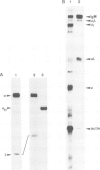
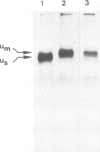
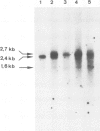
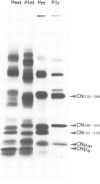
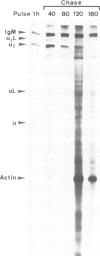
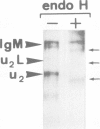
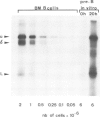
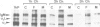Abstract
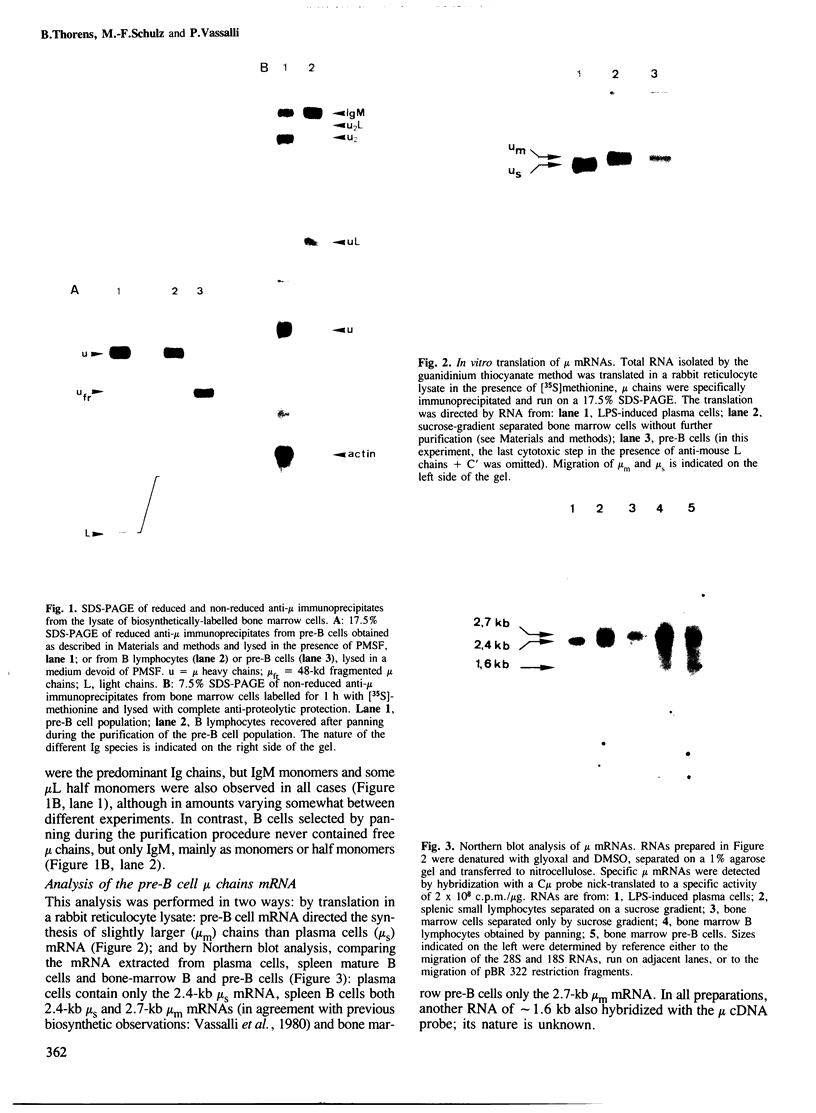
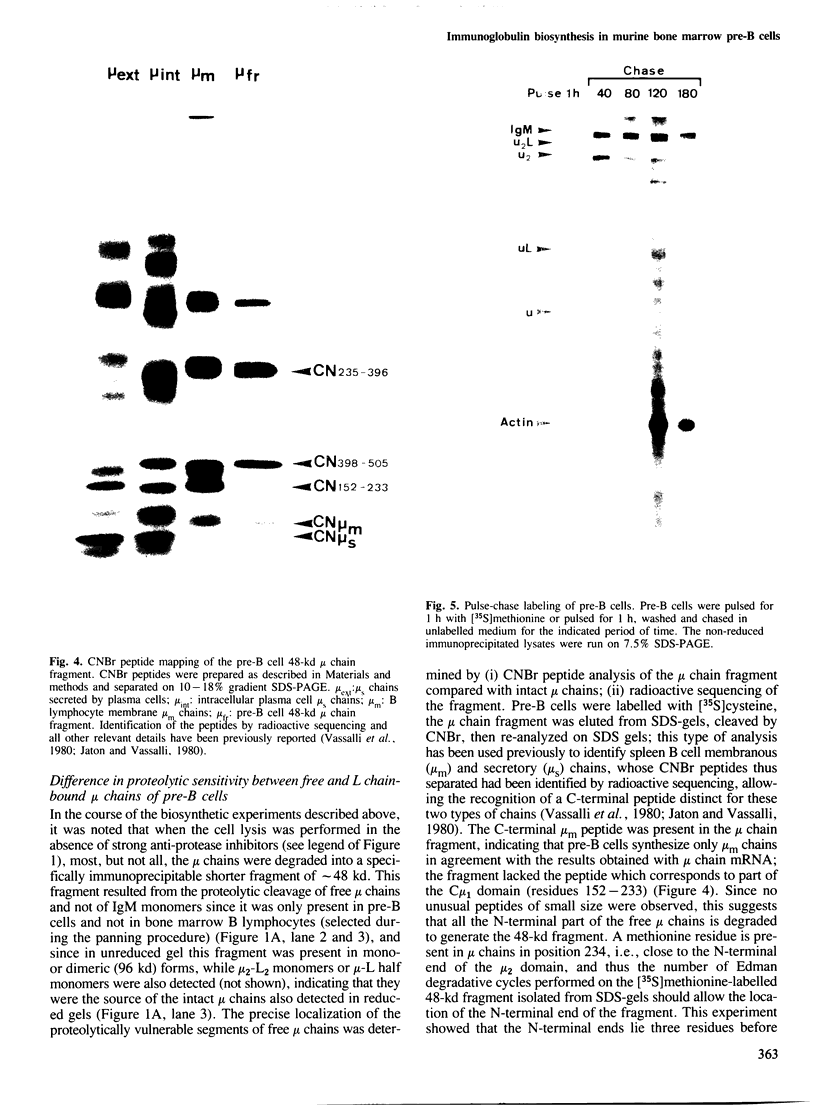
Mouse normal bone marrow pre-B lymphocytes synthesize only membrane mu chains (micron), as shown by mRNA studies and peptide analysis. The micron chains exist in two forms: free micron chains assembled into dimers, or L chain-bound micron chains present in IgM monomers (in the case of 'late pre-B cells', i.e., after productive L chain gene rearrangement). These two forms of molecules are very different in properties, fate and intracellular pathways. Free but not L chain-bound mu chains are highly susceptible to mild proteolysis, which degrades their entire Cmu 1 and VH domains. Free mu chains are rapidly degraded within the lysosomal compartment, which they reach via the cis, avoiding the trans, part of the Golgi complex. In contrast, as soon as mu chains bind to L chains, they are directed towards the 'trans' Golgi compartment, where they undergo terminal glycosylation, then to the cell surface, where they progressively accumulate. It is suggested that the conformation instability of the Cmu 1 and VH domains of the free mu chains plays a critical role in the intracellular targeting of these molecules, as compared with that of L chain-bound mu chains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Arias C., Bell J. R., Lenches E. M., Strauss E. G., Strauss J. H. Sequence analysis of two mutants of Sindbis virus defective in the intracellular transport of their glycoproteins. J Mol Biol. 1983 Jul 25;168(1):87–102. doi: 10.1016/s0022-2836(83)80324-6. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Nucleotide sequence of a cloned cDNA corresponding to secreted mu chain of mouse immunoglobulin. Gene. 1980 Dec;12(1-2):77–86. doi: 10.1016/0378-1119(80)90017-7. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Schwartz M. L., Crystal R. G. Regulation of the production of secretory proteins: intracellular degradation of newly synthesized "defective" collagen. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4746–4750. doi: 10.1073/pnas.77.8.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Schwartz M. L., Rome L. H., Crystal R. G. Lysosomal function in the degradation of defective collagen in cultured lung fibroblasts. Biochemistry. 1984 May 8;23(10):2134–2138. doi: 10.1021/bi00305a005. [DOI] [PubMed] [Google Scholar]
- Brown W. J., Farquhar M. G. The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell. 1984 Feb;36(2):295–307. doi: 10.1016/0092-8674(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Burrows P., LeJeune M., Kearney J. F. Evidence that murine pre-B cells synthesise mu heavy chains but no light chains. Nature. 1979 Aug 30;280(5725):838–840. doi: 10.1038/280838a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. Immunoglobulin gene rearrangement during pre-B cell differentiation. J Mol Cell Immunol. 1983;1(1):31–41. [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- Gathings W. E., Mage R. G., Cooper M. D., Young-Cooper G. O. A subpopulation of small pre-B cells in rabbit bone marrow expresses kappa light chains and exhibits allelic exclusion of b locus allotypes. Eur J Immunol. 1982 Jan;12(1):76–81. doi: 10.1002/eji.1830120114. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983 Mar;96(3):835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L., Levitt D. Analysis of surface mu-chain expression in human lymphoblastoid cell lines that do not produce light chains. J Immunol. 1984 Jan;132(1):502–509. [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaton J. C., Vassalli P. A major structural difference between membrane-bound and secreted IgM of normal mouse spleen cells is located in the C-terminal region of their heavy chains. FEBS Lett. 1980 Jul 28;116(2):277–280. doi: 10.1016/0014-5793(80)80662-4. [DOI] [PubMed] [Google Scholar]
- Kloppel T. M., Kubo R. T., Cain P. S., Browne C. P., Colon S. M., Kearney J. F., Grey H. M. Structural analysis of the mu-chains synthesized by fetal liver hybridomas. J Immunol. 1981 Apr;126(4):1346–1350. [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubagawa H., Gathings W. E., Levitt D., Kearney J. F., Cooper M. D. Immunoglobulin isotype expression of normal pre-B cells as determined by immunofluorescence. J Clin Immunol. 1982 Oct;2(4):264–269. doi: 10.1007/BF00915065. [DOI] [PubMed] [Google Scholar]
- Lancet D., Parham P., Strominger J. L. Heavy chain of HLA-A and HLA-B antigens is conformationally labile: a possible role for beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3844–3848. doi: 10.1073/pnas.76.8.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth K. S., Rosse C., Clagett J. Myelogenous production and maturation of B lymphocytes in the mouse. J Immunol. 1981 Nov;127(5):2027–2034. [PubMed] [Google Scholar]
- Levitt D., Cooper M. D. Mouse pre-B cells synthesize and secrete mu heavy chains but not light chains. Cell. 1980 Mar;19(3):617–625. doi: 10.1016/s0092-8674(80)80038-9. [DOI] [PubMed] [Google Scholar]
- Mains P. E., Sibley C. H. Control of IgM synthesis in the murine pre-B cell line, 70Z/3'. J Immunol. 1982 Apr;128(4):1664–1670. [PubMed] [Google Scholar]
- Mains P. E., Sibley C. H. The requirement of light chain for the surface deposition of the heavy chain of immunoglobulin M. J Biol Chem. 1983 Apr 25;258(8):5027–5033. [PubMed] [Google Scholar]
- Maki R., Kearney J., Paige C., Tonegawa S. Immunoglobulin gene rearrangement in immature B cells. Science. 1980 Sep 19;209(4463):1366–1369. doi: 10.1126/science.6774416. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Fu S. M. Ig biosynthesis in a human pre-B cell line. J Immunol. 1981 Dec;127(6):2609–2611. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen N. L., Nayak D. P., Jones L. V., Compans R. W. Chimeric influenza virus hemagglutinin containing either the NH2 terminus or the COOH terminus of G protein of vesicular stomatitis virus is defective in transport to the cell surface. Proc Natl Acad Sci U S A. 1984 Jan;81(2):395–399. doi: 10.1073/pnas.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opstelten D., Osmond D. G. Pre-B cells in mouse bone marrow: immunofluorescence stathmokinetic studies of the proliferation of cytoplasmic mu-chain-bearing cells in normal mice. J Immunol. 1983 Dec;131(6):2635–2640. [PubMed] [Google Scholar]
- Osmond D. G., Nossal G. J. Differentiation of lymphocytes in mouse bone marrow. II. Kinetics of maturation and renewal of antiglobulin-binding cells studied by double labeling. Cell Immunol. 1974 Jul;13(1):132–145. doi: 10.1016/0008-8749(74)90233-0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Megson M., Owen J. J., Cooper M. D. Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature. 1976 Jan 22;259(5540):224–226. doi: 10.1038/259224a0. [DOI] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser J. E., Vassalli P. Mouse bone marrow lymphocytes and their differentiation. J Immunol. 1974 Sep;113(3):719–728. [PubMed] [Google Scholar]
- Seligmann M., Mihaesco E., Preud'homme J. L., Danon F., Brouet J. C. Heavy chain diseases: current findings and concepts. Immunol Rev. 1979;48:145–167. doi: 10.1111/j.1600-065x.1979.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Siden E., Alt F. W., Shinefeld L., Sato V., Baltimore D. Synthesis of immunoglobulin mu chain gene products precedes synthesis of light chains during B-lymphocyte development. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1823–1827. doi: 10.1073/pnas.78.3.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Lectin-binding sites as markers of Golgi subcompartments: proximal-to-distal maturation of oligosaccharides. J Cell Biol. 1983 Oct;97(4):1243–1248. doi: 10.1083/jcb.97.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P. Plasma cell immunoglobulin M molecules. Their biosynthesis, assembly, and intracellular transport. J Cell Biol. 1979 Nov;83(2 Pt 1):284–299. doi: 10.1083/jcb.83.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli P., Tartakoff A., Pink J. R., Jaton J. C. Biosynthesis of two forms of IgM heavy chains by normal mouse B lymphocytes. Membrane and secretory IgM. J Biol Chem. 1980 Dec 25;255(24):11822–11827. [PubMed] [Google Scholar]
- Vitetta E. S., Melcher U., McWilliams M., Lamm M. E., Phillips-Quagliata J. M., Uhr J. W. Cell surface immunoglobulin. XI. The appearance of an IgD-like molecule on murine lymphoid cells during ontogeny. J Exp Med. 1975 Jan 1;141(1):206–215. doi: 10.1084/jem.141.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. IgD and B cell differentiation. Immunol Rev. 1977;37:50–88. doi: 10.1111/j.1600-065x.1977.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]