Abstract
The M protein is a major virulence factor of Streptococcus pyogenes (group A Streptococcus, GAS). This gram-positive bacterial pathogen is responsible for mild infections, such as pharyngitis, and severe invasive disease, like streptococcal toxic shock syndrome. M protein contributes to GAS virulence in multifarious ways, including blocking deposition of antibodies and complement, helping formation of microcolonies, neutralizing antimicrobial peptides, and triggering a proinflammatory and procoagulatory state. These functions are specified by interactions between M protein and many host components, especially C4BP and fibrinogen. The former interaction is conserved among many antigenically variant M protein types but occurs in a strikingly sequence-independent manner, and the latter is associated in the M1 protein type with severe invasive disease. Remarkably for a protein of such diverse interactions, the M protein has a relatively simple but nonideal α-helical coiled coil sequence. This sequence nonideality is a crucial feature of M protein. Nonideal residues give rise to specific irregularities in its coiled-coil structure, which are essential for interactions with fibrinogen and establishment of a proinflammatory state. In addition, these structural irregularities are reminiscent of those in myosin and tropomyosin, which are targets for crossreactive antibodies in patients suffering from autoimmune sequelae of GAS infection.
12.1 Introduction
The M protein is a central virulence factor of the widespread, gram-positive bacterial pathogen Streptococcus pyogenes (group A Streptococcus, GAS) (Cunningham, 2000). Extending ~500 Å outwards from the GAS surface in the form of hair-like fimbriae (Fischetti, 1989), the M protein is in a prime location to interact with host components and guide the course of infection. GAS is responsible for both mild infections, such as pharyngitis (“strep throat”), as well as severe invasive diseases having high mortality rates (~30%), such as necrotizing fasciitis and streptococcal toxic shock syndrome (STSS) (O’Loughlin et al., 2007). GAS infection, especially if untreated, can also lead to delayed autoimmune diseases, such as acute rheumatic fever (Steer et al., 2009).
M protein contributes to GAS infection in a number of different ways. Most well recognized is the capacity of M protein to block antibodies and complement from being deposited onto the surface of S. pyogenes. This enables GAS to evade phagocytic destruction by leukocytes and survive in whole blood (Carlsson et al., 2003). M protein is also involved in the adhesion of GAS to host cells (Okada et al., 1995), intracellular invasion of host cells (Cue et al., 2000), formation of GAS aggregates that enhance phagocytic resistance and host cell adhesion (Frick et al., 2000), neutralization of antimicrobial peptides (Nilsson et al., 2008; Lauth et al., 2009), and assembly of GAS biofilms (Cho and Caparon, 2005). A growing body of evidence indicates that M protein evokes a proinflammatory and procoagulatory state that causes severe tissue damage reminiscent of the symptoms of STSS (Herwald et al., 2004). M protein is also involved in eliciting crossreactive antibodies in autoimmune sequelae of GAS infection (Cunningham, 2000). The multifarious functions of M protein depend on its interaction with a sizeable number of host components. Curiously for a protein of such diverse interactions, the M protein has a seemingly simple α-helical coiled-coil sequence, albeit one that is nonideal and one that is just beginning to be understood in the light of the first atomic-resolution structure of M protein (McNamara et al., 2008).
12.2 Antigenic Variation and M Types
The M protein was first described as a type-specific substance that could be extracted by acid from virulent strains of GAS (Lancefield, 1928). The type-specificity refers to the antigenic variation of M protein, which has served as the basis for the Lancefield serological typing of GAS strains. M types are now defined genotypically by the first 160 bases of the mature protein, which encode the hypervariable region (HVR) (Fig. 12.1) (Facklam et al., 2002). While greater than 100 M types have been identified, only a few are prevalent in the human population (Steer et al., 2009).
Fig. 12.1.
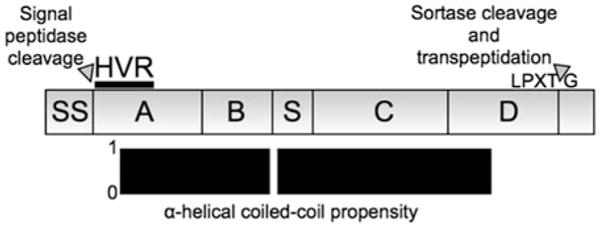
Regions of M protein. Top, the M1 protein type is used as an example to delineate regions. The signal sequence (SS) is removed proteolytically, and the C-terminal “LPXTG” motif is cleaved and covalently attached to the peptidoglycan. Mature M1 is constituted by the A-region, which contains the 50-residue long hypervariable region (HVR); the B-repeats, which consist of ~2.2 imperfect repeats of a 28-residue sequence; the S-region, which is unique to M1; the C-repeats, which consist of 3 imperfect repeats of a 42-residue sequence; and the D-region. Bottom, most of mature M1 has a high propensity for forming an α-helical coiled coil, with a slight break at the end of the B-repeats
The M1 protein type is especially notable not only for being the most prevalent M type but also for being strongly associated with invasive GAS infection and for having proinflammatory properties (Herwald et al., 2004; O’Loughlin et al., 2007). A subclone of the M1T1 serotype has been the leading cause of severe invasive GAS infection worldwide for the past 30 years (Aziz and Kotb, 2008). The M12 and M3 types are also strongly associated with severe invasive disease (O’Loughlin et al., 2007), but less is known about the proinflammatory or procoagulatory properties of these M protein types.
12.3 Bacterial Surface and Released Forms of M Protein
M protein is synthesized in immature form with an N-terminal signal sequence and a C-terminal site for processing by sortase (Fig. 12.1). The N-terminal signal sequence of ~40 residues directs secretion of M protein to the bacterial division septum (Carlsson et al., 2006), and is removed by signal peptidase. The signal sequence is well conserved among M protein types, suggesting a functional importance to secretion at the division septum. Once secreted across the bacterial membrane, M protein is processed by sortase, which cleaves between the Thr and Gly residues in an “LPXTG” motif located close to the C-terminus. Sortase then carries out a transpeptidation reaction in which the Thr residue is covalently attached to the peptidoglycan precursor lipid II, and eventually to the peptidoglycan itself (Navarre and Schneewind, 1999). With the removal of these N- and C-terminal regions, the mature form of M protein contains ~300–400 residues.
M protein is found not only attached to the GAS surface but also as a released molecule. Release may occur through cleavage by the streptococcal cysteine protease SpeB (Berge and Bjorck, 1995). For severe invasive strains which have little or no SpeB expression (Kansal et al., 2000), M protein is released during infection by the action of neutrophil proteases (Herwald et al., 2004). In fact, the greater part of M1 protein in patient samples occurs as released rather than bacterial surface-attached protein (Kahn et al., 2008). Significantly, the released form of M1 retains its proinflammatory and procoagulatory properties (Herwald et al., 2004; Shannon et al., 2007; Kahn et al., 2008; Nilsson et al., 2008).
12.4 Repeats and Nonideal Coiled-Coil Sequences
Two features distinguish the sequence of mature M protein. This is true for both the highly divergent N-terminal half of the protein as well as the more well conserved C-terminal half. The first distinguishing feature is the presence of imperfect repeats in the sequence. Repeats in the sequence divergent N-terminal half are designated A-repeats and B–repeats (Fig. 12.1), but somewhat confusingly, the repeats of two different M types need not be closely related in sequence. A case in point is the B-repeats of M1 and M5, which both bind fibrinogen (Carlsson et al., 2005). The M1 and M5 B-repeats have no obvious relationship to one another (~14% identity), and consistent with this divergence, bind fibrinogen noncompetitively (Ringdahl et al., 2000). In contrast, in the sequence conserved C-terminal half, the 42-residue long C-repeats of different M types are ~70–95% identical. These are followed by a ~90-residue D-region, which has up to ~90% identity between M types. While the D-region is covalently attached to the peptidoglycan and likely buried within it, the C-repeats are exposed and bind host components (Sandin et al., 2006). Why M protein has such a preponderance of repeat sequences is not yet clear.
The second distinguishing feature of the M protein sequence is the presence of an α-helical coiled-coil heptad periodicity (Manjula and Fischetti, 1980). This is true for the divergent N-terminal half, except for the first ~20 residues of the HVR, as well as the conserved C-terminal half, except for the last ~50-residues of the D-region. The heptad periodicity is marked by the recurrence of small hydrophobic residues at the a and d positions. These small hydrophobic residues form the hydrophobic core of the coiled coil through “knobs-into-holes” packing (Fig. 12.2a). Parallel as well as anti-parallel associations of α-helices are possible in coiled coils (Fig. 12.2a), as are homotypic and heterotypic associations. In addition, a number of stoichiometries have been observed for coiled coils.
Fig. 12.2.
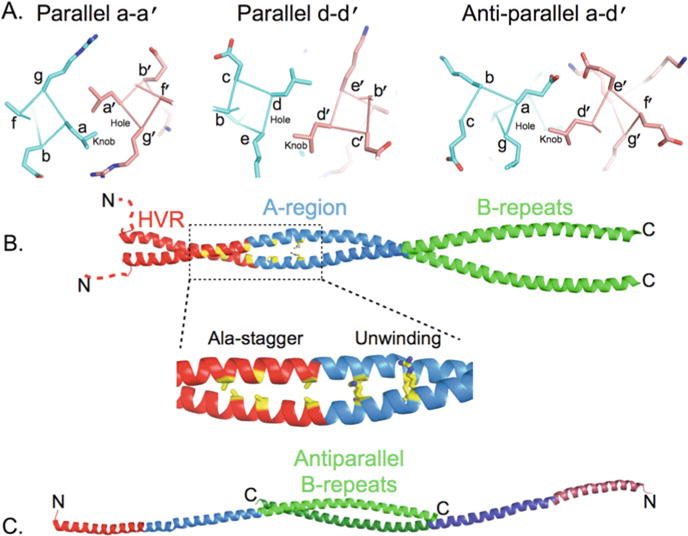
Crystal structure of M protein. (a) Knobs-into-hole packing of residues in the a and d positions, left and middle, respectively, in a dimeric, parallel α-helical coiled coil. Both a and d positions in this example are occupied by Leu, and examples of “knobs” and “holes” are labelled. Right, knobs-into-hole packing of an Asp at the a position with a Leu in the d position in a dimeric, anti-parallel α-helical coiled coil. This and other molecular figures were generated with PyMol (http://www.pymol.org). (b) Structure of the M1AB α-helical coiled-coil dimer. The HVR is in red (with its ~15 initial residues disordered and shown in an arbitrary position as dashed lines), the rest of the A-region in blue, and the B-repeats in green. In yellow, residues that form the Ala-stagger and cause unwinding due to large positively charged residues at a positions. This region is boxed and expanded below. (c) The B-repeats of the M1AB dimer splay apart and engage in an anti-parallel α-helical coiled-coil with the B-repeats of a neighbouring molecule. This anti-parallel interaction between two neighbouring M1AB dimers is shown, with one α-helix of each dimer omitted for clarity
M protein forms a dimeric, parallel α-helical coiled coil structure as directly shown by the crystal structure of a physiologically relevant fragment of M1 protein encompassing the A-region and B-repeats, called M1AB (McNamara et al., 2008). The structure also reveals a substantial number of structural irregularities in the coiled coil (Figs. 12.2b and 12.2c). These structural irregularities stem from a sequence that is far from ideal at the core a and d positions (Nilson et al., 1995; McNamara et al., 2008). The ideal residues for a dimeric, parallel coiled coil at the a and d positions are Val and Leu, respectively (Wagschal et al., 1999; Tripet et al., 2000). Destabilizing residues at a positions are Ala, Gln, His, Lys, Ser, Glu, Arg, and Gly; and at d positions Ala, Trp, Asn, His, Thr, Lys, Asp, Ser, Glu, Arg, and Gly. M protein, regardless of type, has a substantial number of these nonideal residues at predicted a and d positions throughout its length (Nilson et al., 1995; McNamara et al., 2008).
12.5 Structural Irregularities
The consequences of sequence nonidealities in M protein were recently revealed by the crystal structure of M1AB (McNamara et al., 2008). M1AB forms a parallel, dimeric α-helical coiled coil through the A-region, while the B-repeats splay apart and engage in an anti-parallel coiled coil with the B-repeats of an adjoining M1AB molecule in the crystal (Figs. 12.2b and 12.2c). Only two short stretches in M1AB have regular structure. Each of these short segments consists of ~2 heptads (residues 63–79 in the HVR and residues 106–119 of the A-region). The rest of the structure is irregular. These structural irregularities consist of an Ala-stagger due to a cluster of poorly packed Ala residues at contiguous a and d positions in the HVR, superhelical unwinding due to large positively charged residues at consecutive a positions in the A-region, and splaying apart of the entire B-repeats.
Each of these specific structural features has been seen in the α-helical coiled coil portions of myosin, tropomyosin, or both (Brown et al., 2001; Li et al., 2003; Brown et al., 2005; Blankenfeldt et al., 2006). This is notable as patients with acute rheumatic fever have crossreactive antibodies directed against myosin, tropomyosin, and other host α-helical coiled coil proteins (e.g. laminin, keratin, and vimentin) (Cunningham, 2000). This raises the possibility that specific structural irregularities shared between M1, myosin, and tropomyosin are being recognized by crossreactive antibodies rather than the generic “coiled coil-ness” of the structure. In support of this notion, sequence idealization of the B-repeats of M1, in which a and d positions of the B-repeats were substituted with Val and Leu, respectively, resulted in decreased recognition by the crossreactive antibody 36.2.2 (McNamara et al., 2008), noted for its cytoxicity against heart cells (Cunningham et al., 1992). This B-repeat idealized version of M1 retained the capacity to elicit protective immunity (McNamara et al., 2008), suggesting that sequence idealization may be applicable to vaccine design.
While the splaying apart of the B-repeats is indicative of the instability of the coiled coil in this region, it is also possible that the anti-parallel coiled coil reflects a physiologically relevant state in promoting GAS aggregation (Fig. 12.3a). GAS aggregation is involved in the evasion of phagocytosis as well as the formation of microcolonies that adhere better to epithelial cells than single bacterial cells (Caparon et al., 1991; Frick et al., 2000). Evidence exists that M protein dimers dynamically dissociate and reassociate (Akerstrom et al., 1992; Cedervall et al., 1995; Nilson et al., 1995; McNamara et al., 2008), making it possible for M proteins from abutting bacterial cells to associate in anti-parallel form. For the B-repeats and perhaps for other portions of the M protein, the anti-parallel coil form may be more favourable than the parallel one (Nilson et al., 1995; McNamara et al., 2008). This is because the packing in an anti-parallel coiled coil is between a-d′ and d-a′ positions (the prime referring to the residue in the opposing helix) as opposed to the a-a′ and d-d′ packing in a parallel coiled coil (Fig. 12.2a). Anti-parallel packing in the B-repeats of M1 avoids several a-a′ charge-charge clashes that would occur in the parallel conformation. Electron micrographs suggest that this sort of interaction occurs (Phillips et al., 1981), and the importance of M protein to GAS aggregation has been reported (Caparon et al., 1991).
Fig. 12.3.

Functional interactions of M protein. Bacterial surface-bound M protein contributes to (a) GAS aggregation through homotypic interactions, and (b) evasion of phagocytosis through recruitment of fibrinogen or C4BP to the bacterial surface. Bound fibrinogen is also responsible for recruiting plasmin to the GAS surface, which is associated with a transition from localized to invasive infection. (c) M protein released by neutrophil proteases from the bacterial surface interacts with fibrinogen, and M-fibrinogen complexes activate neutrophils via β2 integrins along with IgGs that bind to M protein and interact with FcγRII. Activated neutrophils release the vasodilator heparin binding protein (HBP). (d) M-fibrinogen complexes also activate platelets. This occurs through interaction of these complexes with the integrin GPIIb/IIIa along with IgGs that bind to M protein and interact with FcγRII. Activated platelets in turn lead to further activation of neutrophils and monocytes. (e) M protein synergizes in a TLR2-dependent manner with HBP to activate monocytes, which then secrete proinflammatory cytokines and upregulate the procoagulatory protein tissue factor. (f) M protein is also responsible for neutralizing the antimicrobial effects of cathelicidins in neutrophil extracellular traps (NET)
For myosin and tropomyosin, structural irregularities in the coiled coil are correlated with function. The same appears to be the case for M protein. Idealization of the a and d positions in the B-repeats of M1 was seen to impart greater stability to the molecule but also reduced its affinity for fibrinogen (McNamara et al., 2008). The regularity of a coiled coil, while structurally elegant, appears not to be conducive to conferring specific recognition or conformational flexibility. These features require nonideal sequences and structural irregularity, with the cost of this being a diminution in protein stability. This explains a puzzling feature of M protein, its instability at 37°C (Akerstrom et al., 1992; Cedervall et al., 1995; Nilson et al., 1995; Cedervall et al., 1997; Gubbe et al., 1997; McNamara et al., 2008).
12.6 B-Repeats and Proinflammatory Effects
The B-repeats of several M protein types are crucial to the evasion of phagocytosis through the recruitment of fibrinogen to the GAS surface (Ringdahl et al., 2000; Carlsson et al., 2005). Bound fibrinogen substantially interferes with the formation and deposition of the classical complement pathway C3 convertase, C4bC2a, (even in the absence of immune conditions) on the GAS surface (Fig. 12.3b) (Carlsson et al., 2005). Fibrinogen is also responsible for recruiting plasmin to the GAS surface, which is essential for the transition of an infection from localized to invasive (Sun et al., 2004; Cole et al., 2006). The B-repeats are also immunodominant, although the antibodies elicited by this region are nonopsonic and do not provide protection (Fischetti and Windels, 1988; Huber et al., 1994; Stalhammar-Carlemalm et al., 2007).
A separate role for the B-repeats of released M1 protein in septic shock has been uncovered in recent years (Herwald et al., 2004). Released M1 binds fibrinogen through the B-repeats (Herwald et al., 2004; McNamara et al., 2008), and the resulting M1-fibrinogen complex interacts with β2 integrins (i.e. CD11b/CD18) on the surface of neutrophils (Fig. 12.3c). This brings about neutrophil activation, as seen by an increase of several cell surface markers (e.g. CD11b) and the release of soluble granule components, including heparin binding protein (HBP), the antimicrobial peptide LL-37, MMP-9 (gelatinase), and albumin (Herwald et al., 2004; Soehnlein et al., 2008). This activation event requires both M1 protein and fibrinogen (Herwald et al., 2004; McNamara et al., 2008; Soehnlein et al., 2008), and likely occurs through crosslinking of β2 integrins on the neutrophil surface as deduced from the observation that antibody crosslinking of β2 integrins has the same effects as M1-fibrinogen complexes (Gautam et al., 2000; Herwald et al., 2004; Soehnlein et al., 2008). Neutrophil activation, as monitored by the release of HBP, is substantially enhanced by binding of IgG antibodies directed to the S-region of M1 (between the B-repeats and C-repeats) (Figs. 12.1 and 12.3) (Kahn et al., 2008). These antibodies act through the FcγRII receptor (Kahn et al., 2008). Neutrophil activation is also promoted by the binding of M1-fibrinogen complexes to the low-affinity integrin GPIIb/IIIa on the surface of platelets, in concert with the binding of IgGs directed against M1 to FcγRII (Fig. 12.3d) (Shannon et al., 2007). These interactions lead to platelet activation, aggregation, and generation of thrombi, and activated platelets in turn stimulate neutrophils and monocytes.
The most damaging substance released by activated neutrophils is HBP (also called azurocidin and CAP37). HBP is an inactive serine protease and a member of the serprocidin family of neutrophil cationic proteins (Gautam et al., 2001; Herwald et al., 2004), and is stored in azurophilic granules and secretory vesicles (Tapper et al., 2002). Once released, HBP acts on endothelial cells to cause Ca2+-dependent cytoskeletal rearrangements and intercellular gap formation (Gautam et al., 2001). These events lead in vivo to vasodilation, haemorrhage, and acute pulmonary damage resembling the symptoms of STSS (Herwald et al., 2004; Soehnlein et al., 2008). In agreement with these findings, high plasma levels of HBP in patients have been found to be a strong indicator for the onset of sepsis and circulatory failure (Linder et al., 2009).
HBP also synergizes with M1 protein in bringing about the secretion of the proinflammatory cytokines IL-6, IL-1β, and TNF-α from monocytes (Fig. 12.3e) (Pahlman et al., 2006). HBP acts on β2 integrins on the surface of monocytes, and M1 acts in a TLR2-dependent manner on monocytes (Pahlman et al., 2006). In fact, M1 binds preferentially to monocytes as compared to neutrophils and lymphocytes, and M1 and TLR2 appear to colocalize on the surface of monocytes (Pahlman et al., 2006). Whether this interaction is direct and which portions of M1 protein are involved are not known. M1, as well as some other M protein types, upregulate tissue factor on monocytes to produce a procoagulatory state (Pahlman et al., 2007). The time to clot formation is decreased by these M protein types, with C-terminal portions of M1 (S-region through the C-repeats) being required for this effect (Pahlman et al., 2007).
Lastly and somewhat surprisingly, M1 also acts as a superantigen. M1 stimulates T cell proliferation and causes the release of Th1 type cytokines (TNF-β and IFN-γ). This effect is dependent on class II MHC and shows a preference for certain T cell receptor (TCR) Vβ chains, which is the case for other superantigens (Pahlman et al., 2008). Presumably, M1 crosslinks class II MHC molecules on antigen presenting cells with particular TCRs, but this has not been shown directly and, again, which portions of M1 are involved is not yet known.
The M1 protein type has been intensively studied for proinflammatory and procoagulatory properties due to the prevalence of the M1 type among strains causing invasive disease. Other M protein types have been documented to evoke responses from host cells similar to that of M1. For example, M3, M5, and M49 have also been found to stimulate the release of IL-6 from monocytes (Pahlman et al., 2006). More comparative work will be required to determine whether M1 is especially virulent compared to other M types.
12.7 Hypervariable Region
The very N-terminus of M protein, the HVR (Fig. 12.1), has intriguing functional properties. Within a particular M type, the HVR sequence is quite stable (Facklam et al., 2002). While the HVR is non-immunodominant (Stalhammar-Carlemalm et al., 2007), it specifically elicits protective (i.e. opsonic) antibody responses (Dale et al., 1983; Fischetti and Windels, 1988; Jones and Fischetti, 1988; Persson et al., 2006; Sandin et al., 2006). Due to this protective feature, a multivalent vaccine composed of portions of the HVRs of 26 prevalent M types has been tested and shown to be promising (Dale et al., 2005). The sequence stability of the HVR in the face of immune pressure suggests a functional importance to this region.
Consistent with the HVR having functional significance, deletion of the M5 HVR resulted in a ~50-fold lower competitive index in the liver and spleen of mice (Waldemarsson et al., 2009). The functional basis for the importance of the M5 HVR is unknown. While the M5 HVR binds factor H-like protein 1 (FHL-1) (Johnsson et al., 1998), an alternative splice variant of the complement regulatory protein factor H, the interaction with FHL-1 lacks functional significance (Kotarsky et al., 2001). In contrast to the deletion of the entire HVR, a smaller deletion of residues 3–22 of the M5 HVR had no effect on colonization of the upper respiratory tract of mice (Penfound et al., 2010). This smaller deletion did, however, lead to decreased binding of lipotechoic acid (LTA) to M5 and decreased GAS adherence to epithelial cells (Penfound et al., 2010). LTA displayed on M protein acts as an adhesin (Beachey and Ofek, 1976).
No studies on the virulence of M1 protein lacking its HVR have been carried out, but a functionally important interaction of the M1 HVR with a host component has been identified (Lauth et al., 2009). The M1 HVR provides protection against cathelicidin antimicrobial peptides (i.e. LL-37 and mCRAMP), which kill GAS upon entrapment in neutrophil or mast cell extracellular traps (Fig. 12.3f) (Lauth et al., 2009). The effect is direct as incubation of an M1 fragment containing the HVR results in a dose-dependent decrease of LL-37 in solution. At this point, the structure of the M1 HVR provides little direction in understanding how cathelicidins are recognized. The first ~15 residues of the M1 HVR are disordered (Fig. 12.2b). The following ~4 residues are in random coil conformation, after which the HVR forms an α-helix. This α-helix makes one turn and then engages in a parallel, dimeric coiled coil, at the end of which is the Ala-stagger (Fig. 12.2b). M1 also inhibits a second antimicrobial peptide, this one derived from β2 glycoprotein I, but the region of M1 responsible for this activity has not yet been mapped (Nilsson et al., 2008).
Perhaps the most remarkable feature uncovered for the HVR is the astonishing number of sequence unrelated HVRs that bind the regulatory complement component C4b-binding protein (C4BP) (Morfeldt et al., 2001; Persson et al., 2006). The soluble glycoprotein C4BP increases the rate of dissociation of the C3 convertase and increases the activity of the regulatory complement component factor I (Gigli et al., 1979), which cleaves C3b. The C4BP-binding HVRs, although lacking sequence identity, all bind to the same site on the α chain of C4BP (Accardo et al., 1996; Jenkins et al., 2006). Recruitment of C4BP to the GAS surface by the HVR of M protein is crucial for inhibiting phagocytosis through the interference with deposition of the classical pathway C3 convertase as well as degradation of the C3 convertase, presumably mediated by factor I (Fig. 12.3b) (Carlsson et al., 2003). Furthermore, C4BP competes with opsonising antibodies targeted to the HVR (Berggard et al., 2001; Carlsson et al., 2003).
Sequence analysis has revealed that C4BP-binding HVRs have only four residues that are well conserved, two Glu residues at predicted outward-facing g positions of the heptad and two Leu residues at predicted inward-facing d positions (Fig. 12.2a) (Persson et al., 2006). Individual substitutions of these glutamates in the M22 HVR with Ala had no effect on binding to C4BP, while individual substitutions of the leucines in the M22 HVR with Ala did (Persson et al., 2006). These Glu and Leu residues occur in the ordered coiled-coil portion of the HVR as determined from nuclear magnetic resonance (NMR) spectroscopy evidence; by comparison, the N-terminus is unstructured as it is in the M1 HVR (Andre et al., 2006). Not enough information is available from the NMR data to indicate whether the coiled coil in the C4BP-binding HVRs of M4 and M22 are structurally irregular (Andre et al., 2006). Of note, the predicted coiled-coil register of the M22 HVR and other C4BP-binding HVRs contain several nonideal residues at a and d positions. While C4BP binding is maintained even when the putatively surface-exposed g position glutamates are substituted by Ala, recognition by antibodies is compromised (Persson et al., 2006). This suggests that small variations in the HVR that enable escape from immune pressure are possible without destroying essential functions. The structural basis for the relatively sequence-independent interaction between the HVR and C4BP is currently unknown.
12.8 Concluding Remarks
The M protein is remarkable for the number of different functions in GAS virulence that are specified by its relatively simple, albeit nonideal, α-helical coiled coil sequence. These functions depend on interactions between M protein and a diverse set of host proteins, prominent among these being regulators of the complement system and the blood clotting protein fibrinogen. Deeper knowledge of how the sequence nonideality of M protein relates to its multifarious functions is beginning to be gained. Recent work has shown that sequence nonidealities in M1 result in structural irregularities, and that these structural irregularities are essential for binding fibrinogen and eliciting proinflammatory effects. They also appear to be involved in antibody crossreactivity. A number of important questions remain to be addressed. For example, a detailed accounting of how structural irregularities beget specific recognition and function remains to be furnished. It also remains to be determined whether structural irregularities in M protein are necessary for recognition of other host components, such as C4BP. It will also be important to understand whether the M1 protein type has special proinflammatory and procoagulatory properties that distinguish it from other M protein types. The convergence of structural, biochemical, genetic, and in vivo studies promises to shed light on these questions in the coming years.
References
- Accardo P, Sánchez-Corral P, Criado O, García E, Rodriguez de Córdoba S. Binding of human complement component C4b-binding protein (C4BP) to Streptococcus pyogenes involves the C4b-binding site. J Immunol. 1996;157:4935–4939. [PubMed] [Google Scholar]
- Åkerström B, Lindahl G, Björck L, Lindqvist A. Protein Arp and protein H from group A streptococci. Ig binding and dimerization are regulated by temperature. J Immunol. 1992;148:3238–3243. [PubMed] [Google Scholar]
- André I, Persson J, Blom AM, Nilsson H, Drakenberg T, Lindahl G, Linse S. Streptococcal M protein: structural studies of the hypervariable region, free and bound to human C4BP. Biochemistry. 2006;45:4559–4568. doi: 10.1021/bi052455c. [DOI] [PubMed] [Google Scholar]
- Aziz RK, Kotb M. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg Infect Dis. 2008;14:1511–1517. doi: 10.3201/eid1410.071660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey EH, Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976;143:759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge A, Björck L. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J Biol Chem. 1995;270:9862–9867. doi: 10.1074/jbc.270.17.9862. [DOI] [PubMed] [Google Scholar]
- Berggård K, Johnsson E, Morfeldt E, Persson J, Stalhammar-Carlemalm M, Lindahl G. Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Mol Microbiol. 2001;42:539–551. doi: 10.1046/j.1365-2958.2001.02664.x. [DOI] [PubMed] [Google Scholar]
- Blankenfeldt W, Thomä NH, Wray JS, Gautel M, Schlichting I. Crystal structures of human cardiac β-myosin II S2-Δ provide insight into the functional role of the S2 subfragment. Proc Natl Acad Sci USA. 2006;103:17713–17717. doi: 10.1073/pnas.0606741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Kim KH, Jun G, Greenfield NJ, Dominguez R, Volkmann N, Hitchcock-DeGregori SE, Cohen C. Deciphering the design of the tropomyosin molecule. Proc Natl Acad Sci USA. 2001;98:8496–8501. doi: 10.1073/pnas.131219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Zhou Z, Reshetnikova L, Robinson H, Yammani RD, Tobacman LS, Cohen C. Structure of the mid-region of tropomyosin: bending and binding sites for actin. Proc Natl Acad Sci USA. 2005;102:18878–18883. doi: 10.1073/pnas.0509269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparon MG, Stephens DS, Olsén A, Scott JR. Role of M protein in adherence of group A streptococci. Infect Immun. 1991;59:1811–1817. doi: 10.1128/iai.59.5.1811-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson F, Berggård K, Stålhammar-Carlemalm M, Lindahl G. Evasion of phagocytosis through cooperation between two ligand-binding regions in Streptococcus pyogenes M protein. J Exp Med. 2003;198:1057–1068. doi: 10.1084/jem.20030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson F, Sandin C, Lindahl G. Human fibrinogen bound to Streptococcus pyogenes M protein inhibits complement deposition via the classical pathway. Mol Microbiol. 2005;56:28–39. doi: 10.1111/j.1365-2958.2005.04527.x. [DOI] [PubMed] [Google Scholar]
- Carlsson F, Stålhammar-Carlemalm M, Flärdh K, Sandin C, Carlemalm E, Lindahl G. Signal sequence directs localized secretion of bacterial surface proteins. Nature. 2006;442:943–946. doi: 10.1038/nature05021. [DOI] [PubMed] [Google Scholar]
- Cedervall T, Åkesson P, Stenberg L, Herrmann A, Åkerström B. Allosteric and temperature effects on the plasma protein binding by streptococcal M protein family members. Scand J Immunol. 1995;42:433–441. doi: 10.1111/j.1365-3083.1995.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Cedervall T, Johansson MU, Åkerström B. Coiled-coil structure of group A streptococcal M proteins. Different temperature stability of class A and C proteins by hydrophobic-nonhydrophobic amino acid substitutions at heptad positions a and d. Biochemistry. 1997;36:4987–4994. doi: 10.1021/bi962971q. [DOI] [PubMed] [Google Scholar]
- Cho KH, Caparon MG. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol Microbiol. 2005;57:1545–1556. doi: 10.1111/j.1365-2958.2005.04786.x. [DOI] [PubMed] [Google Scholar]
- Cole JN, McArthur JD, McKay FC, Sanderson-Smith ML, Cork AJ, Ranson M, Rohde M, Itzek A, Sun H, Ginsburg D, Kotb M, Nizet V, Chhatwal GS, Walker MJ. Trigger for group A streptococcal M1T1 invasive disease. FASEB J. 2006;20:1745–1747. doi: 10.1096/fj.06-5804fje. [DOI] [PubMed] [Google Scholar]
- Cue D, Southern SO, Southern PJ, Prabhakar J, Lorelli W, Smallheer JM, Mousa SA, Cleary PP. A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin α5β1-fibronectin-M1 protein complexes. Proc Natl Acad Sci USA. 2000;97:2858–2863. doi: 10.1073/pnas.050587897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW, Antone SM, Gulizia JM, McManus BM, Fischetti VA, Gauntt CJ. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci USA. 1992;89:1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale JB, Penfound T, Chiang EY, Long V, Shulman ST, Beall B. Multivalent group A streptococcal vaccine elicits bactericidal antibodies against variant M subtypes. Clin Diagn Lab Immunol. 2005;12:833–836. doi: 10.1128/CDLI.12.7.833-836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale JB, Seyer JM, Beachey EH. Type-specific immunogenicity of a chemically synthesized peptide fragment of type 5 streptococcal M protein. J Exp Med. 1983;158:1727–1732. doi: 10.1084/jem.158.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam RF, Martin DR, Lovgren M, Johnson DR, Efstratiou A, Thompson TA, Gowan S, Kriz P, Tyrrell GJ, Kaplan E, Beall B. Extension of the Lancefield classification for group A streptococci by addition of 22 new M protein gene sequence types from clinical isolates: emm103 to emm124. Clin Infect Dis. 2002;34:28–38. doi: 10.1086/324621. [DOI] [PubMed] [Google Scholar]
- Fischetti VA. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti VA, Windels M. Mapping the immunodeterminants of the complete streptococcal M6 protein molecule. Identification of an immunodominant region. J Immunol. 1988;141:3592–3599. [PubMed] [Google Scholar]
- Frick IM, Mörgelin M, Björck L. Virulent aggregates of Streptococcus pyogenes are generated by homophilic protein-protein interactions. Mol Microbiol. 2000;37:1232–1247. doi: 10.1046/j.1365-2958.2000.02084.x. [DOI] [PubMed] [Google Scholar]
- Gautam N, Herwald H, Hedqvist P, Lindbom L. Signaling via β2 integrins triggers neutrophil-dependent alteration in endothelial barrier function. J Exp Med. 2000;191:1829–1839. doi: 10.1084/jem.191.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7:1123–1127. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- Gigli I, Fujita T, Nussenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc Natl Acad Sci USA. 1979;76:6596–6600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbe K, Misselwitz R, Welfle K, Reichardt W, Schmidt KH, Welfle H. C repeats of the streptococcal M1 protein achieve the human serum albumin binding ability by flanking regions which stabilize the coiled-coil conformation. Biochemistry. 1997;36:8107–8113. doi: 10.1021/bi962991s. [DOI] [PubMed] [Google Scholar]
- Herwald H, Cramer H, Mörgelin M, Russell W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Björck L. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell. 2004;116:367–379. doi: 10.1016/s0092-8674(04)00057-1. [DOI] [PubMed] [Google Scholar]
- Huber SA, Moraska A, Cunningham M. Alterations in major histocompatibility complex association of myocarditis induced by coxsackievirus B3 mutants selected with monoclonal antibodies to group A streptococci. Proc Natl Acad Sci USA. 1994;91:5543–5547. doi: 10.1073/pnas.91.12.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins HT, Mark L, Ball G, Persson J, Lindahl G, Uhrin D, Blom AM, Barlow PN. Human C4b-binding protein, structural basis for interaction with streptococcal M protein, a major bacterial virulence factor. J Biol Chem. 2006;281:3690–3697. doi: 10.1074/jbc.M511563200. [DOI] [PubMed] [Google Scholar]
- Johnsson E, Berggård K, Kotarsky H, Hellwage J, Zipfel PF, Sjöbring U, Lindahl G. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J Immunol. 1998;161:4894–4901. [PubMed] [Google Scholar]
- Jones KF, Fischetti VA. The importance of the location of antibody binding on the M6 protein for opsonization and phagocytosis of group A M6 streptococci. J Exp Med. 1988;167:1114–1123. doi: 10.1084/jem.167.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn F, Mörgelin M, Shannon O, Norrby-Teglund A, Herwald H, Olin AI, Björck L. Antibodies against a surface protein of Streptococcus pyogenes promote a pathological inflammatory response. PLoS Pathog. 2008;4:e1000149. doi: 10.1371/journal.ppat.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansal RG, McGeer A, Low DE, Norrby-Teglund A, Kotb M. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect Immun. 2000;68:6362–6369. doi: 10.1128/iai.68.11.6362-6369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarsky H, Gustafsson M, Svensson HG, Zipfel PF, Truedsson L, Sjöbring U. Group A streptococcal phagocytosis resistance is independent of complement factor H and factor H-like protein 1 binding. Mol Microbiol. 2001;41:817–826. doi: 10.1046/j.1365-2958.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- Lancefield RC. The antigenic complex of streptococcus haemolyticus: I. Demonstration of a Type-Specific Substance in Extracts of Streptococcus Haemolyticus. J Exp Med. 1928;47:91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauth X, von Köckritz-Blickwede M, McNamara CW, Myskowski S, Zinkernagel AS, Beall B, Ghosh P, Gallo RL, Nizet V. M1 protein allows group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J Innate Immun. 2009;1:202–214. doi: 10.1159/000203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Brown JH, Reshetnikova L, Blazsek A, Farkas L, Nyitray L, Cohen C. Visualization of an unstable coiled coil from the scallop myosin rod. Nature. 2003;424:341–345. doi: 10.1038/nature01801. [DOI] [PubMed] [Google Scholar]
- Linder A, Christensson B, Herwald H, Björck L, Åkesson P. Heparin-binding protein: an early marker of circulatory failure in sepsis. Clin Infect Dis. 2009;49:1044–1050. doi: 10.1086/605563. [DOI] [PubMed] [Google Scholar]
- Manjula BN, Fischetti VA. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980;151:695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara C, Zinkernagel AS, Macheboeuf P, Cunningham MW, Nizet V, Ghosh P. Coiled-coil irregularities and instabilities in group A Streptococcus M1 are required for virulence. Science. 2008;319:1405–1408. doi: 10.1126/science.1154470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfeldt E, Berggård K, Persson J, Drakenberg T, Johnsson E, Lindahl E, Linse S, Lindahl G. Isolated hypervariable regions derived from streptococcal M proteins specifically bind human C4b-binding protein: implications for antigenic variation. J Immunol. 2001;167:3870–3877. doi: 10.4049/jimmunol.167.7.3870. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson BH, Frick IM, Åkesson P, Forsén S, Björck L, Åkerström B, Wikström M. Structure and stability of protein H and the M1 protein from Streptococcus pyogenes. Implications for other surface proteins of gram-positive bacteria. Biochemistry. 1995;34:13688–13698. doi: 10.1021/bi00041a051. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Wasylik S, Mörgelin M, Olin AI, Meijers JC, Derksen RH, de Groot PG, Herwald H. The antibacterial activity of peptides derived from human β-2 glycoprotein I is inhibited by protein H and M1 protein from Streptococcus pyogenes. Mol Microbiol. 2008;67:482–492. doi: 10.1111/j.1365-2958.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Okada N, Liszewski MK, Atkinson JP, Caparon M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci USA. 1995;92:2489–2493. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, Beall B, Harrison LH, Reingold A, Van Beneden C. Active Bacterial Core Surveillance T. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45:853–862. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- Påhlman LI, Malmström E, Mörgelin M, Herwald H. M protein from Streptococcus pyogenes induces tissue factor expression and pro-coagulant activity in human monocytes. Microbiology. 2007;153:2458–2464. doi: 10.1099/mic.0.2006/003285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Påhlman LI, Mörgelin M, Eckert J, Johansson L, Russell W, Riesbeck K, Soehnlein O, Lindbom L, Norrby-Teglund A, Schumann RR, Björck L, Herwald H. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J Immunol. 2006;177:1221–1228. doi: 10.4049/jimmunol.177.2.1221. [DOI] [PubMed] [Google Scholar]
- Påhlman LI, Olin AI, Darenberg J, Mörgelin M, Kotb M, Herwald H, Norrby-Teglund A. Soluble M1 protein of Streptococcus pyogenes triggers potent T cell activation. Cell Microbiol. 2008;10:404–414. doi: 10.1111/j.1462-5822.2007.01053.x. [DOI] [PubMed] [Google Scholar]
- Penfound TA, Ofek I, Courtney HS, Hasty DL, Dale JB. The NH(2)-Terminal region of Streptococcus pyogenes M5 protein confers protection against degradation by proteases and enhances mucosal colonization of mice. J Infect Dis. 2010;201:1580–1588. doi: 10.1086/652005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Beall B, Linse S, Lindahl G. Extreme sequence divergence but conserved ligand-binding specificity in Streptococcus pyogenes M protein. PLoS Pathog. 2006;2:e47. doi: 10.1371/journal.ppat.0020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GN, Jr, Flicker PF, Cohen C, Manjula BN, Fischetti VA. Streptococcal M protein: α-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci USA. 1981;78:4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringdahl U, Svensson HG, Kotarsky H, Gustafsson M, Weineisen M, Sjöbring U. A role for the fibrinogen-binding regions of streptococcal M proteins in phagocytosis resistance. Mol Microbiol. 2000;37:1318–1326. doi: 10.1046/j.1365-2958.2000.02062.x. [DOI] [PubMed] [Google Scholar]
- Sandin C, Carlsson F, Lindahl G. Binding of human plasma proteins to Streptococcus pyogenes M protein determines the location of opsonic and non-opsonic epitopes. Mol Microbiol. 2006;59:20–30. doi: 10.1111/j.1365-2958.2005.04913.x. [DOI] [PubMed] [Google Scholar]
- Shannon O, Hertzén E, Norrby-Teglund A, Mörgelin M, Sjöbring U, Björck L. Severe streptococcal infection is associated with M protein-induced platelet activation and thrombus formation. Mol Microbiol. 2007;65:1147–1157. doi: 10.1111/j.1365-2958.2007.05841.x. [DOI] [PubMed] [Google Scholar]
- Soehnlein O, Oehmcke S, Ma X, Rothfuchs AG, Frithiof R, van Rooijen N, Mörgelin M, Herwald H, Lindbom L. Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur Respir J. 2008;32:405–412. doi: 10.1183/09031936.00173207. [DOI] [PubMed] [Google Scholar]
- Stålhammar-Carlemalm M, Waldemarsson J, Johnsson E, Areschoug T, Lindahl G. Nonimmunodominant regions are effective as building blocks in a streptococcal fusion protein vaccine. Cell Host Microbe. 2007;2:427–434. doi: 10.1016/j.chom.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9:611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjobring U, Ginsburg D. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305:1283–1286. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- Tapper H, Karlsson A, Mörgelin M, Flodgaard H, Herwald H. Secretion of heparin-binding protein from human neutrophils is determined by its localization in azurophilic granules and secretory vesicles. Blood. 2002;99:1785–1793. doi: 10.1182/blood.v99.5.1785. [DOI] [PubMed] [Google Scholar]
- Tripet B, Wagschal K, Lavigne P, Mant CT, Hodges RS. Effects of side-chain characteristics on stability and oligomerization state of a de novo-designed model coiled-coil: 20 amino acid substitutions in position “d”. J Mol Biol. 2000;300:377–402. doi: 10.1006/jmbi.2000.3866. [DOI] [PubMed] [Google Scholar]
- Wagschal K, Tripet B, Lavigne P, Mant C, Hodges RS. The role of position a in determining the stability and oligomerization state of α-helical coiled coils: 20 amino acid stability coefficients in the hydrophobic core of proteins. Protein Sci. 1999;8:2312–2329. doi: 10.1110/ps.8.11.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldemarsson J, Stålhammar-Carlemalm M, Sandin C, Castellino FJ, Lindahl G. Functional dissection of Streptococcus pyogenes M5 protein: the hypervariable region is essential for virulence. PLoS One. 2009;4:e7279. doi: 10.1371/journal.pone.0007279. [DOI] [PMC free article] [PubMed] [Google Scholar]


