Abstract
Growing evidence from field studies has linked daily stressors to dysregulated patterns of diurnal cortisol. Less is known about whether naturally-occurring positive events in everyday life are associated with diurnal cortisol. The objectives of this study were to evaluate daily positive events as predictors of between-person differences and within-person (day-to-day) variations in diurnal cortisol parameters, in addition to daily positive events as buffers against the associations between daily stressors and cortisol. In the National Study of Daily Experiences, 1,657 adults ages 33–84 (57% female) reported daily experiences during telephone interviews on 8 consecutive evenings. Saliva samples were collected 4 times per day on 4 interview days and assayed for cortisol. Multilevel models were used to estimate associations of daily positive events with cortisol awakening response (CAR), diurnal cortisol slope, and area under the curve (AUC). At the between-person level, people who experienced more frequent positive events exhibited a steeper diurnal cortisol slope, controlling for daily stressors, daily affect, and other covariates. At the within-person level, positive events in the morning (but not prior-night or afternoon/evening events) predicted steeper decline in cortisol across that day; positive events were also marginally associated with lower same-day AUC. Associations were not mediated by daily positive affect, and positive events did not buffer against stressor-related cortisol alterations. These findings indicate that individual differences and day-to-day variations in daily positive events are associated with diurnal cortisol patterns, independent of stressors and affect.
Keywords: daily positive events, daily stress, positive affect, salivary cortisol, HPA axis
1. Introduction
Cortisol—a key hormone produced by the hypothalamic-pituitary-adrenal (HPA) axis—has wide-ranging effects across multiple physiological systems to mobilize the body when faced with a physical or psychological stressor (Sapolsky et al., 1986). Short-term increases (i.e., reactivity) in cortisol are necessary for mounting an adaptive bodily response to acute stressors; however, diminished or excessive cortisol reactivity or the inability to sufficiently terminate the glucocorticoid cascade are thought to reflect maladaptive stress responses (Sapolsky et al., 1986). This is supported by considerable evidence on psychological stress and dysregulated HPA axis activity (Dickerson and Kemeny, 2004; Miller et al., 2007). Chronic stress is associated with alterations in the diurnal pattern of cortisol, as indicated by either an increased or a blunted rise in cortisol in the first hour after waking (cortisol awakening response; CAR) (Chida and Steptoe, 2009), with features of the person and stressor (e.g., time since stressor onset, controllability) accounting for some of these variations (Miller et al., 2007). A robust CAR may be an adaptive response signifying the anticipation of daily challenges, although higher CAR (Adam et al., 2014) as well as smaller CAR (Nederhof et al., 2015) are risk factors for future onset of mental disorders. Blunted CAR and diminished total cortisol output are found in cases of burnout, prolonged stress, and trauma (Chida and Steptoe, 2009; Heim et al., 2000). Furthermore, chronic stress is linked to flatter decline in cortisol across the day (Miller et al., 2007), which in turn is associated with elevated inflammation (DeSantis et al., 2012), shorter telomere length (Tomiyama et al., 2012), and greater mortality risk (Kumari et al., 2011). It is therefore important to identify characteristics of individuals and their environments that are linked to variations in cortisol patterns.
Field studies that combine self-reports of daily experiences with salivary cortisol assessments can provide unique insights into how people navigate stress in real-life settings (Adam and Kumari, 2009; Almeida et al., 2009). Intensive repeated assessments, such as daily diary or experience sampling, allow for the examination of between-person differences (e.g., Do people who encounter more stressors have dysregulated cortisol profiles, compared to less-stressed people?) and within-person variation from one occasion to the next (e.g., Is cortisol altered on days when a stressor occurs, relative to stressor-free days?). Recently, results from nearly 1,700 adults in the National Study of Daily Experiences—from which data for the current study are also drawn—demonstrated that people who experienced more frequent daily stressors exhibited a steeper diurnal cortisol slope. At the within-person level, total cortisol output (as indexed by area under the curve; AUC) was higher on days when stressors occurred compared to stressor-free days (Stawski et al., 2013). Other field studies have also shown between-person and/or within-person associations of daily stress and affect with alterations in cortisol (Adam et al., 2006; Chida and Steptoe, 2009; Jacobs et al., 2007; Smyth et al., 1998). Yet, in contrast to the literature on daily stress and affect, less research has examined whether individual differences and day-to-day variations in protective factors—particularly positive events—play adaptive roles for cortisol.
Daily positive events are favorable or desirable events, such as having a positive social interaction or spending time in nature, that are external to a person’s emotional states and reflect transactions with the environment (Sin et al., 2015; Zautra et al., 2005). People who experience more frequent daily positive events tend to have higher positive affect (Zautra et al., 2005), better health behaviors (Sin et al., 2015, 2017; Tomfohr et al., 2011), lower body mass index (Sin et al., 2015), and lower levels of inflammation (Bajaj et al., 2016; Jain et al., 2007; Sin et al., 2015), compared to those who report fewer daily positive events. The previously observed links between daily positive events and reduced inflammation may be due, at least in part, to normative HPA axis activity and less glucocorticoid resistance (Miller et al., 2002; Rohleder, 2012). Daily positive events—and the positive emotions they produce—might be directly associated with cortisol, in addition to buffering against the influences of stressors on cortisol. Previous empirical and theoretical work have described the role of positive experiences in attenuating cortisol reactivity to acute and chronic stressors (Bostock et al., 2011; Ditzen et al., 2008; Pressman et al., 2009), promoting positive reappraisals and problem-focused coping in the context of stress (Folkman and Moskowitz, 2000), and cultivating psychosocial resources (e.g., positive social relationships, self-efficacy) that can offset future stress (Fredrickson, 1998; Hobfoll, 1989).
Positive affect is a potential key mechanism linking positive events to cortisol patterns. Daily positive events are associated with increases in positive affect when they occur (Charles et al., 2010; Zautra et al., 2005). Positive affect, in turn, has been linked to cortisol, although previous findings have been inconsistent. When comparing between-persons, findings from several studies suggest that people with higher trait-like or aggregated momentary positive affect had smaller CAR than those with lower positive affect (Brummett et al., 2009; Chida and Steptoe, 2009; Miller et al., 2016; Steptoe et al., 2007). Greater positive affect has been associated with steeper diurnal slopes between-persons (Hoyt et al., 2015; Miller et al., 2016), but null results have also been reported for diurnal slopes (Brummett et al., 2009; Slatcher et al., 2015), CAR (Hoyt et al., 2015), AUC (Miller et al., 2016), and cortisol levels averaged across a day (Steptoe et al., 2007). Findings are also equivocal at the within-person level, such that state positive affect has been linked to lower-than-usual momentary salivary cortisol levels (i.e., cortisol reactivity) (Smyth et al., 1998) and lower same-day AUC (Nater et al., 2010; Polk et al., 2005), whereas other studies have found no associations of state positive affect with cortisol reactivity (Jacobs et al., 2007; van Eck et al., 1996) or the diurnal rhythm (Adam et al., 2006). Based on trends discerned from this mixed literature, we tentatively expected daily positive affect to mediate the associations of daily positive events with smaller CAR and steeper diurnal slopes at the between-person level, as well as the relationship between daily positive events with reduced same-day AUC at the within-person level.
To our knowledge, few studies have examined the associations between naturally-occurring positive events and salivary cortisol in daily life. In a sample of 47 participants with major depression and 39 healthy participants, momentary positive events assessed 10 times per day were unrelated to cortisol reactivity across 6 days of observation (Peeters et al., 2003). Because cortisol was only examined at the moment-level in that study, it remains unclear whether positive events—directly or in interaction with stressors—are associated with the diurnal rhythm of cortisol (i.e., CAR, diurnal cortisol slope) or total cortisol output. In another study, AUC was reduced on days when couples had greater exchange of physical affection with one another, relative to days with little or no physical affection; positive couple interactions also reduced AUC levels associated with chronic work stressors (Ditzen et al., 2008). Thus, there is initial evidence suggesting that positive events may be beneficial under conditions of heightened chronic stress, but none of the research to date has examined whether daily positive events buffer against the influence of same-day stressors on diurnal cortisol rhythms.
The overarching goals of the current study were to investigate the potential main effects and stress-buffering effects of daily positive events with diurnal salivary cortisol. In a national sample of 1,657 midlife and older adults, participants reported daily experiences during telephone interviews on eight consecutive evenings and completed a saliva collection protocol on four of those days (Almeida et al., 2009). First, we evaluated daily positive events as predictors of between-person differences and within-person (day-to-day) variations in diurnal cortisol patterns. Second, daily positive affect was tested as a potential mediator of the links between daily positive events and cortisol. Lastly, we examined whether daily positive events mitigated the associations of daily stressors with cortisol.
2. Methods
2.1. Sample and procedure
The Midlife in the United States Study (MIDUS) is a national survey designed to examine the roles of behavioral, psychological, and social factors in aging and health. We used data from the second wave of MIDUS because cortisol and daily positive events were not measured during the first wave. The parent MIDUS study comprised of 4,963 English-speaking adults ages 33 to 86 across the US and an additional 592 African Americans from Milwaukee.
A random subsample of 2,022 respondents enrolled in a daily diary substudy called the National Study of Daily Experiences, which consisted of brief semi-structured telephone interviews on eight consecutive evenings (Almeida et al., 2002). Starting on Day 2 of the study, participants collected saliva samples four times per day for four consecutive days, totaling 16 saliva samples per participant (Almeida et al., 2009). The saliva samples were collected upon waking, 30-min post-waking, before lunch, and before bed.
Of the 2,022 daily diary participants, 1,735 (86%) provided valid cortisol samples (mean = 15.51 samples per person, SD = 1.35). A total of 26,902 cortisol samples were obtained across 6,789 days. We excluded cortisol samples where the cortisol level was >60 nmol/L (1.46%), the time stamp was missing (1.28%), or the lunch sample was ≥10 nmol/L more than the 30-min post-waking sample (suggesting that participants ate before collecting their saliva, 1.82%). We further excluded cortisol samples from days when participants woke before 4 AM (3.14%) or after 12 PM (0.67%), or days when <15 or >60 min elapsed between the first two samples (indicators of noncompliance that influence assessment of the awakening response, 9.74%). An additional 28 cortisol samples (0.10%) were excluded from analyses due to missing participant data on daily positive events, daily stressors, or covariates. This resulted in a final analytic sample of 1,657 participants with 5,602 days of cortisol collection and 21,557 salivary cortisol samples. Findings were unchanged when we ran a sensitivity analysis restricted to 1,508 participants who each provided at least 50% (eight) useable cortisol samples.
2.2. Daily positive events and stressors
During nightly telephone interviews, participants were asked whether any of these five positive events had occurred in the past 24 hours: (a) positive interpersonal interaction, (b) positive experience at work, school, or at a volunteer position, (c) positive experience at home, (d) network positive event (i.e., positive event experienced by a close friend or relative), and (e) any other positive event (Charles et al., 2010; Sin et al., 2015). For example, positive interpersonal interactions were assessed with the question, “Did you have an interaction with someone that most people would consider particularly positive (for example, sharing a good laugh with someone, or having a good conversation) since we spoke yesterday?” Participants were also asked to report what time the events happened. A day was considered to be a “positive event day” if the participant endorsed at least one positive event. Positive events were entered as a dichotomous variable (1 = positive event day, 0 = no positive event that day) when examined as a within-person predictor.1 As a between-person predictor, the frequency of positive events was computed as the percent of study days during which at least one positive event occurred (Seltzer et al., 2009; Sin et al., 2015).
Daily stressors were assessed using the Daily Inventory of Stressful Events (Almeida et al., 2002). Participants reported whether the following stressors had occurred in the past 24 hours: (a) argument, (b) avoided an argument, (c) stressor at work or school, (d) stressor at home, (e) discrimination, (f) network stressor (i.e., stressful event that happened to a close friend or family member), and (g) any other stressor. Examples of items included the following: “Did you have an argument or disagreement with anyone since this time yesterday?” and “Since this time yesterday, did anything happen at home (other than what you already mentioned) that most people would consider stressful?” A dichotomous variable was created to indicate whether at least one stressor had occurred that day (i.e., stressor day) or if no stressors had occurred (i.e., stressor-free day). At the between-person level, the frequency of daily stressors was computed as the percent of study days during which at least one stressor occurred (Sin et al., 2016).
2.3. Salivary cortisol
Participants received a Home Saliva Collection Kit prior to their initial telephone interview. The kit contained a detailed instruction sheet and 16 numbered and color-coded salivette collection devices (Sarstedt, Nümbrecht, Germany). Interviewers reviewed the collection procedures with the participants during the first interview and answered any questions. Participants were instructed to collect four saliva samples per day on Days 2–5: immediately upon waking, 30 min after waking, before lunch, and before bed. Participants recorded the exact time of each saliva sample on a form sent with the collection kit, in addition to reporting the sampling times to study staff during the nightly telephone interviews. The sampling times reported on the forms and in telephone interviews were correlated above 0.90 for each of the four sampling occasions.
After completing the saliva collection protocol, participants shipped the salivettes to the MIDUS Biological Core at the University of Wisconsin, Madison, where they were stored at −60 °C. For analysis, the salivettes were thawed and centrifuged at 3000 rpm for five minutes. Cortisol concentrations were determined with commercially-available luminescence immunoassay (IBL, Hamburg, Germany), with intra-assay and inter-assay coefficients of variation below 5% (Dressendörfer et al., 1992). Additional details on salivary cortisol assessment in the National Study of Daily Experiences were provided elsewhere (Almeida et al., 2009; Karlamangla et al., 2013; Stawski et al., 2013).
Based on prior research linking daily stressors or emotions to diurnal cortisol (Adam et al., 2006; Polk et al., 2005; Stawski et al., 2013), we were primarily interested in three indices of diurnal cortisol: (1) CAR as indexed by the increase in cortisol from waking to 30 min later; (2) linear slope from waking to bedtime (as well as the quadratic slope, indicating the rate of deceleration); and (3) total cortisol output as indexed by AUC with respect to ground. Cortisol was natural log transformed prior to analyses to correct for positive skew in the distribution.
2.4. Covariates
Analyses included covariates that have previously been identified as potential confounding factors with regard to psychosocial well-being and cortisol. These included demographics (age, gender, race, and education), self-rated health, depression, and optimism that were obtained by a telephone survey as part of the parent MIDUS Study. Self-rated physical health was scored as 4 = excellent, 3 = very good, 2 = good, 1 = fair, and 0 = poor. Major depression in the past year was determined by the presence of depressed mood or anhedonia (loss of interest) most of the day, nearly every day, and at least four other associated symptoms (e.g., fatigue, appetite or sleep disturbances, trouble concentrating, feeling worthless, suicidal thoughts) during a 2-week period (Wang et al., 2000). Optimism was assessed with one item on a 0–3 scale, whereby participants rated how much the word “optimistic” (or “hopeful about how things will turn out”) described them. Response choices were a lot, somewhat, a little, or not at all; higher scores referred to greater optimism. The results were unchanged when, instead of using one item for optimism, we controlled for the 6-item Life Orientation Test-Revised (Scheier et al., 1994) obtained in a subset of 1,613 participants. A dummy-coded variable indicated the use of medications known to influence cortisol: steroid inhalers, steroid medications, medications containing cortisone, oral contraceptives, other hormonal medications, antidepressants, and/or anti-anxiety medications. A variable for smoking was created by averaging the daily number of cigarettes smoked across the interview days.
Furthermore, analyses controlled for wake time and daily affect at both the within-person and between-person levels (i.e., averaged across days). Daily positive and negative affect were assessed using measures developed for MIDUS (Kessler et al., 2002; Mroczek and Kolarz, 1998). On a 5-point scale ranging from “none of the time” to “all of the time,” participants rated how frequently they had experienced 13 positive emotions (e.g., cheerful, calm and peaceful, enthusiastic, attentive) and 14 negative emotions (e.g., nervous, upset, frustrated, hopeless). Daily positive and negative affect were calculated by averaging ratings for the items within each subscale. Cronbach’s alpha ranged from 0.93 to 0.95 for daily positive affect and from 0.84 to 0.88 for daily negative affect across the eight study days.
2.5. Data analysis
Multilevel modeling (Raudenbush and Bryk, 2002) was used to examine the associations of daily positive events with diurnal cortisol parameters. Multilevel modeling accounts for the non-independence of observations and permits the evaluation of within- and between-person predictors of diurnal cortisol parameters (Adam et al., 2006; Stawski et al., 2013). The within-person analyses linked daily experiences (i.e., positive events, stressors, and affect) to same-day cortisol on the four days during which salivary cortisol was collected. For the between-person analyses, however, daily experiences were averaged across all eight interview days to better capture a person’s typical exposure to daily events and their trait-like daily affect. Within-person variables were person-mean centered, such that the person’s mean score across days was subtracted from their daily score (e.g., Positive Event Daydi - Positive Event Day.i). Between-person variables were grand-mean centered. This form of centering allowed the within-person effects to be interpreted as deviations from a person’s own mean and the between-person effects to be interpreted as a person’s deviation from the sample mean (Hoffman and Stawski, 2009).
To estimate CAR and the diurnal cortisol slope, we used a 3-level model to account for the nesting of cortisol samples within days and within persons. Time since waking was used as the time metric, such that the intercept was set to the cortisol level at waking. Level 1 variables were those that changed with each cortisol sample at the moment-level (e.g., time since waking), Level 2 within-person variables were those that varied from day-to-day (e.g., positive event day, stressor day, wake time), and Level 3 included between-person variables that were relatively stable (e.g., demographics, optimism, depression) or that were aggregated across all interview days (e.g., frequency of positive events and stressors). Because cortisol was natural log transformed, the intercept (i.e., cortisol level at waking) can be interpreted in the original units of nmol/L after back-transformation. The parameter estimates for the slopes are interpreted as percent change in cortisol after applying the transformation B%change = exp(Bestimate)−1 (Adam et al., 2006). At Level 1, cortisol at time t on day d for person i was modeled as:
The intercept (π0di) reflected log-transformed cortisol level at waking. CAR was coded as a dummy variable, in which the 30-min post-waking sample was assigned a value of 1 and the other samples were set to 0. Thus, the coefficient for CAR (π1di) reflected the percent change in cortisol between the waking and 30-min post-waking cortisol samples. π2di and π3di reflected the linear and quadratic percent changes in cortisol, respectively, per hour since waking. etdi was the residual term.
Intraindividual daily variables (e.g., person-mean centered positive event day) were included at Level 2 as predictors of the Level 1 intercept and slopes:
The intercepts at Level 2 represented individual i’s average log-transformed cortisol level at waking (β00i), average percent change in cortisol for CAR (β10i), average linear percent rate of cortisol decline (β20i), and average percent rate of deceleration in cortisol (β30i). The coefficients β01i, β11i, β21i, and β31i reflected changes in the waking cortisol sample, CAR, linear slope, and rate of deceleration associated with the time-varying effect of a positive event. A random effect (r0di) allowed the intercept to vary within-persons across days. Within-person covariates included wake time, stressor day, and daily positive and negative affect.
Between-person variables were entered at Level 3 as predictors of the Level 2 intercepts:
The coefficients γ000, γ100, γ200, and γ300 represented the sample averages for log-transformed cortisol level at waking and percent changes in cortisol for CAR and the linear and quadratic slopes. γ001, γ101, γ201, and γ301 reflected the between-person associations of positive event frequency with the average log-transformed cortisol level at wake-up, and percent changes in cortisol for CAR and the linear and quadratic slopes. u00i and u20i were random effects that allowed the intercept and linear slope to vary across persons. Level 3 also included person-means (aggregated across days) for wake time, stressor frequency, number of cigarettes smoked, and daily affect. Covariates were also entered for age, gender, education level (high school or less, some college, or Bachelor’s degree or higher), self-rated physical health (0–4 scale), medication use (yes/no), depression (yes/no), and optimism (0–3 scale).
AUC with respect to ground was computed from the log-transformed cortisol data using the formula described by Pruessner, Kirschbaum, Meinlschmid, and Hellhammer (2003). AUC was then analyzed in a 2-level model that accounted for the nesting of days within persons. Level 1 contained within-person variables and Level 2 contained between-person variables:
At Level 1, AUC on day d for person i was a function of an intercept reflecting the person’s average AUC (π0i), a slope reflecting the change in AUC associated with the occurrence of a positive event (π1i), and a within-person residual representing the difference between the person’s actual and predicted AUC that day (edi). At Level 2, the Level 1 intercept was a function of the sample average AUC (β00), the between-person association of positive event frequency with AUC (β01), and a random effect allowing the AUC intercept to vary across participants (r0i). Also, the Level 1 slope (π1i) was determined by the sample average slope (β10).
We included Positive Events x Stressors interaction terms at both the between- and within-person levels of analysis to test whether daily positive events mitigated the association between daily stressors and cortisol. In addition, to better understand the direction of effects, we ran lagged analyses and tested the timing of positive events (i.e., events occurring in the prior night, in the morning, or in the afternoon/evening) in relation to cortisol patterns at the within-person level.
If daily positive events and positive affect predicted cortisol at the within-person level, we planned to run lower-level “1-1-1” or “2-1-1” mediation models (Kenny et al., 2003) using the simultaneous modeling approach described by Bauer and colleagues (Bauer et al., 2006). If daily positive events and positive affect were associated with cortisol parameters at the between-person level, then an upper-level “2-2-1” mediation analysis would be conducted using standard procedures (Baron and Kenny, 1986). In particular, the following conditions must hold to establish positive affect as a mediator: (a) daily positive events will be associated with daily positive affect; (b) daily positive affect will be associated with the cortisol parameter of interest; and (c) the relationship between daily positive events and cortisol will be reduced or eliminated after adding daily positive affect to the model.
Models were estimated using full information maximum likelihood estimation in SAS 9.4 PROC MIXED, which makes use of all available data in the estimation of parameters and can flexibly handle missing data (Raudenbush and Bryk, 2002). For the 3-level models, even when a person was missing time-varying data for some occasions, his or her complete cases for other occasions were still included in analyses. However, calculation of AUC required no missing data on the four cortisol samples and saliva collection times across the day; thus, the sample size for AUC analyses was reduced from 1,657 to 1,639 participants (99%) and from 5,602 days of observation to 4,910 days (88%). Intraclass correlation coefficients (ICC; between-person level variance/total variance) were estimated using multilevel linear models for continuous variables and multilevel logistic regression for dichotomous variables (Snijders and Bosker, 2011).
3. Results
3.1. Descriptives
The sample of 1,657 participants had a mean age of 56 years (range: 33-84 years old), 43% were male, and 40% had a Bachelor’s degree or higher (Table 1). On average, participants reported positive events on 71% of interview days and stressors on 40% of interview days. The mean daily positive affect score of 2.74 indicated that participants experienced positive affect nearly “most of the time,” whereas the negative affect score of 0.19 corresponded to experiencing negative affect close to “none of the time.” Greater frequency of daily positive events was associated with older age, female gender, higher educational attainment, greater daily positive affect and dispositional optimism, better self-rated health, use of cortisol-altering medications, and less smoking. The frequency of daily positive events was unrelated to daily negative affect at the between-person level. People who reported more positive events also tended to experience more daily stressors (r = 0.27). Much of the variance in daily positive events and stressors were attributable to day-to-day differences within individuals. The ICCs indicated that between-person differences accounted for only 30% of the variance in daily positive events and 19% of the variance in daily stressors. By contrast, 76% of the variance in daily positive affect and 50% of the variance in daily negative affect were attributable to between-person differences.
Table 1.
Characteristics of 1,657 participants
| Correlations with Daily Events
|
|||
|---|---|---|---|
| Participant Characteristics | Mean (SD) or N (%) | Daily Positive Events | Daily Stressors |
| Demographics | |||
| Age, years | 56.44 (12.11) | 0.08** | −0.24*** |
| Male | 718 (43%) | −0.07** | −0.10*** |
| Educational attainment | 0.23*** | 0.23*** | |
| Up to high school diploma or GED | 495 (30%) | ||
| Some college or associate degree | 499 (30%) | ||
| Bachelor’s degree or higher | 660 (40%) | ||
| Daily experiencesa | |||
| Percent of days with ≥1 positive event | 71% (27%) | ------- | 0.27*** |
| Percent of days with ≥1 stressor | 40% (26%) | 0.27*** | ------- |
| Daily positive affect (range: 0–4) | 2.74 (0.70) | 0.12*** | −0.32*** |
| Daily negative affect (range: 0–4) | 0.19 (0.24) | −0.03 | 0.43*** |
| Physical health and behaviors | |||
| Self-rated physical health (range: 0–4) | 2.60 (1.00) | 0.18*** | 0.01 |
| Use of cortisol-altering medications | 620 (37%) | 0.06* | 0.11*** |
| Mean cigarettes smoked per day | 1.78 (5.39) | −0.06* | 0.01 |
| Psychological characteristics | |||
| Depression | 167 (10%) | −0.05* | 0.13*** |
| Optimism (range: 0–3) | 2.41 (0.69) | 0.16*** | −0.01 |
Daily experiences (i.e., daily affect and the frequency of daily positive events and stressors) were person-means averaged across up to eight interview days.
p < 0.001,
p < 0.01,
p < 0.05
Descriptive statistics for saliva collection times and salivary cortisol are shown in Table 2. There was substantial variability between-persons and within-persons across days in cortisol levels, such that the ICCs were 0.42 for the waking sample, 0.47 for the 30-min post-waking sample, 0.36 for the pre-lunch sample, and 0.32 for the bedtime sample. The mean AUC was 25.66 log nmol/L (SD = 8.13), and 56% of the variance in AUC was due to between-person differences.
Table 2.
Descriptive statistics for salivary cortisol (N = 1,657 participants, 5,602 days, and 21,557 cortisol samples)
| Collection times | Mean time (SD) | |
|---|---|---|
| Waking | 06:45 AM (69 min) | |
| 30-min post-waking | 07:19 AM (70 min) | |
| Before lunch | 12:42 PM (67 min) | |
| Before bed | 10:30 PM (72 min) | |
|
| ||
| Cortisol levels (nmol/L)a | Mean (SD) | |
|
| ||
| Waking | 15.03 (6.94) | |
| 30-min post-waking | 21.10 (9.19) | |
| Before lunch | 6.98 (3.79) | |
| Before bed | 3.30 (3.49) | |
|
| ||
| Total cortisol output (log nmol/L) | Mean (SD) | |
|
| ||
| Area under the curve with respect to ground (AUC) | 25.66 (8.13) | |
|
| ||
| Diurnal cortisol rhythm (log nmol/L)b | Estimate (SE) | Interpretation |
|
| ||
| Intercept (cortisol level at waking) | 2.530 (0.014) | 12.55 nmol/L |
| Cortisol awakening response (CAR) | 0.447 (0.010) | +56% from waking |
| Time since waking (linear slope), per hour | −0.149 (0.003) | −14% per hour |
| Time since waking2 (quadratic slope), per hour | 0.002 (0.0002) | +0.20% per hour |
Note. Standard deviations were calculated between persons.
The non-transformed mean cortisol levels are shown here. Cortisol was natural log transformed for subsequent analyses. AUC was computed using log-transformed cortisol from 1,639 participants.
The diurnal rhythm of cortisol was modeled in an unconditional 3-level model to obtain estimates for CAR, Time Since Waking, and Time Since Waking2. The intercept can be interpreted in the original units after back-transformation, whereas the parameter estimates for the slopes are interpreted as percent change in cortisol after applying the transformation B%change = exp(Bestimate)−1 (Adam et al., 2006).
An unconditional multilevel model was run to obtain estimates for the expected diurnal pattern (Table 2). Cortisol was approximately 12.55 nmol/L at waking, increased 56% in the 30 min after waking, declined at a rate of 14% per hour after waking, and decelerated 0.20% per hour thereafter. We had good reliability (ρ = 0.82) for estimating between-person differences in diurnal slopes (Hruschka et al., 2005).
3.2. Daily positive events predicting diurnal cortisol rhythm
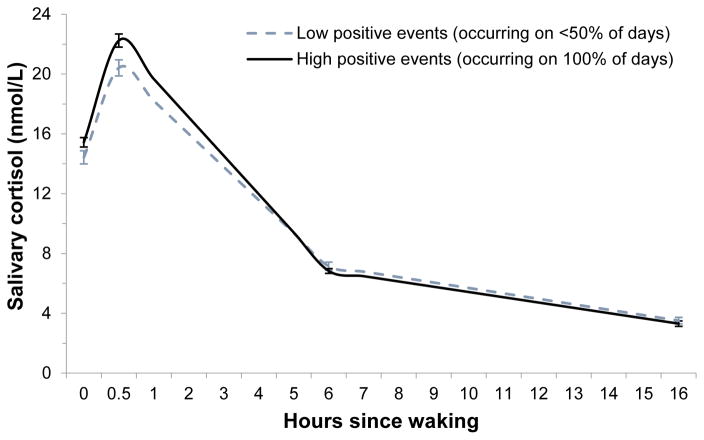
Figure 1 illustrates the observed cortisol levels for people with lower versus higher frequency of daily positive events. For the primary analyses, we first ran 3-level models to examine the between- and within-person associations of daily positive events with CAR and the diurnal cortisol slope. Daily positive events were unrelated to cortisol parameters at the within-person level, controlling for wake time (Model 1 in Table 3). However, daily positive events were significantly associated with cortisol parameters at the between-person level. In particular, people with 1-SD higher positive event frequency had 1.19 nmol/L higher cortisol level at waking (e0.175 = 1.19 nmol/L, p < 0.001), 3.73% steeper linear decline in cortisol across the day (e−0.038−1 = −3.73%, p < 0.001), and 0.20% faster rate of deceleration (e0.002−1 = 0.20%, p < 0.01), although positive event frequency was not related to CAR. The between-person associations of daily positive events with the linear and quadratic slopes remained significant after adjusting for daily stressors and affect, age, gender, education, self-rated health, medication use, smoking, depression, and dispositional optimism (Models 2 and 3 in Table 3).
Figure 1. Mean cortisol levels across the day by daily positive events.
For illustrative purposes, the figure depicts mean observed levels of cortisol and standard errors for people in the top quartile of positive events (i.e., positive events reported everyday) compared to those in the bottom quartile (i.e., positive events reported on less than 50% of days).
Table 3.
Three-level models of daily positive events and diurnal cortisol rhythm (log nmol/L) in 1,657 participants
| Cortisol level at waking, π0di | Cortisol awakening response, π1di | Time since waking, π2di | Time since waking2, π3di | |
|---|---|---|---|---|
|
|
|
|
|
|
| Fixed Effect | Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) |
| Model 1: Minimally-adjusted model | ||||
| Intercept | 2.532 (0.014)*** | 0.448 (0.010)*** | −0.149 | 0.002 |
| Wake time (WP) | 0.013 (0.011) | −0.075 (0.015)*** | −0.028 (0.003)*** | 0.001 (0.0002)*** |
| Wake time (BP) | −0.012 (0.012) | −0.007 (0.009) | − 0.012 (0.004)*** | 0.001 (0.0002) *** |
| Daily positive events (WP) | 0.002 (0.023) | 0.012 (0.029) | − 0.006 (0.007) (0.003)*** | 0.0002 (0.0004) (0.0001)*** |
| Daily positive events (BP) | 0.175 (0.051)*** | 0.016 (0.038) | −0.038 (0.011)*** | 0.002 (0.001)** |
| Model 2: Model including daily stressors and between-person interaction | ||||
| Intercept | 2.536 (0.014)*** | 0.439 (0.011)*** | −0.150 (0.003)*** | 0.002 (0.0002)*** |
| Wake time (WP) | 0.014 (0.011) | −0.074 (0.015)*** | −0.027 (0.004)*** | 0.001 (0.0002)*** |
| Wake time (BP) | −0.013 (0.012) | −0.007 (0.009) | −0.011 (0.003)*** | 0.001 (0.0001)*** |
| Daily positive events (WP) | 0.003 (0.023) | 0.011 (0.029) | −0.006 (0.007) | 0.0002 (0.0004) |
| Daily positive events (BP) | 0.156 (0.055)** | 0.037 (0.041) | −0.027 (0.012)* | 0.001 (0.001)* |
| Daily stressors (WP) | 0.006 (0.019) | 0.032 (0.025) | 0.017 (0.006)** | −0.001 (0.0004)** |
| Daily stressors (BP) | 0.032 (0.057) | 0.003 (0.043) | −0.026 (0.012)* | 0.001 (0.001) |
| Positive events x Stressors (BP) | −0.219 (0.214) | 0.432 (0.162)** | 0.084 (0.046)† | −0.004 (0.002)† |
| Model 3: Fully-adjusted model | ||||
| Intercept | 2.468 (0.031)*** | 0.503 (0.024)*** | −0.155 (0.007)*** | 0.003 (0.0004)*** |
| Wake time (WP) | 0.015 (0.011) | −0.074 (0.015)*** | −0.028 (0.004)*** | 0.001 (0.0002)*** |
| Wake time (BP) | −0.003 (0.012) | −0.010 (0.009) | −0.011 (0.003)*** | 0.001 (0.0002)*** |
| Daily positive events (WP) | 0.005 (0.023) | 0.011 (0.029) | −0.007 (0.007) | 0.0002 (0.0004) |
| Daily positive events (BP) | 0.066 (0.058) | −0.002 (0.044) | −0.025 (0.013)* | 0.002 (0.001)** |
| Daily stressors (WP) | 0.003 (0.020) | 0.031 (0.026) | 0.015 (0.007)* | −0.001 (0.0004)* |
| Daily stressors (BP) | 0.046 (0.066) | 0.073 (0.051) | −0.003 (0.014) | −0.0003 (0.001) |
| Positive events x Stressors (BP) | −0.203 (0.211) | 0.352 (0.164)* | 0.073 (0.046) | −0.004 (0.003) |
| Daily positive affect (WP) | 0.001 (0.026) | −0.010 (0.033) | 0.0004 (0.008) | 0.0001 (0.0005) |
| Daily positive affect (BP) | −0.065 (0.025)** | 0.014 (0.019) | 0.012 (0.005)* | −0.0005 (0.0003) |
| Daily negative affect (WP) | 0.026 (0.048) | −0.007 (0.060) | 0.010 (0.015) | −0.0005 (0.001) |
| Daily negative affect (BP) | 0.015 (0.076) | −0.071 (0.060) | 0.013 (0.017) | 0.0005 (0.001) |
| Age (per 10 years) | 0.040 (0.012)*** | 0.025 (0.009)** | 0.014 (0.003)*** | −0.0004 (0.001)** |
| Gender (1 = Male) | 0.104 (0.028)*** | −0.091 (0.022)*** | 0.014 (0.006)* | − 0.001 (0.0003)** |
| Education (ref: High school) | ----- | ----- | ----- | ----- |
| Some college | 0.056 (0.036) | −0.059 (0.028)* | −0.004 (0.008) | 0.0002 (0.0004) |
| Bachelor’s degree or higher | 0.100 (0.036)** | −0.035 (0.028) | −0.014 (0.008)† | 0.0004 (0.0004) |
| Self−rated physical health | 0.092 (0.015)*** | 0.012 (0.012) | −0.015 (0.003)*** | 0.0004 (0.0002)* |
| Medication use (1 = yes) | −0.069 (0.029)* | 0.015 (0.022) | 0.006 (0.006) | −0.0001 (0.0003) |
| Mean daily cigarettes | −0.004 (0.003) | 0.004 (0.002)* | 0.003 (0.001)*** | −0.0001 (0.000)* |
| Depression (1 = yes) | −0.096 (0.049)† | 0.028 (0.038) | 0.026 (0.011)* | −0.002 (0.001)** |
| Optimism | 0.009 (0.021) | 0.002 (0.016) | 0.010 (0.005)* | −0.001 (0.002)** |
Note. WP: within-person, BP: between-person. Momentary (Level 1) predictors were uncentered, within-person (Level 2) predictors were centered at the person-mean, and between-person (Level 3) predictors were centered at the grand mean. Cortisol awakening response (CAR) was estimated from a dummy-coded variable (1 = 30-minutes post-waking sample, 0 = all other cortisol samples). Time Since Waking and Time Since Waking2 referred to linear and quadratic changes, respectively, in cortisol per hour since waking. Higher values on cortisol parameters indicated higher cortisol at wake-up, steeper CAR, flatter slope, or faster rate of deceleration. Within-person and cross-level interactions for Daily Positive Events x Stressors were non-significant and therefore not included in the models presented. Level 3 random effects for intercept and Time Since Waking were significant at p < 0.001.
p < 0.001,
p < 0.01,
p < 0.05,
p < 0.10
Daily stressors were linked to flatter cortisol slopes within-persons and steeper slopes between-persons (Model 2 in Table 3). We then examined whether daily positive events might buffer against the links between daily stressors and cortisol. Controlling for wake time, daily positive events interacted with daily stressors at the between-person level to predict CAR (Interaction: Est. = 0.432, SE = 0.162, p < 0.01). Individuals who encountered both positive events and stressors frequently tended to have a steeper CAR, whereas those who experienced frequent stressors but fewer positive events had a flatter CAR (simple slope for positive events effect among people with more stressors: Est. = 0.150, SE = 0.066, p = 0.02). After adjusting for covariates (Model 3 in Table 3), this simple slope was no longer significant although the interaction term remained predictive of CAR. A trend emerges in the fully-adjusted model in which higher positive event frequency was marginally associated with smaller CAR in individuals who encountered fewer stressors (simple slope for positive events effect among people with fewer stressors: Est. = −0.093, SE = 0.052, p = 0.07). There were no other significant interactions between daily positive events and daily stressors for predicting the diurnal cortisol rhythm.
3.3. Daily positive events predicting log AUC
Next, daily positive events were tested as between- and within-person predictors of log AUC in 2-level models. As shown in Table 4, daily positive events were marginally associated with lower same-day AUC (within-person effect in minimally-adjusted model: Est. = -0.472, SE = 0.262, p = 0.07). This trend persisted after adjusting for all covariates (Est. = −0.504, SE = 0.263, p = 0.06). Consistent with a previous study using this cohort (Stawski et al., 2013), there was also a within-person effect of daily stressors on elevated AUC (Table 4). At the between-person level, however, individual differences in positive events and stressors were not associated with AUC. There were no significant interactions between daily stressors and positive events on AUC at either the between- or within-person levels.
Table 4.
Two-level models of daily positive events and AUC (log nmol/L) in 1,639 participants
| Fixed Effect | Estimate (SE) |
|---|---|
| Model 1: Minimally-adjusted model | |
| Intercept | 25.790 (0.194)*** |
| Wake time (WP) | −2.131 (0.130)*** |
| Wake time (BP) | −1.277 (0.169)*** |
| Daily positive events (WP) | −0.472 (0.262)† |
| Daily positive events (BP) | 0.926 (0.716) |
| Model 2: Model including stressors | |
| Intercept | 25.782 (0.194)*** |
| Wake time (WP) | −2.106 (0.130)*** |
| Wake time (BP) | −1.254 (0.169)*** |
| Daily positive events (WP) | −0.469 (0.262)† |
| Daily positive events (BP) | 1.228 (0.744)† |
| Daily stressors (WP) | 0.632 (0.223)** |
| Daily stressors (BP) | −1.135 (0.783) |
| Model 3: Fully-adjusted model | |
| Intercept | 25.340 (0.425)*** |
| Wake time (WP) | −2.089 (0.130)*** |
| Wake time (BP) | −1.121 (0.166)*** |
| Daily positive events (WP) | −0.504 (0.263)† |
| Daily positive events (BP) | 0.162 (0.765) |
| Daily stressors (WP) | 0.591 (0.237)* |
| Daily stressors (BP) | 0.741 (0.883) |
| Daily positive affect (WP) | 0.316 (0.303) |
| Daily positive affect (BP) | −0.266 (0.343) |
| Daily negative affect (WP) | 0.424 (0.561) |
| Daily negative affect (BP) | 0.872 (1.049) |
| Age (per 10 years) | 1.578 (0.166)*** |
| Gender (1 = Male) | 1.576 (0.391)*** |
| Education (ref: High school) | |
| Some college | 0.157 (0.496) |
| Bachelor’s degree or higher | 0.267 (0.497) |
| Self−rated physical health | 0.449 (0.209)* |
| Medication use (1 = yes) | −0.919 (0.395)* |
| Mean daily cigarettes | 0.191 (0.036)*** |
| Depression (1 = yes) | −0.689 (0.681) |
| Optimism | 0.317 (0.288) |
Note. WP: within-person, BP: between-person. Within-person (Level 1) predictors were centered at the person-mean, and between-person (Level 2) predictors were centered at the grand mean. AUC with respect to ground was calculated using natural log transformed cortisol values. Daily Positive Events x Stressors interactions were non-significant and therefore were not included in the models presented. Level 2 random effects for intercept were significant in all models at p < 0.001.
p < 0.001,
p < 0.01,
p < 0.05,
p < 0.10
To ensure that results were not driven by the morning rise in cortisol, we ran a sensitivity analysis in which AUC was calculated without the morning peak (30-min post-waking) cortisol sample. The results were similar to those obtained before: daily positive events predicted lower same-day AUC (fully-adjusted: Est = −0.549, SE = 0.263, p = 0.04), daily stressors predicted higher same-day AUC (fully-adjusted: Est = 0.549, SE = 0.237, p = 0.02), and there were no between-person associations of daily positive events or stressors with AUC.
3.4. Do positive events precede changes in cortisol?
We conducted two types of within-person analyses to better understand the direction of associations. First, we ran lagged analyses in which positive events reported on yesterday evening’s interview were tested as predictors of today’s cortisol. There were no significant within-person effects in these lagged analyses.
Second, we examined the timing of positive events reported on today’s interview. Positive events were dummy-coded into three variables based on the time of occurrence: (a) last night after yesterday evening’s interview, (b) this morning before 12 PM, or (c) this afternoon/evening after 12 PM. Because participants could report multiple positive events, it was possible to have different events that were coded as happening at various times on the same day. Positive events in the morning (reported on 1,664 days, or 30% of all 5,602 days) predicted a steeper decline in cortisol that day (fully-adjusted: Est. = −0.014, SE = 0.007, p = 0.04) and faster rate of deceleration (fully-adjusted: Est. = 0.001, SE = 0.0004, p < 0.05), but was not associated with same-day cortisol level at waking, CAR, or AUC. Positive events that happened on the prior night (N = 1,348 days; 24%) or in the afternoon (N = 2,161 days; 39%) were not related to any cortisol parameters within-persons. Overall, these results suggest that positive events in the morning were predictive of cortisol decline across the day, although positive events did not “carry over” to predict next-day cortisol. By contrast, there was no evidence that cortisol earlier in the day (e.g., cortisol level at waking, CAR) was associated with subsequent positive events.
3.5. Daily positive affect as a potential mediator
Daily positive affect was not related to any cortisol measures at either the within- or between-person levels before covariate adjustment. In addition, the associations between daily positive events and cortisol were relatively unchanged before and after inclusion of daily positive affect in the models. Thus, we concluded that daily positive affect was not a mediator.
4. Discussion
The current study examined the within- and between-person associations of daily positive events with diurnal cortisol, as well as their potential stress-buffering effects. Using daily diary data from a national sample of 1,657 midlife and older adults, we found that people who experienced more frequent daily positive events tended to have a cortisol profile characterized by a steeper daily decline in cortisol. At the within-person level, positive events in the morning—but not prior-day or afternoon/evening events—predicted a steeper decline in cortisol across that day. Positive events were also marginally associated with lower same-day AUC. These associations were independent of daily stressors and were not mediated by daily positive affect. There was limited evidence for stress-buffering effects. Overall, these findings support the protective role of daily positive events for steeper diurnal cortisol slopes (between- and within-persons) and lower AUC on days when these events occur.
Counter to our predictions, positive events did not buffer the within-person associations of daily stressors on same-day flatter cortisol slopes and higher AUC. There was only modest evidence of stress-buffering effects between-persons and before covariate adjustment, such that people who frequently encountered both positive events and stressors tended to have a steeper CAR compared to those who encountered frequent stressors but few positive events. High exposure to daily stressors and few accompanying positive experiences might reflect chronic strain, in line with previous research showing blunted CAR among people with prolonged stress, burnout, and post-traumatic stress disorder (Chida and Steptoe, 2009; Fries et al., 2009). Unexpectedly, after adjusting for covariates, a simple slope effect showed that positive event frequency was marginally associated with smaller CAR among people with fewer stressors. This is inconsistent with the stress-buffering hypothesis, yet some previous research has linked positive affect with smaller CAR (Chida and Steptoe, 2009). The stress-buffering effects of positive experiences might be more evident among people with elevated psychological distress or those undergoing life difficulties (Cohen and Hoberman, 1983; Nezlek and Plesko, 2003; Ong et al., 2004; Takano et al., 2013), but was perhaps less relevant for counteracting stressors in our healthy community-based sample.
We did not find support for our hypothesis that daily positive affect would mediate the associations between daily positive events and diurnal cortisol parameters. Given the inconsistent literature on positive affect and cortisol, it was perhaps unsurprising that positive affect was not related to cortisol measures in an unadjusted model (and was associated with flatter diurnal slopes after covariate adjustment, which may have been driven by lower cortisol level at wake-up). A limitation of our positive affect measure, however, was the inability to group items into subscales (e.g., low versus high arousal) which may be differentially related to cortisol patterns (Hoyt et al., 2015). Besides average levels of affect, dynamic aspects of positive affect—such as affective reactivity to and recovery from positive events—might be important in explaining the association between daily positive events and cortisol (Ong and Ram, 2017).
A number of psychological and social factors may serve as pathways linking daily positive events to diurnal cortisol rhythms. At the between-person level, positive experiences can help cultivate resources such as social support, skills, and a sense of mastery (Fredrickson, 1998; Hobfoll, 1989; Pressman et al., 2009). For example, positive social interactions might influence same-day alterations in cortisol (e.g., steeper slope and lower AUC), as well as accrue over time to build social relationships that can be drawn upon during times of stress. In addition to psychosocial resources, stable characteristics of individuals or their environments may be responsible for both exposure to daily positive events and patterns of diurnal salivary cortisol. In our study and others (Charles et al., 2010; Gunaydin et al., 2016; Zautra et al., 2005), people who experienced more daily positive events also reported more daily stressors. The correlation between positive events and stressors may reflect more roles and social engagement, which can have favorable influences on health. Psychological, social, and behavioral processes surrounding positive events may serve as mechanisms at the within-person level, including appraisals, affective responses, sharing news of the positive events with others, and feelings of being in control (Miller et al., 2007).
This study is one of the first to examine within-person associations of daily positive events with diurnal cortisol rhythms. We found that positive events in the morning were associated with a steeper decline in cortisol across that day. When considering positive events that occurred at any time of the day, there was a marginal association between positive events and same-day lower AUC but not for CAR. These findings are in line with a previous study showing that AUC was lower on days when couples had more intimacy than usual (Ditzen et al., 2008). In another field study, family caregivers of individuals with dementia who had a “burned out” cortisol pattern (blunted CAR and lower AUC) showed increased CAR and AUC on days when the individual with dementia attended adult day services, relative to days when caregivers provided most or all of the care themselves (Klein et al., 2014). Their results, particularly for CAR, suggest that anticipating an easier day might restore normative cortisol patterns in a high-stress population. Our study extends these prior findings by demonstrating that even minor and commonplace positive events across different life domains—such as having a good conversation, receiving a compliment, or accomplishing a task at work—can produce alterations in cortisol on days when these events occur (within-persons) and if they occur frequently over time (between-persons).
It is important to determine whether daily positive events influence cortisol rhythms or vice versa. As mentioned previously, we found that positive events in the morning predicted steeper declines in cortisol across that day, whereas neither cortisol level at waking nor CAR predicted same-day positive events. Although positive events in the morning might have preceded the subsequent decline in cortisol across the day, these events nevertheless happened concurrent to cortisol in the morning. We therefore cannot entirely rule out the influences of cortisol on daily positive events. In another study, for instance, within-person increases in cortisol predicted subsequent increases in positive emotional states (Hoyt et al., 2016). Our end-of-day assessments of daily experiences were limited in their sensitivity to capture very minor events or the precise timing of event occurrence. Future efforts to disentangle the direction of these effects in daily life would require ecological momentary assessments of positive events and cortisol across the day.
There are currently no clinical cutoffs for determining the health significance for differences in diurnal cortisol patterns. Some insight comes from a study of 4,047 middle-aged British adults in the Whitehall II cohort, which found that 1-SD flatter diurnal cortisol slope was associated with 87% increased risk of cardiovascular mortality and 30% increased risk of all-cause mortality across six years of follow-up (Kumari et al., 2011). CAR was not predictive of mortality risk, however. The difference in cortisol slopes between those who died versus survived was 0.0147 nmol/L per hour in the Whitehall II study, suggesting that even very small differences in cortisol slopes might be relevant for health. As we do not yet know the clinical significance of the cortisol levels we observed, additional work is needed to examine the associations between diurnal cortisol patterns and prospective health outcomes, as well as testing cortisol as a pathway linking daily positive events to subsequent health.
Several limitations should be considered when interpreting the results of this study. First, our measure of daily positive events inquired about the occurrence of any events in five broad categories (social interactions, work, home, network, and other). We did not have information regarding participants’ perceptions, emotions, and behaviors specific to the positive events. Future research on daily positive events could benefit from more in-depth assessments of different types of positive events and subjective experiences of these events. Second, we did not assess individual differences in chronotype, which may have been an important confounding factor (Kudielka et al., 2006). Third, although the data came from a large national study of U.S. adults, the sample was nevertheless predominantly white and college-educated. Previous work in MIDUS and other studies have shown that African Americans and those with lower socioeconomic status have relatively flatter diurnal cortisol slopes (Cohen et al., 2006; DeSantis et al., 2015; Karlamangla et al., 2013). Given that social disadvantage increases vulnerability to daily stressors and prevents the development of positive psychosocial resources (Almeida et al., 2005; Matthews et al., 2010), future investigations could evaluate daily experiences as mechanisms linking social disadvantage to altered diurnal cortisol patterns.
5. Conclusion
Despite being a common feature in daily life, the potential roles of daily positive events in diurnal cortisol patterns and stress processes are not well-understood. We found that daily positive events were associated with steeper diurnal cortisol slopes at both the between- and within-person levels, in addition to marginally lower AUC within-persons. However, there was limited evidence to support daily positive events as buffers against stress-related cortisol alterations in this healthy population-based sample of midlife and older adults. These results demonstrate that positive events represent an important dimension of daily life that are not captured by assessments of stressors or affect and that have independent associations with diurnal cortisol. Our findings raise the possibility that efforts to engender more positive experiences in daily life may be protective for diurnal cortisol rhythms. Further work is needed to test possible mechanisms linking positive events to diurnal cortisol, including cognitive, affective, and behavioral responses to positive events. Given that much of the variability in daily positive events, daily stressors, and cortisol were attributable to within-person variation, future research should consider contextual influences in people’s day-to-day lives that influence health and well-being.
Supplementary Material
Highlights.
Daily positive events, daily stressors, and salivary cortisol assessed in N = 1,657
People with more frequent positive events had steeper diurnal cortisol slopes
Within-persons, positive events in morning associated with same-day steeper slope
Within-persons, positive events marginally associated with same-day lower AUC
Limited support for stress-buffering effects of positive events
Acknowledgments
Nancy Sin was supported by National Institute on Aging (NIA) grant F32AG048698. Longitudinal follow-up of the Midlife in the United States (MIDUS) investigation was supported by NIA grant P01-AG020166. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. We thank the staff of the Clinical Research Centers at the University of Wisconsin-Madison, UCLA, and Georgetown University for their support in conducting this study. The MIDUS Biomarker Project was supported by the following grants: M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program and 1UL1RR025011 (UW) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Role of the Funding Sources The funding sources had no involvement in the study design; data collection, analysis, or interpretation; nor the writing and submission of this manuscript.
Footnotes
Participants reported an average of 1.13 positive events per day (SD = 0.67) and 0.52 stressors per day (SD = 0.44). Because it was less common to encounter multiple positive events or multiple stressors within a day, we focused on whether any of these events had occurred. The findings were unchanged when we ran a sensitivity analysis using the number of daily positive events and stressors as predictors of cortisol (Supplementary Tables 1 and 2).
Conflict of Interest The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience – cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Adam EK, Vrshek-Schallhorn S, Kendall AD, Mineka S, Zinbarg RE, Craske MG. Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up–2013 Curt Richter Award Winner. Psychoneuroendocrinology. 2014;44:47–59. doi: 10.1016/j.psyneuen.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography Soc Biol. 2009;55:219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Neupert SD, Banks SR, Serido J. Do daily stress processes account for socioeconomic health disparities? J Gerontol B Psychol Sci Soc Sci. 2005;60:S34–S39. doi: 10.1093/geronb/60.special_issue_2.s34. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The Daily Inventory of Stressful Events An Interview-Based Approach for Measuring Daily Stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Bajaj A, John-Henderson NA, Cundiff JM, Marsland AL, Manuck SB, Kamarck TW. Daily social interactions, close relationships, and systemic inflammation in two samples: Healthy middle-aged and older adults. Brain Behav Immun. 2016;58:152–164. doi: 10.1016/j.bbi.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Preacher KJ, Gil KM. Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: New procedures and recommendations. Psychol Methods. 2006;11:142–163. doi: 10.1037/1082-989X.11.2.142. [DOI] [PubMed] [Google Scholar]
- Bostock S, Hamer M, Wawrzyniak AJ, Mitchell ES, Steptoe A. Positive emotional style and subjective, cardiovascular and cortisol responses to acute laboratory stress. Psychoneuroendocrinology. 2011;36:1175–1183. doi: 10.1016/j.psyneuen.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Kuhn CM, Siegler IC, Williams RB. Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology. 2009;46:862–869. doi: 10.1111/j.1469-8986.2009.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Luong G, Almeida DM, Ryff C, Sturm M, Love G. Fewer ups and downs: Daily stressors mediate age differences in negative affect. J Gerontol B Psychol Sci Soc Sci. 2010;65B:279–286. doi: 10.1093/geronb/gbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hoberman HM. Positive Events and Social Supports as Buffers of Life Change Stress. J Appl Soc Psychol. 1983;13:99–125. doi: 10.1111/j.1559-1816.1983.tb02325.x. [DOI] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Racial and ethnic differences in diurnal cortisol rhythms: are they consistent over time? Psychosom. Med. 2015;77:6–15. doi: 10.1097/PSY.0000000000000131. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, DiezRoux AV, Hajat A, Aiello AE, Golden SH, Jenny NS, Seeman TE, Shea S. Associations of salivary cortisol levels with inflammatory markers: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2012;37:1009–1018. doi: 10.1016/j.psyneuen.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Hoppmann C, Klumb P. Positive couple interactions and daily cortisol: on the stress-protecting role of intimacy. Psychosom Med. 2008;70:883–889. doi: 10.1097/PSY.0b013e318185c4fc. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. Am Psychol. 2000;55:647–654. doi: 10.1037/0003-066X.55.6.647. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. What good are positive emotions? Rev. Gen Psychol. 1998;2:300–319. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gunaydin G, Selcuk E, Ong AD. Trait Reappraisal Predicts Affective Reactivity to Daily Positive and Negative Events. Front Psychol. 2016:7. doi: 10.3389/fpsyg.2016.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/S0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hobfoll SE. Conservation of resources: A new attempt at conceptualizing stress. Am Psychol. 1989;44:513–524. doi: 10.1037/0003-066X.44.3.513. [DOI] [PubMed] [Google Scholar]
- Hoyt LT, Craske MG, Mineka S, Adam EK. Positive and negative affect and arousal: cross-sectional and longitudinal associations with adolescent cortisol diurnal rhythms. Psychosom Med. 2015;77:392–401. doi: 10.1097/PSY.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt LT, Zeiders KH, Ehrlich KB, Adam EK. Positive upshots of cortisol in everyday life. Emotion. 2016;16:431. doi: 10.1037/emo0000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between-and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Myin-Germeys I, Derom C, Delespaul P, van Os J, Nicolson NA. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biol Psychol. 2007;74:60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Jain S, Mills PJ, Von Känel R, Hong S, Dimsdale JE. Effects of perceived stress and uplifts on inflammation and coagulability. Psychophysiology. 2007;44:154–160. doi: 10.1111/j.1469-8986.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawski RS, Almeida DM. Daytime trajectories of cortisol: Demographic and socioeconomic differences—Findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DA, Korchmaros JD, Bolger N. Lower level mediation in multilevel models. Psychol Methods. 2003;8:115–128. doi: 10.1037/1082-989X.8.2.115. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, Walters EE, Zaslavsky AM. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- Klein LC, Kim K, Almeida DM, Femia EE, Rovine MJ, Zarit SH. Anticipating an Easier Day: Effects of Adult Day Services on Daily Cortisol and Stress. The Gerontologist. 2014:1–11. doi: 10.1093/geront/gnu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Federenko IS, Hellhammer DH, Wüst S. Morningness and eveningness: the free cortisol rise after awakening in “early birds” and “night owls.” Biol. Psychol. 2006;72:141–146. doi: 10.1016/j.biopsycho.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of Diurnal Patterns in Salivary Cortisol with All-Cause and Cardiovascular Mortality: Findings from the Whitehall II Study. J Clin Endocrinol Metab. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections? Ann. N Y Acad Sci. 2010;1186:146–173. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037/0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller KG, Wright AGC, Peterson LM, Kamarck TW, Anderson BA, Kirschbaum C, Marsland AL, Muldoon MF, Manuck SB. Trait positive and negative emotionality differentially associate with diurnal cortisol activity. Psychoneuroendocrinology. 2016;68:177–185. doi: 10.1016/j.psyneuen.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. J Pers Soc Psychol. 1998;75:1333–1349. doi: 10.1037/0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Nater UM, Hoppmann C, Klumb PL. Neuroticism and conscientiousness are associated with cortisol diurnal profiles in adults--role of positive and negative affect. Psychoneuroendocrinology. 2010;35:1573–1577. doi: 10.1016/j.psyneuen.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Nederhof E, van Oort FVA, Bouma EMC, Laceulle OM, Oldehinkel AJ, Ormel J. Predicting mental disorders from hypothalamic-pituitary-adrenal axis functioning: a 3-year follow-up in the TRAILS study. Psychol Med. 2015;45:2403–2412. doi: 10.1017/S0033291715000392. [DOI] [PubMed] [Google Scholar]
- Nezlek JB, Plesko RM. Affect- and self-based models of relationships between daily events and daily well-being. Pers Soc Psychol Bull. 2003;29:584–596. doi: 10.1177/0146167203029005004. [DOI] [PubMed] [Google Scholar]
- Ong AD, Bergeman CS, Bisconti TL. The role of daily positive emotions during conjugal bereavement. J Gerontol B Psychol Sci Soc Sci. 2004;59:P168–P176. doi: 10.1093/geronb/59.4.p168. [DOI] [PubMed] [Google Scholar]
- Ong AD, Ram N. Fragile and Enduring Positive Affect: Implications for Adaptive Aging. Gerontology. 2017;63:263–269. doi: 10.1159/000453357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30:261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Matthews KA, Cohen S, Martire LM, Scheier M, Baum A, Schulz R. Association of enjoyable leisure activities with psychological and physical well-being. Psychosom Med. 2009;71:725. doi: 10.1097/PSY.0b013e3181ad7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems. Psychoneuroendocrinology. 2012;37:307–316. doi: 10.1016/j.psyneuen.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Sin NL, Almeida DM, Crain TL, Kossek EE, Berkman LF, Buxton OM. Bidirectional, Temporal Associations of Sleep with Positive Events, Affect, and Stressors in Daily Life Across a Week. Ann Behav Med. 2017:1–14. doi: 10.1007/s12160-016-9864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Graham-Engeland JE, Almeida DM. Daily positive events and inflammation: Findings from the National Study of Daily Experiences. Brain Behav Immun. 2015;43:130–138. doi: 10.1016/j.bbi.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Sloan RP, McKinley PS, Almeida DM. Linking Daily Stress Processes and Laboratory-Based Heart Rate Variability in a National Sample of Midlife and Older Adults. Psychosom Med. 2016;78:573–582. doi: 10.1097/PSY.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatcher RB, Selcuk E, Ong AD. Perceived Partner Responsiveness Predicts Diurnal Cortisol Profiles 10 Years Later. Psychol Sci. 2015 doi: 10.1177/0956797615575022. 0956797615575022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23:353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Snijders TA, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. 2. SAGE; 2011. [Google Scholar]
- Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: timing is everything. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i71–81. doi: 10.1093/geronb/gbq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski RS, Cichy KE, Piazza JR, Almeida DM. Associations among daily stressors and salivary cortisol: Findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2654–2665. doi: 10.1016/j.psyneuen.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Gibson EL, Hamer M, Wardle J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology. 2007;32:56–64. doi: 10.1016/j.psyneuen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Takano K, Sakamoto S, Tanno Y. Ruminative self-focus in daily life: Associations with daily activities and depressive symptoms. Emotion. 2013;13:657–667. doi: 10.1037/a0031867. [DOI] [PubMed] [Google Scholar]
- Tomfohr L, Ancoli-Israel S, Pung MA, Natarajan L, Dimsdale JE. Uplifts and sleep. Behav Sleep Med. 2011;9:31–37. doi: 10.1080/15402002.2011.533992. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, Chan J, Dhabhar FS, Wolkowitz O, Kirschbaum C, Blackburn E, Epel E. Does cellular aging relate to patterns of allostasis?: An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav. 2012;106:40–45. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Wang PS, Berglund P, Kessler RC. Recent care of common mental disorders in the United States : prevalence and conformance with evidence-based recommendations. J Gen Intern Med. 2000;15:284–292. doi: 10.1046/j.1525-1497.2000.9908044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Affleck GG, Tennen H, Reich JW, Davis MC. Dynamic approaches to emotions and stress in everyday life: Bolger and Zuckerman reloaded with positive as well as negative affects. J Pers. 2005;73:1511–1538. doi: 10.1111/j.0022-3506.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.