version 9.4 (SAS Institute Inc, Cary, NC) at a two-sided α=0.05 to determine statistical significance.
RESULTS
Of 1,225 children in the intervention arm of RESTORE, we excluded 24 with tracheostomies and 159 who never had an ERT after passing the screen (Figure 1). The remaining 1,042 children had at least one ERT performed (median: 2, interquartile range [IQR]: 1–3, maximum: 19) for a total of 2,735 ERTs. Of these, 444 (43%) passed their first ERT. Compared with children who passed their first ERT, those who failed were younger, more likely to have an obstructive lung disease, less likely to have received early neuromuscular blockade, and on mechanical ventilation for fewer days prior to their first ERT and had lower PRISM III-12 scores, less sedative exposure on the day prior to the ERT, and more severe lung disease on the day of the ERT (Table 2). In multivariable analysis, younger age at PICU admission and lower opioid exposure on the day before the first ERT were associated with higher risk of ERT failure. The most common reason for failing the first ERT was respiratory rate out of range (38%) Of 704 children with more than one ERT, 333 (47%) passed their second ERT, while 192 of 409 children (47%) passed their third ERT. (Supplemental Digital Content Tables 1, 2)
Figure 1.
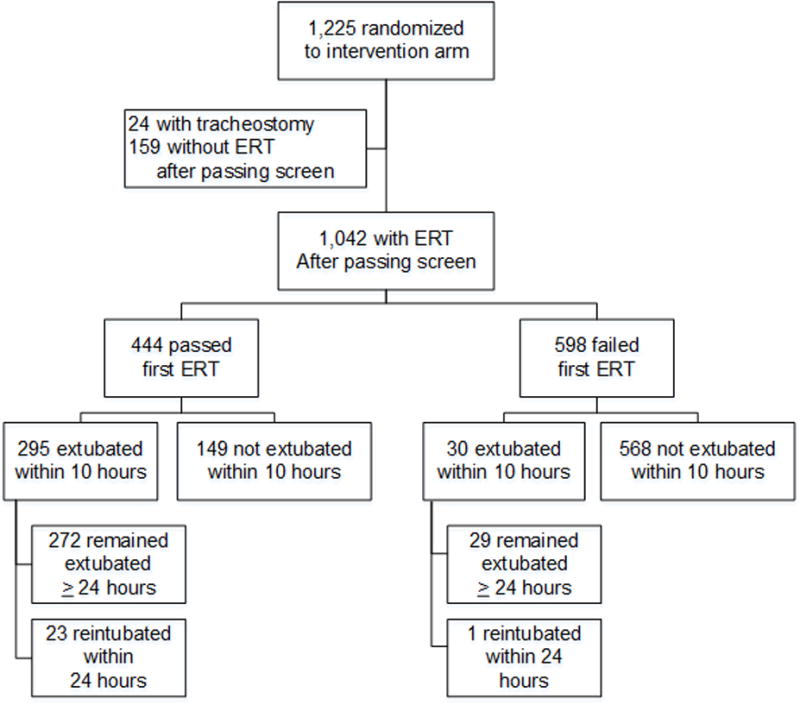
Flow diagram for patients in the primary cohort
Legend: ERT, extubation readiness test.
Table 2.
Characteristics of Children According to First Extubation Readiness Test Result
| Characteristic | Passed ERT (n = 444) |
Failed ERT (n = 598) |
pa |
|---|---|---|---|
| Age at PICU admission (IQR), y | 1.8 (0.6–7.7) | 0.6 (0.2–4.4) | <0.001 |
| Female, n (%) | 205 (46) | 265 (44) | 0.61 |
| Non-Hispanic white, n/total (%) | 221/438 (50) | 304/596 (51) | 0.65 |
| Cognitive impairment (baseline PCPC score >1), n (%)b | 107 (24) | 118 (20) | 0.049 |
| Functional impairment (baseline POPC score >1), n (%)b | 125 (28) | 141 (24) | 0.08 |
| PRISM III-12 score | 7 (3–12) | 5.5 (2–11) | 0.03 |
| Risk of mortality based on PRISM III-12 score, % | 2.9 (1.0–10.2) | 2.2 (0.9–7.7) | 0.12 |
| Primary diagnosis category, n (%)c | <0.001 | ||
| Restrictive lung disease | 264 (59) | 295 (49) | |
| Obstructive lung disease | 180 (41) | 303 (51) | |
| Neuromuscular blockade for the entire duration of days 0 to 2, n (%) | 37 (8) | 25 (4) | 0.007 |
| Days of mechanical ventilation prior to day of first ERT | 3 (1–6) | 2 (1–5) | 0.009 |
| Sedative exposure on day before first ERTd | |||
| Opioid exposure, mg/kg | 2.5 (1.2–4.7) | 1.8 (0.8–3.5) | <0.001 |
| Benzodiazepine exposure, mg/kg | 2.3 (1.1–4.5) | 1.8 (0.9–3.6) | 0.005 |
| Number of sedative classes, mean ± SDe | 2.3 ± 0.7 | 2.1 ± 0.5 | 0.006 |
| PARDS on day of first ERT, n (%)f | <0.001 | ||
| At risk (OI <4.0 or OSI <5.0) | 361 (81) | 422 (71) | |
| Mild (OI 4.0–7.9 or OSI 5.0–7.4) | 78 (18) | 163 (27) | |
| Moderate (OI 8.0–15.9 or OSI 7.5–12.2) | 5 (1) | 12 (2) | |
| Severe (OI ≥16.0 or OSI ≥12.3) | 0 | 1 (<1) | |
| SBS score at start of first ERT, n (%) | 0.23 | ||
| −3/−2 | 15 (3) | 42 (7) | |
| −1/0 | 389 (89) | 492 (84) | |
| +1/+2 | 33 (8) | 50 (9) | |
| Missing | 7 | 14 |
ARF, acute respiratory failure; ERT, extubation readiness test; IQR, interquartile range; OI, oxygenation index; OSI, oxygen saturation index; PARDS, pediatric acute respiratory syndrome; PCPC, Pediatric Cerebral Performance Category; PICU, pediatric intensive care unit; POPC, Pediatric Overall Performance Category; PRISM III-12, Pediatric Risk of Mortality III score from first 12 hours in the PICU; SBS, State Behavioral Scale; SD, standard deviation.
Values are median (IQR) unless otherwise specified.
P values for the comparison between groups were calculated using linear, logistic, and cumulative logit regression accounting for PICU as a cluster variable using generalized estimating equations for log-transformed continuous, binary, and ordinal variables, respectively.
PCPC and POPC range from 1 to 6, with higher categories indicating greater impairment.
Diagnoses for restrictive lung disease: pneumonia, sepsis, aspiration pneumonia, pulmonary edema, thoracic trauma, pulmonary hemorrhage, ARF post bone marrow transplant, acute chest syndrome/sickle cell disease, pertussis, pneumothorax (non-trauma), ARF related to multiple blood transfusions, lymphangiectasia, pulmonary embolism. Diagnoses for obstructive lung disease: bronchiolitis, asthma, laryngotracheobronchitis, acute exacerbation of lung disease (cystic fibrosis or bronchopulmonary dysplasia).
Two children passed first ERT on day 0, so no data available for day before first ERT.
Different sedative classes include opioids, benzodiazepines, α2-adrengenic agonists (dexmedetomidine or clonidine), propofol, barbiturates (pentobarbital or phenobarbital), ketamine, and chloral hydrate.
Oxygenation index was calculated as ([FIO2 × mean airway pressure]/PaO2 × 100). When an arterial blood gas measurement was not available, SpO2 was used to estimate PaO2 in order to calculate OSI ([FIO2 × mean airway pressure]/SpO2 × 100). Lower scores reflect better oxygenation.
Of the 444 children who passed the first ERT, 295 (66%) were extubated within 10 hours of starting the test. The most common reasons for not extubating were physician preference (26%) and excessive secretions (25%). Children who were not extubated were younger and more likely to have received early neuromuscular blockade and had more severe lung disease on the day of the ERT than those who were extubated (Supplemental Digital Content Tables 3, 4).
In addition to the 295 children who were extubated within 10 hours of passing their first ERT, 30 children were extubated within 10 hours of failing their first ERT. Seventeen of these 30 children passed a second ERT prior to extubation. We included these 325 children in our primary cohort.
A total of 1,093 children had a planned extubation. Of these, 861 (79%) had an ERT started within 10 hours of extubation and comprised our secondary cohort. An additional 138 children had an ERT started >10 hours prior to extubation. Children who did not have an ERT within 10 hours of extubation had worse baseline PCPC and POPC scores, were less likely to have an obstructive lung disease, and had more severe lung disease on the day of their first extubation. A total of 788 (92%) passed this ERT. Children who failed the ERT had lower PRISM III-12 scores, were on mechanical ventilation for more days prior to extubation, and had less opioid exposure on the day before extubation than those who passed the ERT. In multivariable analysis, lower PRISM III-12 score and more days on mechanical ventilation prior to extubation were associated with higher risk of ERT failure. The most common reason for failing this ERT was respiratory rate out of range (49%) (Supplemental Digital Content Figure 1 and Tables 1, 2, 5, 6).
Extubation Failures
Among children in the primary cohort who passed their first ERT and were extubated, 23 (8%) failed extubation. The reasons for failing extubation were upper airway obstruction (65%), lower respiratory dysfunction resulting in impaired gas exchange (22%), excessive secretions (9%), and tachypnea (4%). Children who failed extubation were younger, had lower risk of mortality, and had more agitated State Behavioral Scale scores at the start of the ERT than those who were successfully extubated (Table 3). Of 272 children who were successfully extubated, 37 (14%) were placed on NIV post-extubation. One of 30 children (3%) who failed the first ERT but were extubated failed extubation. Of those who passed a second and third ERT and were extubated within 10 hours of starting the ERT, 21 of 247 (9%) and 5 of 128 (4%) failed extubation, respectively.
Table 3.
Characteristics of Children with Planned Extubations Within 10 Hours of Passing First Extubation Readiness Test According to Extubation Result
| Characteristic | Successful Extubation (n = 272) |
Reintubated within 24 h (n = 23) |
pa |
|---|---|---|---|
| Age at PICU admission (IQR), y | 2.5 (0.6–10.3) | 1.3 (0.2–3.3) | <0.001 |
| Female, n (%) | 123 (45) | 8 (35) | 0.41 |
| Non-Hispanic white, n/total (%) | 140/271 (52) | 14/23 (61) | 0.29 |
| Cognitive impairment (baseline PCPC score >1), n (%)b | 65 (24) | 8 (35) | 0.27 |
| Functional impairment (baseline POPC score >1), n (%)b | 77 (28) | 8 (35) | 0.56 |
| PRISM III-12 score | 7 (3–12) | 4 (0–7) | <0.001 |
| Risk of mortality based on PRISM III-12 score, % | 2.9 (1.0–9.8) | 1.3 (0.5–4.3) | <0.001 |
| Primary diagnosis category, n (%) | 0.21 | ||
| Restrictive lung disease | 162 (60) | 10 (43) | |
| Obstructive lung disease | 110 (40) | 13 (57) | |
| Days of mechanical ventilation prior to day of first ERT | 3 (1–6) | 3 (1–7) | 0.77 |
| Sedative exposure on day before first ERTc | |||
| Opioid exposure, mg/kg | 2.5 (1.1–4.7) | 2.5 (0.5–4.2) | 0.57 |
| Benzodiazepine exposure, mg/kg | 2.4 (1.1–4.4) | 2.1 (0.6–5.0) | 0.69 |
| Number of sedative classesd | 2 (2–3) | 2 (2–2) | 0.84 |
| PARDS on day of first ERT, n (%)e | 0.86 | ||
| At risk (OI <4.0 or OSI <5.0) | 230 (85) | 20 (87) | |
| Mild (OI 4.0–7.9 or OSI 5.0–7.4) | 40 (15) | 1 (4) | |
| Moderate (OI 8.0–15.9 or OSI 7.5–12.2) | 2 (<1) | 2 (9) | |
| Severe (OI ≥16.0 or OSI ≥12.3) | 0 | 0 | |
| SBS score at start of first ERT, n (%) | 0.002 | ||
| −3/−2 | 9 (3) | 0 | |
| −1/0 | 239 (90) | 17 (74) | |
| +1/+2 | 17 (6) | 6 (26) | |
| Missing | 7 | 0 | |
| Non-invasive ventilation immediately post-extubation, n (%)f | 37 (14) | 7 (30) | 0.053 |
ERT, extubation readiness test; IQR, interquartile range; OI, oxygenation index; OSI, oxygen saturation index; PARDS, pediatric acute respiratory syndrome; PCPC, Pediatric Cerebral Performance Category; PICU, pediatric intensive care unit; POPC, Pediatric Overall Performance Category; PRISM III-12, Pediatric Risk of Mortality III score from first 12 hours in the PICU; SBS, State Behavioral Scale.
Values are median (IQR) unless otherwise specified.
P values for the comparison between groups were calculated using linear, logistic, and cumulative logit regression accounting for PICU as a cluster variable using generalized estimating equations for log-transformed continuous, binary, and ordinal variables, respectively.
PCPC and POPC range from 1 to 6, with higher categories indicating greater impairment.
One child passed ERT on day 0, so no data available for day before first ERT.
Different sedative classes include opioids, benzodiazepines, α2-adrengenic agonists (dexmedetomidine or clonidine), propofol, barbiturates (pentobarbital or phenobarbital), ketamine, and chloral hydrate.
Oxygenation index was calculated as ([FIO2 × mean airway pressure]/PaO2 × 100). When an arterial blood gas measurement was not available, SpO2 was used to estimate PaO2 in order to calculate OSI ([FIO2 × mean airway pressure]/SpO2 × 100). Lower scores reflect better oxygenation.
Non-invasive ventilation includes biphasic positive airway pressure or continuous positive airway pressure ≥5 cm H2O.
Among children in the secondary cohort who passed the ERT prior to their first planned extubation, 52 (7%) failed extubation. Those who failed extubation were younger and had lower PRISM III-12 scores than those who were successfully extubated. Of 736 children who were successfully extubated, 101 (14%) were placed on NIV post-extubation. Of the 73 children (10%) who failed their ERT but were extubated, 7 (10%) failed extubation. (Supplemental Digital Content Table 7)
In multivariable analysis, younger age, worse baseline PCPC score, lower PRISM III-12 score, and use of NIV post-extubation were associated with extubation failure in both cohorts (Supplemental Digital Content Table 8).
Diagnostic Accuracy of the ERT
In our primary cohort, the PPV of the first ERT was 92% (Table 4). The negative predictive value, sensitivity, and specificity were 3%, 90%, and 4%, respectively. When the use of NIV post-extubation was added to the definition of extubation failure, the PPV was 80%.
Table 4.
Accuracy of the Extubation Readiness Test
| Positive Predictive Value | Negative Predictive Value |
Sensitivity | Specificity | |
|---|---|---|---|---|
| Primary cohort (first ERT, n = 325) | ||||
| Successful extubation vs Reintubated within 24 h |
92% (89–95%) |
3% (0–17%) |
90% (86–93%) |
4% (0–21%) |
| Successful extubation vs Reintubated within 24 h or non-invasive ventilation immediately post-extubation |
80% (75–84%) |
20% (8–39%) |
91% (87–94%) |
9% (3–19%) |
| Secondary cohort (first planned extubation, n = 861) | ||||
| Successful extubation vs Reintubated within 24 h |
93% (91–95%) |
8% (3–17%) |
92% (90–93%) |
10% (4–21%) |
| Successful extubation vs Reintubated within 24 h or non-invasive ventilation immediately post-extubation |
81% (78–83%) |
21% (12–32%) |
92% (89–94%) |
9% (5–14%) |
ERT, extubation readiness test.
Data presented as value (exact 95% confidence interval).
In our secondary cohort, the PPV of the ERT was 93% (Table 3). When the use of NIV post-extubation was added to the definition of extubation failure, the PPV was 81%.
Timing of Extubation
In the secondary cohort, the ERT was most often (59%) started between 04:00H and 07:59H, while an additional 36% were started between 08:00H and 15:59H. Nearly half (46%) of first planned extubations occurred between 12:00H and 15:59H in children who had an ERT, with an additional 39% extubated between 08:00H and 11:59H. This compares with 34% and 19% for these time periods in children who did not have an ERT. Children who had an ERT were extubated earlier in the day with less variability (median: 12:15H, IQR: 10:45H-14:18H) compared to those who did not have an ERT (median: 14:54H, IQR: 11:42H-16:42H; p<0.001 for location shift and p<0.001 for variability shift). (Supplemental Digital Content Figure 2)
DISCUSSION
In this analysis of data from a large multicenter cluster randomized trial, we report that if a child passed the ERT and was extubated within 10 hours of starting the ERT, the probability of remaining extubated for at least 24 hours was 92–93%. Performing an ERT was associated with extubations occurring earlier during the day. Our findings demonstrate the potential benefits of using an ERT screen and test in practice and in clinical trials.(12)
In our multivariable analyses, we found that younger children were more likely to fail the ERT and fail extubation, which is consistent with other studies.(3, 5) It is unclear if extubation failure in these children is due to patient factors such as weight, motor strength, or smaller airways. We also found that lower PRISM III-12 score was associated with ERT and extubation failure, although these associations may be statistically but not clinically significant. Lower opioid exposure on the day before the first ERT was also associated with ERT failure, which may reflect the complex relationship of wakefulness, pain, and agitation that was demonstrated in the RESTORE trial.(5)
Our study provides some insight into potential barriers to the routine use of ERT screening and testing. The most common reasons for not extubating a child despite their passing an ERT were physician preference and excessive secretions. Children who were not extubated were younger. This is consistent with our findings in children who failed extubation and may have played a role in physician preference not to extubate. A survey of pediatric critical care practitioners reported that most clinicians assess the highly subjective character of tracheal secretions when determining a child’s readiness for extubation.(13) The association between secretion volume and successful extubation is unclear.(14) Also unclear is whether pulmonary secretions are better removed by endotracheal suctioning or by spontaneous effective coughing without an artificial airway. Objective measures of inadequate secretion management may facilitate future clinical decision-making.
Future modifications in the RESTORE ERT may also improve its predictive value. Specifically, with the common use of high flow, high humidity nasal cannula and other forms of NIV, children may be successfully extubated despite tachypnea, the most common reason for failing the RESTORE ERT.(1, 15) Additional tests and monitoring, such as respiratory inductance plethysmography and esophageal manometry, may better predict upper airway obstruction post-extubation.(16)
Given that so few patients were extubated after failing the RESTORE ERT, we are only confident in our reporting of the test’s PPV with and without NIV included in the definition of extubation failure. Passing the RESTORE ERT can identify, with good accuracy, children who can be successfully extubated, but the test can say little about those who fail the ERT. Our reported PPV is similar to other pediatric ERTs,(14, 17–19) but these studies may have overestimated their PPV by including children without respiratory disease. The RESTORE ERT slightly improved on the 87% PPV reported by Randolph et al.(4)
Although the RESTORE ERT was designed to identify children who are ready for extubation in the setting of a clinical trial, benefits can be applied in practice. When the ERT was performed per protocol, children were extubated earlier in the day which may improve PICU workflow.
Our study has several limitations. First, a third of children who passed their first ERT were not extubated within 10 hours of the test and this could have affected the estimates of the diagnostic accuracy. Second, the RESTORE ERT may overestimate extubation success due to the level of pressure support used during the test.(2) Unfortunately, we were unable to determine the association between level of pressure support and extubation success because these data were not collected during the trial. Third, we included the use of NIV in our secondary definition of extubation failure; however, we did not standardize its use or collect data on the level or rationale for the NIV support provided. Finally, the decision to reintubate a child was not standardized in RESTORE.
CONCLUSION
In this secondary analysis of data from a multicenter trial, we described the accuracy of an ERT when applied to children with acute respiratory failure from lower respiratory tract disease who are spontaneously breathing with an oxygenation or oxygen saturation index ≤6. Passing the RESTORE ERT can identify, with reasonable accuracy, children who will be successfully extubated. Performance of this test is associated with extubation earlier during the day.
Supplementary Material
Supplemental Digital Content Figure 1. Flow diagram for patients in the secondary cohort.
Legend: ERT, extubation readiness test.
Supplemental Digital Content Figure 2. Timing of extubation.
Legend: ERT, extubation readiness test. Panel a shows the distribution of times of day for extubation for the 861 patients with an ERT within 10 hours prior to their first planned extubation. Panel b shows the distribution of time of day for extubation for the 232 patients with no ERT within 10 hours prior to their first planned extubation.
Acknowledgments
Institutions where work was performed: Advocate Children’s Hospital-Oak Lawn, Oak Lawn, IL; Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Children’s Hospital and Medical Center, Omaha, NE; Children’s Mercy Hospital, Kansas City, MO; Children’s Hospital of Orange County, Orange, CA; Cohen Children’s Medical Center of New York, Hyde Park, NY; Children’s Medical Center of Dallas, Dallas, TX; Lucile Packard Children’s Hospital Stanford, Palo Alto, CA; Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE; Oregon Health & Science University Doernbecher Children’s Hospital, Portland, OR; Primary Children’s Hospital, Salt Lake City, UT; St. Louis Children’s Hospital, St. Louis, MO; University of California Davis Medical Center, Sacramento, CA; University of California at San Francisco Benioff Children’s Hospital at Oakland, Oakland, CA; University of Massachusetts Memorial Children’s Medical Center, Worcester, MA; University of Arizona Medical Center, Tucson, AZ; Yale-New Haven Children’s Hospital, New Haven, CT.
Financial support: The Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) study was supported by grants from the National Heart, Lung, and Blood Institute and the National Institute of Nursing Research, National Institutes of Health (grant U01 HL086622 to Dr. Curley and U01 HL086649 to Dr. Wypij).
Dr. Schwarz received support for article research from the National Institutes of Health (NIH). His institution received funding from the NIH. Dr. Asaro received support for article research from the NIH. Her institution received funding from the NIH/NHLBI. Dr. Wypij received support for article research from the NIH. His institution received funding from the National Heart, Lung, and Blood Institute. Dr. Curley received support for article research from the NIH and received funding from SBS Consultant for Hospira, Inc.
Footnotes
No reprints requested.
Copyright form disclosures:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
RESTORE STUDY INVESTIGATORS
The RESTORE study investigators include: Martha A.Q. Curley (Principal Investigator; School of Nursing and the Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; Critical Care and Cardiovascular Program, Boston Children’s Hospital, Boston, MA); David Wypij, (Principal Investigator – Data Coordinating Center; Department of Biostatistics, Harvard T.H. Chan School of Public Health; Department of Pediatrics, Harvard Medical School; Department of Cardiology, Boston Children’s Hospital, Boston, MA); Geoffrey L. Allen (Children’s Mercy Hospital, Kansas City, MO); Derek C. Angus (Clinical Research, Investigation, and Systems Modeling of Acute Illness Center, Pittsburgh, PA); Lisa A. Asaro (Department of Cardiology, Boston Children’s Hospital, Boston, MA); Judy A. Ascenzi (The Johns Hopkins Hospital, Baltimore, MD); Scot T. Bateman (University of Massachusetts Memorial Children’s Medical Center, Worcester, MA); Santiago Borasino (Children’s Hospital of Alabama, Birmingham, AL); Cindy Darnell Bowens (Children’s Medical Center of Dallas, Dallas, TX); G. Kris Bysani (Medical City Children’s Hospital, Dallas, TX); Ira M. Cheifetz (Duke Children’s Hospital, Durham, NC); Allison S. Cowl (Connecticut Children’s Medical Center, Hartford, CT); Brenda L. Dodson (Department of Pharmacy, Boston Children’s Hospital, Boston, MA); E. Vincent S. Faustino (Yale-New Haven Children’s Hospital, New Haven, CT); Lori D. Fineman (University of California San Francisco Benioff Children’s Hospital at San Francisco, San Francisco, CA); Heidi R. Flori (University of California at San Francisco Benioff Children’s Hospital at Oakland, Oakland, CA); Linda S. Franck (University of California at San Francisco School of Nursing, San Francisco, CA); Rainer G. Gedeit (Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI); Mary Jo C. Grant (Primary Children’s Hospital, Salt Lake City, UT); Andrea L. Harabin (National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD); Catherine Haskins-Kiefer (Florida Hospital for Children, Orlando, FL); James H. Hertzog (Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE); Larissa Hutchins (The Children’s Hospital of Philadelphia, Philadelphia, PA); Aileen L. Kirby (Oregon Health & Science University Doernbecher Children’s Hospital, Portland, OR); Ruth M. Lebet (School of Nursing, University of Pennsylvania, Philadelphia, PA); Michael A. Matthay (University of California at San Francisco School of Medicine, San Francisco, CA); Gwenn E. McLaughlin (Holtz Children’s Hospital, Jackson Health System, Miami, FL); JoAnne E. Natale (University of California Davis Children’s Hospital, Sacramento, CA); Phineas P. Oren (St. Louis Children’s Hospital, St. Louis, MO); Nagendra Polavarapu (Advocate Children’s Hospital-Oak Lawn, Oak Lawn, IL); James B. Schneider (Cohen Children’s Medical Center of New York, Hyde Park, NY); Adam J. Schwarz (Children’s Hospital of Orange County, Orange, CA); Thomas P. Shanley (C. S. Mott Children’s Hospital at the University of Michigan, Ann Arbor, MI); Shari Simone (University of Maryland Medical Center, Baltimore, MD); Lewis P. Singer (The Children’s Hospital at Montefiore, Bronx, NY); Lauren R. Sorce (Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL); Edward J. Truemper (Children’s Hospital and Medical Center, Omaha, NE); Michele A. Vander Heyden (Children’s Hospital at Dartmouth, Dartmouth, NH); R. Scott Watson (Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute, Seattle, WA); Claire R. Wells (University of Arizona Medical Center, Tucson, AZ).
References
- 1.Blackwood B, Murray M, Chisakuta A, et al. Protocolized versus non-protocolized weaning for reducing the duration of invasive mechanical ventilation in critically ill paediatric patients. Cochrane Database Syst Rev. 2013;7:CD009082. doi: 10.1002/14651858.CD009082.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newth CJ, Venkataraman S, Willson DF, et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. 2009;10(1):1–11. doi: 10.1097/PCC.0b013e318193724d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston C, da Silva P. Weaning and extubation in pediatrics. Curr Respir Med Rev. 2012;8(1):68–78. [Google Scholar]
- 4.Randolph AG, Wypij D, Venkataraman ST, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: A randomized controlled trial. JAMA. 2002;288(20):2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 5.Curley MA, Wypij D, Watson RS, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: A randomized clinical trial. JAMA. 2015;313(4):379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curley MA, Arnold JH, Thompson JE, et al. Clinical trial design–effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome. J Crit Care. 2006;21(1):23–32. doi: 10.1016/j.jcrc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curley MA, Fackler JC. Weaning from mechanical ventilation: Patterns in young children recovering from acute hypoxemic respiratory failure. Am J Crit Care. 1998;7(5):335–345. [PubMed] [Google Scholar]
- 8.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 9.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S23–40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 11.Curley MA, Harris SK, Fraser KA, et al. State Behavioral Scale: A sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7(2):107–114. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings BM, Noviski N. Pediatric extubation readiness: Faith-based practice or amenable to standardization? Respir Care. 2014;59(3):445–446. doi: 10.4187/respcare.03103. [DOI] [PubMed] [Google Scholar]
- 13.Mhanna MJ, Anderson IM, Iyer NP, et al. The use of extubation readiness parameters: A survey of pediatric critical care physicians. Respir Care. 2014;59(3):334–339. doi: 10.4187/respcare.02469. [DOI] [PubMed] [Google Scholar]
- 14.Laham JL, Breheny PJ, Rush A. Do clinical parameters predict first planned extubation outcome in the pediatric intensive care unit? J Intensive Care Med. 2015;30(2):89–96. doi: 10.1177/0885066613494338. [DOI] [PubMed] [Google Scholar]
- 15.Sinha IP, McBride AK, Smith R, et al. CPAP and high-flow nasal cannula oxygen in bronchiolitis. Chest. 2015;148(3):810–823. doi: 10.1378/chest.14-1589. [DOI] [PubMed] [Google Scholar]
- 16.Khemani RG, Hotz J, Morzov R, et al. Evaluating risk factors for pediatric post-extubation upper airway obstruction using a physiology-based tool. Am J Respir Crit Care Med. 2016;193(2):198–209. doi: 10.1164/rccm.201506-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavez A, dela Cruz R, Zaritsky A. Spontaneous breathing trial predicts successful extubation in infants and children. Pediatr Crit Care Med. 2006;7(4):324–328. doi: 10.1097/01.PCC.0000225001.92994.29. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson LP, Walsh BK, Munhall D, et al. A spontaneous breathing trial with pressure support overestimates readiness for extubation in children. Pediatr Crit Care Med. 2011;12(6):e330–335. doi: 10.1097/PCC.0b013e3182231220. [DOI] [PubMed] [Google Scholar]
- 19.Foronda FK, Troster EJ, Farias JA, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: A randomized controlled trial. Crit Care Med. 2011;39(11):2526–2533. doi: 10.1097/CCM.0b013e3182257520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content Figure 1. Flow diagram for patients in the secondary cohort.
Legend: ERT, extubation readiness test.
Supplemental Digital Content Figure 2. Timing of extubation.
Legend: ERT, extubation readiness test. Panel a shows the distribution of times of day for extubation for the 861 patients with an ERT within 10 hours prior to their first planned extubation. Panel b shows the distribution of time of day for extubation for the 232 patients with no ERT within 10 hours prior to their first planned extubation.


