Abstract
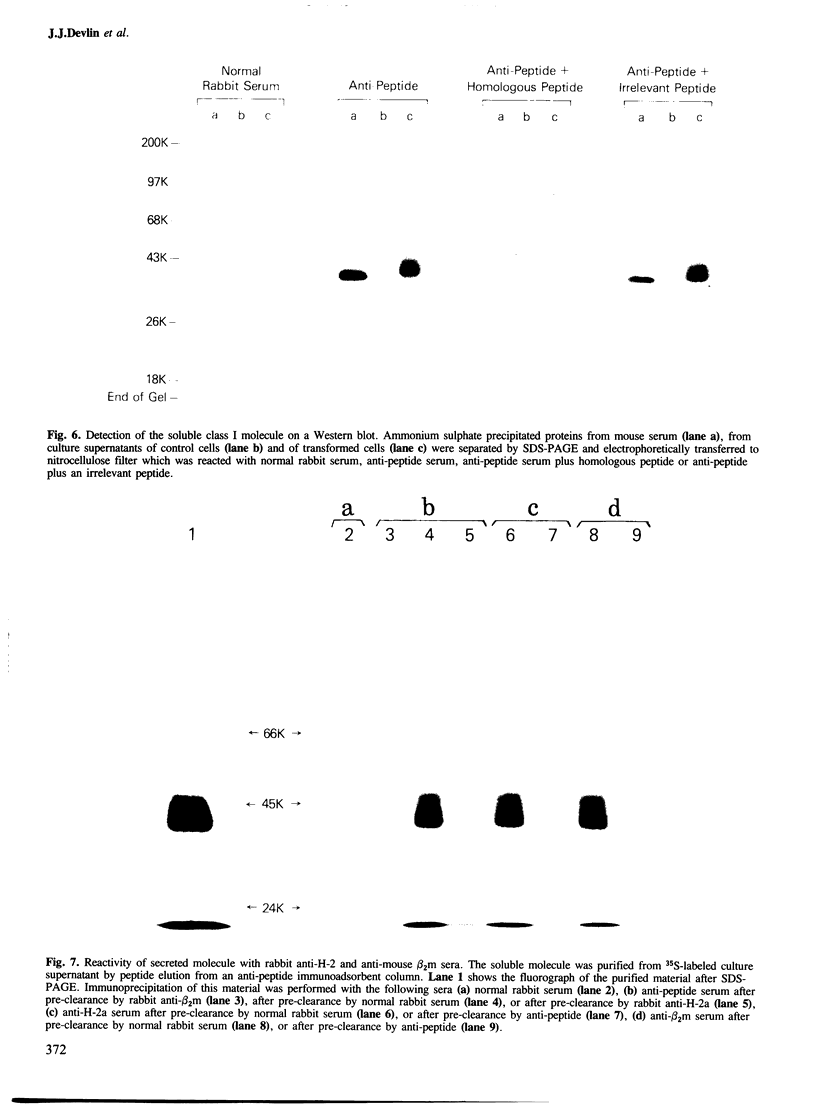
The DNA sequence of the Q10 genes appears to be highly conserved amongst strains of mice and has only been found to be transcribed in the liver. An examination of the nucleotide sequence of the exon that normally encodes the transmembrane domain of class I molecules suggested that the Q10 gene encodes a secreted protein. We have established this by showing that L cells transformed with an expression vector containing the Q10 gene secrete a class I molecule which was identified with an antiserum raised against a peptide predicted by the Q10 transmembrane exon. Both the L cell-derived Q10 molecule and a class I protein immunoprecipitated from serum with this anti-peptide antiserum have mol. wts. of approximately 38 000; the Q10 molecule secreted by L cells is heterogeneous in mol. wt. This heterogeneity was drastically reduced after endoglycosidase F treatment, suggesting that Q10 molecules secreted into the serum by the liver may be glycosylated differently from those secreted by L cells. Endoglycosidase F treatment of both the L cell and serum forms of the soluble molecule yielded two products with mol. wts. of approximately 32 000 and 35 000; this is consistent with the observation that the predicted Q10 protein sequence has two potential glycosylation sites. In contrast to previous published results, the Q10 molecule reacted with rabbit anti-H-2 antisera which is consistent with its greater than 80% homology to the classical transplantation antigens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aley S. B., Barnwell J. W., Daniel W., Howard R. J. Identification of parasite proteins in a membrane preparation enriched for the surface membrane of erythrocytes infected with Plasmodium knowlesi. Mol Biochem Parasitol. 1984 May;12(1):69–84. doi: 10.1016/0166-6851(84)90045-8. [DOI] [PubMed] [Google Scholar]
- Cosman D., Khoury G., Jay G. Three classes of mouse H-2 messenger RNA distinguished by analysis of cDNA clones. Nature. 1982 Jan 7;295(5844):73–76. doi: 10.1038/295073a0. [DOI] [PubMed] [Google Scholar]
- Cosman D., Kress M., Khoury G., Jay G. Tissue-specific expression of an unusual H-2 (class I)-related gene. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4947–4951. doi: 10.1073/pnas.79.16.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan E. P., Schwartz B. D., Cullen S. E. Murine I-Ak alpha-chain subspecies with glycosylation differences. J Immunol. 1982 May;128(5):2019–2025. [PubMed] [Google Scholar]
- Cullen S. E., Kindle C. S., Shreffler D. C., Cowing C. Differential glycosylation of murine B cell and spleen adherent cell Ia antigens. J Immunol. 1981 Oct;127(4):1478–1484. [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Kress M., Cosman D., Khoury G., Jay G. Secretion of a transplantation-related antigen. Cell. 1983 Aug;34(1):189–196. doi: 10.1016/0092-8674(83)90149-6. [DOI] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E., Barra Y., Jay G. Detection of a secreted form of the murine H-2 class I antigen with an antibody against its predicted carboxyl terminus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1216–1220. doi: 10.1073/pnas.81.4.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Kress M., Jay G., Flavell R. A. A nonpolymorphic class I gene in the murine major histocompatibility complex. Cell. 1984 Jan;36(1):139–144. doi: 10.1016/0092-8674(84)90082-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Norstedt G., Gelinas R. E., Hammer R. E., Brinster R. L. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983 Nov 18;222(4625):809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Isolation of H-2 alloantigens solubilized by the detergent NP-40. J Immunol. 1971 Nov;107(5):1363–1367. [PubMed] [Google Scholar]
- Soloski M. J., Uhr J. W., Vitetta E. S. Primary structural studies of the Qa-2 alloantigen: implications for the evolution of the MHC. Nature. 1982 Apr 22;296(5859):759–761. doi: 10.1038/296759a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Moore K. W., Frelinger J. G., Sher B. T., Shen F. W., Boyse E. A., Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. doi: 10.1016/0092-8674(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto A., Steinmetz M., Hood L. Genetic mapping in the major histocompatibility complex by restriction enzyme site polymorphisms: most mouse class I genes map to the Tla complex. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3425–3429. doi: 10.1073/pnas.80.11.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Stockert E., Old L. J., Nathenson S. G. Structural comparisons of TL antigens derived from normal and leukemia cells of Tl+ and TL- strains and relationship to genetically linked H-2 major histocompatibility complex products. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7078–7082. doi: 10.1073/pnas.78.11.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]









