Abstract
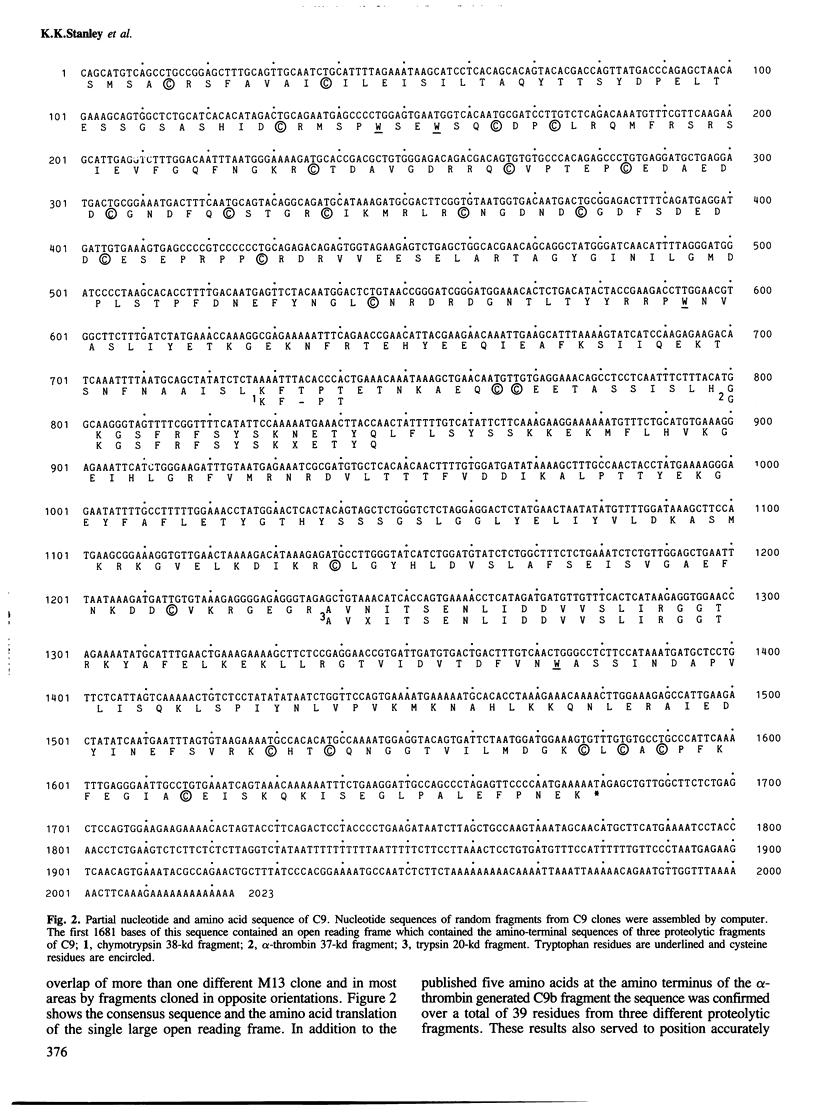
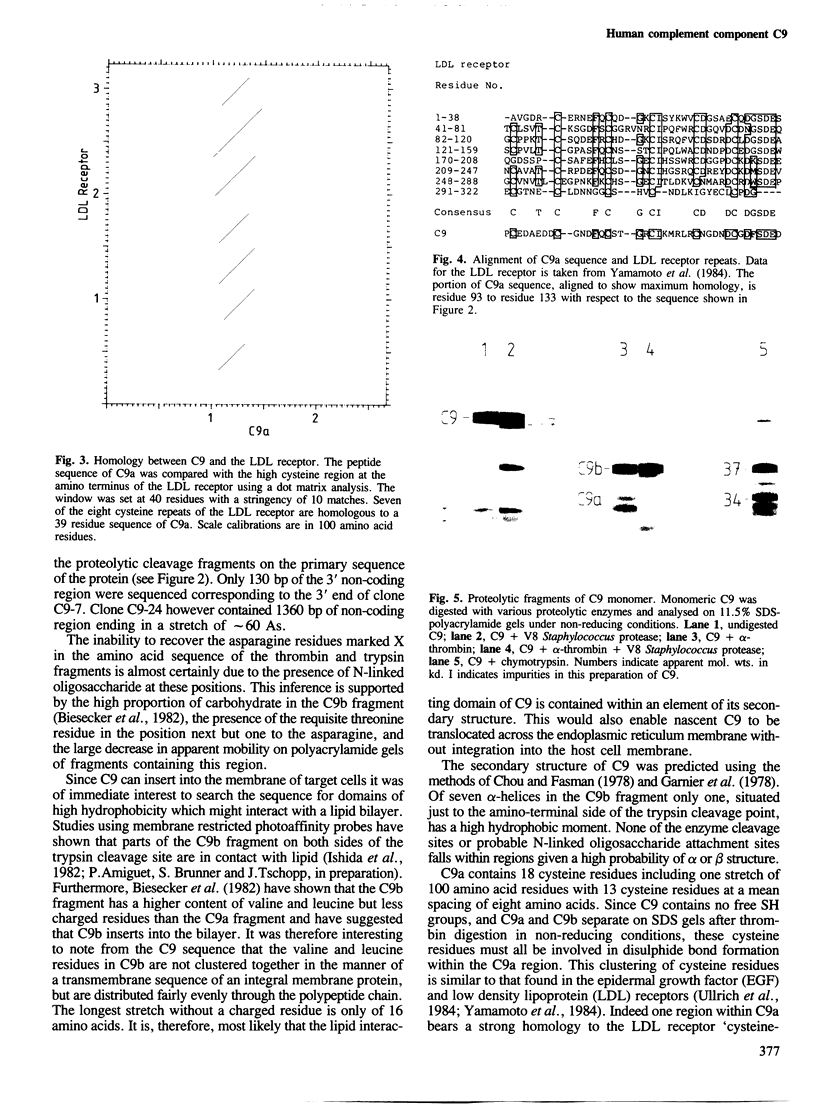
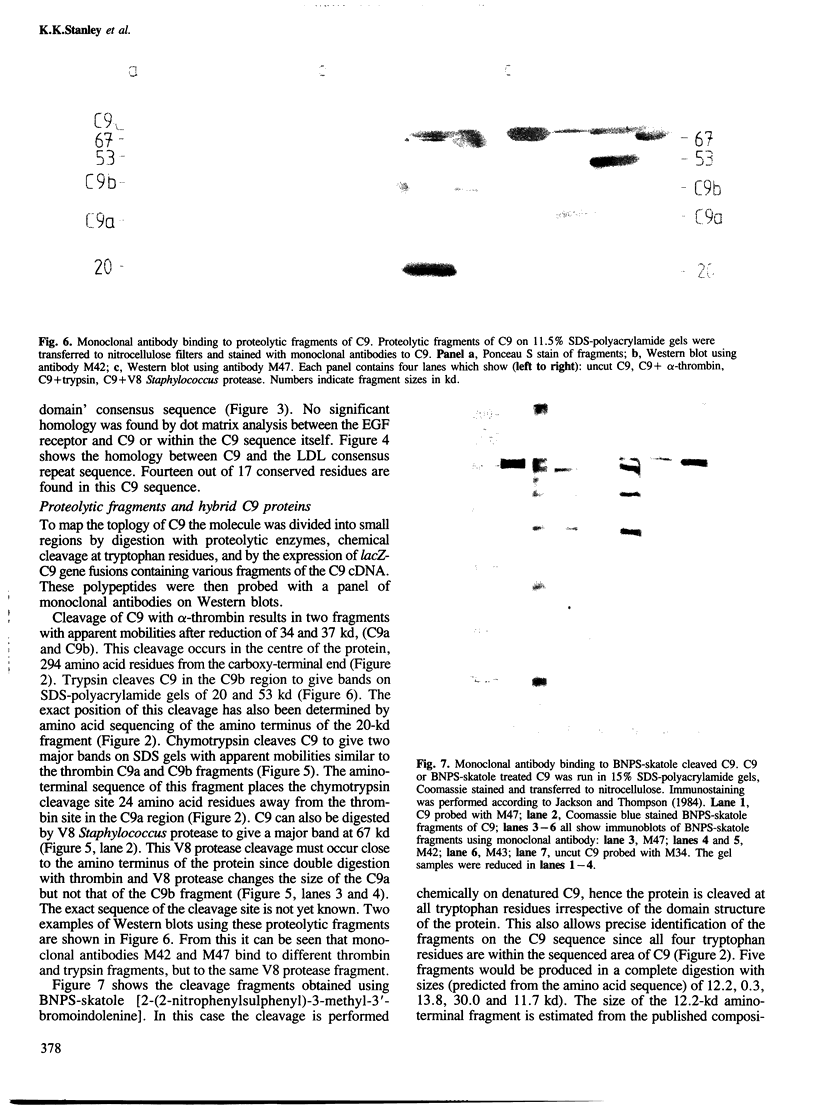
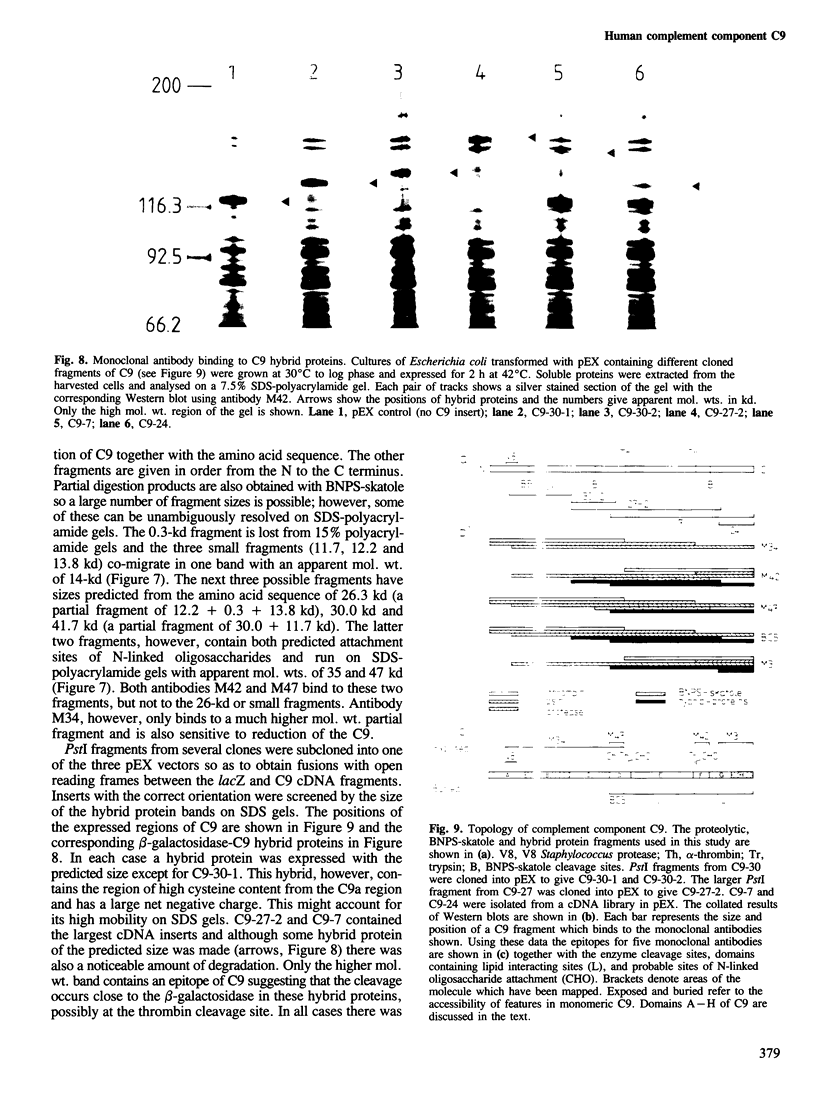
A partial nucleotide sequence of human complement component C9 cDNA representing 94% of the coding region of the mature protein is presented. The amino acid sequence predicted from the open reading frame of this cDNA concurs with the amino acid sequence at the amino-terminal end of three proteolytic fragments of purified C9 protein. No long stretches of hydrophobic residues are present, even in the carboxy-terminal half of the molecule which reacts with lipid-soluble photoaffinity probes. Monoclonal antibody epitopes have been mapped by comparing overlapping fragments of C9 molecule to which the antibodies bind on Western blots. Several of these epitopes map to small regions containing other surface features (e.g., proteolytic cleavage sites and N-linked oligosaccharide). The amino-terminal half of C9 is rich in cysteine residues and contains a region with a high level of homology to the LDL receptor cysteine-rich domains. A model for C9 topology based on these findings is proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Ey P., Bhakdi-Lehnen B. Isolation of the terminal complement complex from target sheep erythrocyte membranes. Biochim Biophys Acta. 1976 Feb 6;419(3):445–457. doi: 10.1016/0005-2736(76)90258-3. [DOI] [PubMed] [Google Scholar]
- Biesecker G., Gerard C., Hugli T. E. An amphiphilic structure of the ninth component of human complement. Evidence from analysis of fragments produced by alpha-thrombin. J Biol Chem. 1982 Mar 10;257(5):2584–2590. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D. C., Jaton J. C. Identical VL region sequences of two antibodies from two outbred rabbits exhibiting complete idiotypic cross-reactivity and probably the same antigen-binding site fine structure. J Immunol. 1978 Sep;121(3):1194–1198. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Drenth J., Low B. W., Richardson J. S., Wright C. S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10;255(7):2652–2655. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hadding U., Müller-Eberhard H. J. The ninth component of human complement: isolation, description and mode of action. Immunology. 1969 Jun;16(6):719–735. [PMC free article] [PubMed] [Google Scholar]
- Ishida B., Wisnieski B. J., Lavine C. H., Esser A. F. Photolabeling of a hydrophobic domain of the ninth component of human complement. J Biol Chem. 1982 Sep 25;257(18):10551–10553. [PubMed] [Google Scholar]
- Lundblad R. L., Kingdon H. S., Mann K. G. Thrombin. Methods Enzymol. 1976;45:156–176. doi: 10.1016/s0076-6879(76)45017-6. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Daw R. A., Siddle K., Luzio J. P., Campbell A. K. Immunoaffinity purification of human complement component C9 using monoclonal antibodies. J Immunol Methods. 1983 Nov 25;64(3):269–281. doi: 10.1016/0022-1759(83)90434-9. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Luzio J. P., Campbell A. K. Inhibition of complement-induced [14C]sucrose release by intracellular and extracellular monoclonal antibodies to C9: evidence that C9 is a transmembrane protein. Biochem Biophys Res Commun. 1984 Jan 30;118(2):616–622. doi: 10.1016/0006-291x(84)91347-0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Tschoop J., Müller-Eberhard H. J. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. doi: 10.1084/jem.156.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Circular polymerization of the ninth component of complement. Ring closure of the tubular complex confers resistance to detergent dissociation and to proteolytic degradation. J Biol Chem. 1982 Dec 25;257(24):15204–15212. [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc Natl Acad Sci U S A. 1982 Jan;79(2):574–578. doi: 10.1073/pnas.79.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan M., Darbre A. Identification of amino acid thiohydantoins directly by thin-layer chromatography and indirectly by gas-liquid chromatography after hydrolysis. Biochem J. 1975 Jun;147(3):435–438. doi: 10.1042/bj1470435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. I., Packman C. H., Leddy J. P. Inhibition of the lytic action of cell-bound terminal complement components by human high density lipoproteins and apoproteins. J Clin Invest. 1983 Apr;71(4):795–808. doi: 10.1172/JCI110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J. Circular polymerization of the membranolytic ninth component of complement. Dependence on metal ions. J Biol Chem. 1984 Aug 25;259(16):10569–10573. [PubMed] [Google Scholar]
- Tschopp J., Engel A., Podack E. R. Molecular weight of poly(C9). 12 to 18 C9 molecules form the transmembrane channel of complement. J Biol Chem. 1984 Feb 10;259(3):1922–1928. [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J. Ultrastructure of the membrane attack complex of complement. Heterogeneity of the complex caused by different degree of C9 polymerization. J Biol Chem. 1984 Jun 25;259(12):7857–7863. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. S., Gavilanes F., Peterson D. L. Primary structure of wheat germ agglutinin isolectin 2. Peptide order deduced from X-ray structure. Biochemistry. 1984 Jan 17;23(2):280–287. doi: 10.1021/bi00297a017. [DOI] [PubMed] [Google Scholar]
- Wright C. S. The crystal structure of wheat germ agglutinin at 2-2 A resolution. J Mol Biol. 1977 Apr 25;111(4):439–457. doi: 10.1016/s0022-2836(77)80063-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Migita S. Proteolysis of the monomeric and dimeric C5b-9 complexes of complement: alteration in the susceptibility to proteases of the C9 subunits associated with C5b-9 dimerization. J Immunol. 1981 Aug;127(2):423–426. [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]