Abstract
Globally, lung cancer remains the leading cause of cancer-related death. Annual low-dose computed tomography has been recommended as a screening test for early detection of lung cancers. Implementing this screening strategy is expected to challenge pulmonologist to confirm the nature of the increasing number of detected pulmonary nodules. Clinicians are obliged to use the less invasive and most efficient and safe means to set diagnoses. Hence, the field of diagnostic modalities, especially the advanced diagnostic bronchoscopy is witnessing rapid evolution to fulfill these unmet needs. This review highlights the available diagnostic modalities, describes their advantages and discusses the limitations of each technique. It also suggests an integrated diagnostic algorithm based on the best available evidence. A search of the PubMed database was conducted using relevant terms described at methodology; only articles in English were reviewed by November 2016.
Keywords: Bronchoscopic modalities, image-guided transthoracic needle aspiration, interventional pulmonology, lung cancer, navigation bronchoscopy, peripheral lung nodule
Lung cancer remains the second most common malignancy affecting both genders.[1] It accounts for more deaths than any other malignancy, with 1.59 million deaths/year worldwide.[2] Early diagnosis of lung cancer heavily influences the disease's prognosis: The medium 5 years survival in stage IA nonsmall cell lung cancer (NSCLC) is 59 months compared to only 4 months in stage IV disease.[3]
Different screening strategies in the past failed to detect lung cancer early enough to allow for mortality reduction. It was not until the American National Lung Screening Trial (NLST) has shown a 20% reduction of the lung cancer-related mortality by using a low-dose chest CT (LDCT) in high-risk group of patients, that a true screening plan emerged as a possibility.[4] Thereafter, guidelines from many scientific societies recommend an annual LDCT screening of individuals aged 55–74 having a pretest high risk to develop lung cancer.[5]
Following these recommendations, the number of the detected peripheral lung nodules and other lesions is expected to increase significantly. In fact, NLST study showed that 39.1% of the patients in the low-dose CT arm had at least one positive screening result.[4] As the CT specificity for lung cancer remains disappointingly low, these nodules require further evaluation and confirmation, either by noninvasive follow-up imaging or by invasive modalities such as CT-guided fine-needle aspiration, bronchoscopic biopsies, or even surgical excision.
Peripheral lung nodules have always been a challenge to bronchoscopists. Studies have shown that the sensitivity of bronchoscopy ranges from 63% to 34% for peripheral lung nodules depending on whether their size is more or less than 2 cm, respectively.[6] To improve the unacceptably low sensitivity of the standard bronchoscopic approache, innovative bronchoscopic modalities have been developed to facilitate accurate localization of the peripheral pulmonary lesions (PPLs). In this article, we are presenting a review of the contemporary modalities for diagnosis of peripheral lung lesions.
Methods
A search of the PubMed database was conducted using the terms; “peripheral pulmonary nodule,” “Peripheral lung lesions,” “transthoracic needle aspiration,” “transcutaneous needle aspiration,” “CT-Guided transthoracic lung biopsy,” “bronchoscopy,” “flexible bronchoscopy,” “fiber optic bronchoscopy,” “navigation bronchoscopy,” “endobronchial ultrasound,” “radial probe EBUS,” “virtual bronchoscopy,” “virtual bronchoscopic navigation,” “thin bronchoscopy,” “ultrathin bronchoscopy,” “electromagnetic navigation,” and “trans-parenchymal nodule access.” Only articles in English were reviewed by November 2016.
Indications for sampling peripheral pulmonary lesions
The American College of Chest Physicians (ACCP) guidelines emphasize on using the simplest interventional method possible to confirm the diagnosis of lung cancer.[6] Bronchoscopy has high sensitivity for centrally located tumors, where histology diagnosis can be obtained in approximately 90% of cases with standard bronchoscopic techniques (forceps biopsy, bronchial brushing, or washing); therefore, it is the recommended method.[7] On the contrary, the diagnostic yield of bronchoscopy for the peripheral (nonendoscopically visible) lesions is low, especially in nodules below 2 cm of diameter.
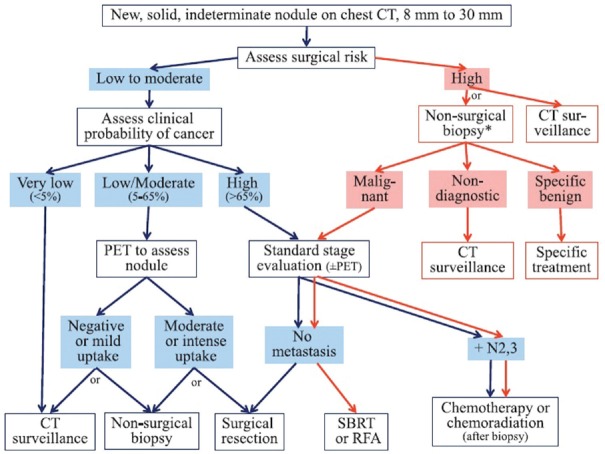
For peripheral nodules larger than 8 mm, ACCP guidelines suggest an algorithm based on the pretest probability of malignancy.[8] Despite the presence of different modules to estimate the pretest probability of malignancy, the module developed by Swensen et al.[9] is the most extensively validated one. This module is based on six variables which include older age, smoking history (current or past), history of extrathoracic cancer in the past 5 years, diameter of the nodule by millimeters, presence of speculation, and upper lobe location (upper lobes being of increased risk). ACCP guidelines recommend surveillance with serial CT when the clinical probability of malignancy is very low (<5%).[8] When pretest probability of malignancy is low to moderate (5%–65%) functional imaging (positron emission tomography scan) is suggested. When the probability of malignancy is high (more than 65%), surgical diagnosis is indicated[8] [Figure 1].
Figure 1.
Algorithm for the management of pulmonary nodules. Diagnosis and management of lung cancer 3rd ed: American College of Chest Physicians guidelines[8]
Nonsurgical biopsy is advised in the following situations:[8]
In low to moderate (~10%–60%) clinical probability of malignancy
When discordance between clinical pretest probability and imaging tests occurs
If malignant growth on serial subsequent imaging is documented
On patient request to confirm the malignant diagnosis before surgery
When physician is suspecting a benign diagnosis that requires specific medical treatment.
Selection of the biopsy modality (transthoracic or bronchoscopic access) is left for the clinician to tailor according to clinical variables, complication risk, equipment availability, and expertise.
Expert clinicians can suspect malignant lesions with high accuracy; however, a diagnosis of lung cancer should not be made without definitive pathology. Targeted therapies of specific mutations have revolutionized the treatment of the lung cancer and change its dismal prognosis. That carries the bronchoscopist an additional task of obtaining a sample large enough for supplemental immunohistochemical and genetic analysis. For these reasons, specific emphasis is currently put in diagnostic modalities for the diagnosis and staging of malignant PPLs.
Diagnostic modalities
Image guided transthoracic needle aspiration
Fluoroscopy, ultrasound (US), or CT scan has been used to guide the biopsy needle, CT-guided transthoracic needle aspiration (CT-TTNA) being the most commonly used modality. Both fine-needle aspiration and core biopsy for cytological and histologic evaluation can be obtained through the transthoracic approaches. TTNA can virtually reach any region of the lung; however, technical difficulties can be encountered in lesions located beneath a bone or a vascular structure with possible increase in the complication rate.[10]
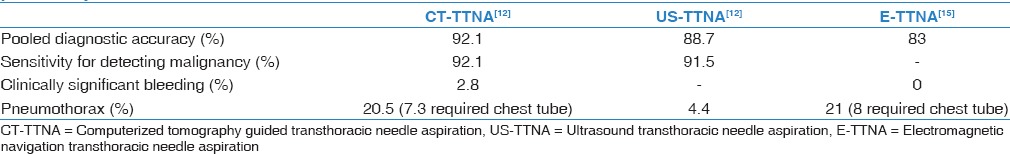
The sensitivity and diagnostic accuracy of image-guided TTNA are high [Table 1]. Nevertheless, these procedures carry a substantial risk for complications in particular pneumothorax which requires chest tube treatment in 7.3% of the cases. The distance of the nodule from the entry point is the major determinant to the pneumothorax risk.[11] However, chest tube placement was more required in patients' suffering from emphysema and obstructive lung disease.[11] Studies have shown that US-TTNA has better safety profile than CT-TTNA; this can be explained by the fact that US guided biopsies are only performed on nodules that have a direct contact with pleura.[12]
Table 1.
Comparison of image guided transthoracic needle aspiration modalities for diagnosing peripheral pulmonary lesion
Seeding and spreading of cancer cells through the needle tract is another serious concern; a study demonstrated a 60% frequency of malignant cell spread.[13] The growth of these cells to mass has only been reported in scarce case reports.[14]
The electromagnetic navigation-TTNA (E-TTNA) is a novel modality to access peripheral lung nodules via transthoracic approach. In a recently published pilot study E-TTNA was performed in 23 out of 24 enrolled subjects. The risk of pneumothorax was comparable to other transthoracic modalities, however, higher than transbronchial ones. 21% of these cases developed pneumothoraces, and 8% of them required drainage. No bleeding or hemoptysis events were recorded. The diagnostic yield of E-TTNA was 83% when used alone and increased to 87% and 92% when combined with navigation bronchoscopy (NB) and NB with endobronchial US (EBUS) respectively.[15] Pooled data from published literature are presented in Table 1.
Bronchoscopic modalities
Conventional flexible bronchoscopy
Lung lesions are termed peripheral if they are located within 3 cm of a costal pleural surface[16] (in the outer third of the lung).[17] These lesions are not visible by the conventional flexible bronchoscopy; hence, the poor diagnostic results achieved bronchoscopically compared to central lesions.[6] Diagnostic yield for the unguided flexible bronchoscopy in such lesions reported to be <20%.[18] Using more than one sampling technique adds to the diagnostic value of bronchoscopy[19] [Figure 2].
Figure 2.
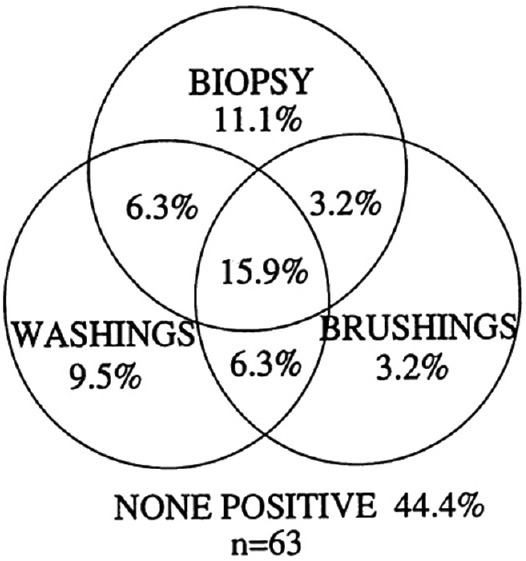
Diagnostic value of combined conventional bronchoscopic techniques for sampling peripheral pulmonary lesion. Adapted from source 19
Nondiagnostic or nonconclusive procedures have always been a major concern for the physicians and included among the “side effects” of suboptimal bronchoscopy. Given the availability of the contemporary bronchoscopic navigation modalities, it is no more acceptable to blindly “fish” for a peripheral nodule without any guidance, especially if the nodule size is <2 cm.
Navigation bronchoscopy
Fluoroscopy guided flexible bronchoscopy
Fluoroscopy has been used since the early 70's to navigate the bronchoscope to endoscopically unseen lesions.[20] Despite fluoroscopy use and by combining all tissue acquisition techniques (brushing, transbronchial needle aspiration [TBNA], transbronchial biopsies [TBB] and bronchoalveolar lavage [BAL]) bronchoscopy only achieves an overall sensitivity of 78% whereas for lesions <2 cm is 34%.[6]
Many factors affect the sensitivity of bronchoscopic biopsies. These factors are either related to the lesion itself as location, size, and nature of the lesion (either benign or malignant) or to the method of sample acquisition (technique used and number of biopsies).
The size of the peripheral lesion is the most influencing factor on the sensitivity of the bronchoscopic biopsies. Studies have shown that conventional bronchoscopy loses more than 50% of its sensitivity when the size of the peripheral lesion is <2 cm.[6] On the contrary, the presence of bronchus sign (CT finding of an airway leading to the lesion) significantly increases the diagnostic yield of the bronchoscopic procedure.[17]
Lesion size, location, and malignant nature are factors which cannot be modified by the bronchoscopist, however, diagnostic accuracy of bronchoscopy can be increased by combining different sampling techniques and by obtaining more biopsies. Among the available sampling methods, TBNA has the highest sensitivity (65%), whereas the poorest sensitivity (43%) is reported with BAL/washings.[6] TBB and brushing have a sensitivity of 57% and 54%, respectively.[6] The diagnostic accuracy of TBB increases in a stepwise manner through the sixth forceps biopsy.[21]
The main drawback for the wider application of this method apart of its cost and implementation logistics is the increased radiation exposure for both the patient and the medical personnel.[22] A prolonged learning curve is required for trainee to master this technique, which is usually available at a highly specialized well-equipped reference centers [Figure 3].
Figure 3.
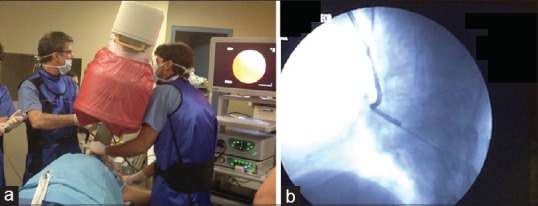
(a) The setting needed to perform fluoroscopic guided bronchoscopy. (b) Bronchoscopic biopsy of a peripheral lesion under fluoroscopic guidance
Radial probe-endobronchial ultrasound
Radial probe-EBUS is an US transducer probe that can be inserted through the working channel of a standard flexible bronchoscope to generate a 360° (radial) ultrasonic view.[23] At standard frequency of 20 MHz, the spatial resolution is <1 mm and penetration depth is 4–5 cm.[23] Normal lung tissue has been described as “a snow storm appearance,” whereas the peripheral lesions appear darker (hypo-echoic) and more homogenous.
After a careful review of the CT image a flexible bronchoscope is advanced to the targeted sub-segment with or without fluoroscopy aid. Once the scope is in wedge position, the EBUS probe is inserted via a disposable guide sheath (extended working channel) through the working channel of the scope. EBUS probe has to be advanced in different bronchi where the lesion is suspected to be found. Once the peripheral lesion is identified the guide sheath is fixed in place, the radial probe is removed, and the biopsy tools are introduced to sample the lesion.[24,25]
A meta-analysis of 16 studies (1420 subjects) reports an overall sensitivity of RP-EBUS of 73%.[18] RP-EBUS thus has higher sensitivity than fluoroscopic guidance in peripheral lesions <2 cm. In fact, a prospective randomized trial comparing RP-EBUS-guided TBLB to conventional TBLB, confirmed the superiority of the RP-EBUS with a diagnostic yield of 79% versus 55%, respectively.[26] However, many prospective studies have shown both modalities being inferior to CT-TTNA [Table 2].
Table 2.
Comparison of diagnostic performance of various combinations of bronchoscopic and guiding modalities
On the other hand, the advantage of the bronchoscopic over the transthoracic approach is its safety profile. RP-EBUS has a clinically significant bleeding risk close to zero, and 1% risk of pneumothorax compared to 2.8% and 20.5% respectively [Table 1].[12,18] The second advantage of the bronchoscopic approach is the possibility of thorough examination of the airways which can lead to discovery of endoscopically visible lesions in up to 12.6% of the cases.[27] Adding to the above, this approach has the advantage to potentially stage the lung cancer in the same procedure by using convex EBUS-TBNA for the assessment of the mediastinal lymph nodes. Thus, a complete single bronchoscopic examination can promptly and safely provide diagnostic information for both peripheral and centrally located lesions as well as mediastinal involvement.
When using the RP-EBUS searching for a small lung nodule, positioning the probe within the bronchus inside the lesion is crucial as failing to do so, significantly decreases the diagnostic yield.[28] If the bronchoscopist manages to position the probe within the lesion, the diagnostic yield is expected to be 83% compared to 61% and 4% if the probe is adjacent or far from the lesion, respectively.[28] Use of the TBNA through the extended working channel increases the diagnostic yield of the conventional techniques especially if the bronchoscopist was not able to position the probe within the lesion[29] [Figure 4].
Figure 4.
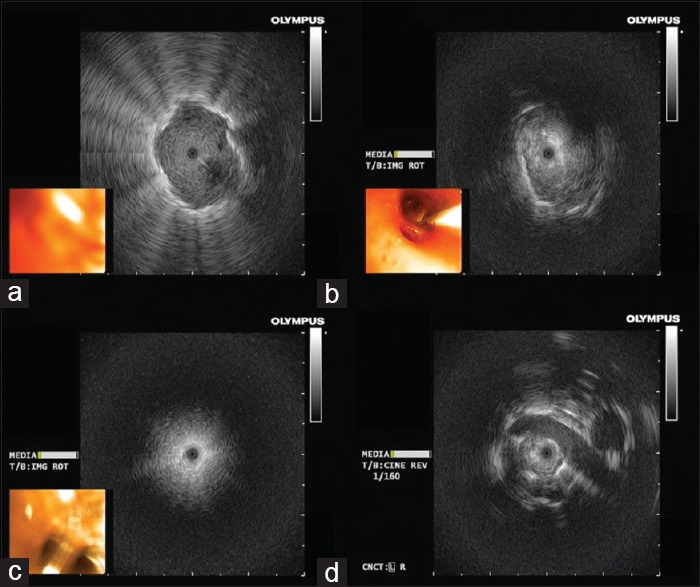
(a) Ultrasound radial probe located within the lesion, (b) ultrasound radial probe adjacent to the lesion (from 4 o'clock to 9 o'clock), (c) is radial probe ultrasonic view in normal lung parenchyma, (d) a vascular structure in relation to the lesion
Diagnostic accuracy of RP-EBUS TBB increases with each added biopsy. In a recent study, at least 5 biopsies were needed to achieve a diagnostic yield of 97%, thereafter the diagnostic yield of the biopsies plateaued.[28]
A major disadvantage to this technique is the lack of navigation system to guide the bronchoscope within the bronchial tree. The operator has to map his way based on the previously obtained CT images. In several studies, RP-EBUS was unable to localize the lesion in 11%–24% of the cases.[30]
Another disadvantage to RP-EBUS is the cost of the disposable single use guidance sheath that is added to the cost of the radial probe which can be used for approximately 30–50 times.[31] In a recent trial, the investigators instead of using the sheath combined the RP-EBUS with fluoroscopic guidance thus reducing the cost and attaining a diagnostic accuracy of 72.5%.[31] The technique included localization of the lesion under fluoroscopy and examination of the corresponding bronchial segments by the radial US probe. In case of fluoroscopically invisible lesion, the suspected area was located by studying the CT scan.
Virtual bronchoscopic navigation and ultrathin bronchoscopes
Conventionally, the bronchoscopist has to study the two-dimensional CT images and mentally reconstruct a three-dimensional (3D) image of the bronchial anatomy before planning his path to the targeted peripheral lesion. Virtual bronchoscopy (VB) is a noncontrast CT imaging-based technique where CT images of specific resolution and thickness are processed by a software which creates a virtual 3D bronchial tree map.
Virtual bronchoscopic navigation (VBN) is a modality that utilizes VB imaging to guide the bronchoscope into a PPL. The process of performing VBN includes three steps:
Creating a VB image
Synchronizing VB image with real-time bronchoscopic image
Sampling the PPL by biopsy, brush and lavage.
The major disadvantage of this system is the lack of real-time tracing of the scope during navigation and inability of confirming the location within the lesion; hence other modalities as fluoroscopy, RP-EBUS are usually combined with VBN.
In a prospective study, patients were randomized to VBN-assisted RP-EBUS or non-VBN-assisted RP-EBUS group; both groups utilized a guide sheath and fluoroscopy. VBN-assisted RP-EBUS group has a higher diagnostic yield (80.4%) and shorter examination duration (median 24 min) compared to non-VBN-assisted RP-EBUS arm with diagnostic yield of 67% and median duration of 26.2 min.[32]
Ultrathin bronchoscope (UB) (≤2.8 mm outer diameter) seems a reasonable addition to enable the bronchoscopist to reach as far as the VBN bronchial map leads, as in some cases regular size scope cannot follow the indicated route. However, a smaller working channel is the direct consequence of smaller size scopes and hence smaller biopsy samples are expected. UB was able to visualize an extra 10% of peripheral lesions which could not be visualized using standard scope.[33] The combination of both UB and VBN has been studied in a prospective study where the investigators used a UB and randomized the patients into VBN-assisted and non-VBN assisted group. In this study, VBN assisted UB was not shown to significantly improve the diagnostic yield for PPL.[34] The investigators further conducted a subgroup analysis which suggested that VBN combined with UB could improve the diagnostic yield when the lesion was located in the right upper lobe; at the peripheral third of the lung or was invisible on CXR.[34] In another prospective, multicenter, randomized study, a new prototype ultrathin hybrid broncho-fiber video scope (Y-0025; Olympus Medical Systems, Tokyo, Japan) of 3mm outer diameter was compared to 4mm thin bronchoscope using a multimodality approach (EBUS, VBN, and fluoroscopic guidance). The investigators found this UB which has a 1.7 mm working channel (enabling passing of a 1.4 mm radial ultrasonic probe) and a 1.5 mm biopsy forceps, when compared to the 4 mm scope both using an RP approach, had a significant higher diagnostic yield (74% vs. 59%, respectively).[35]
The idea of combining the available diagnostic modalities is not new. In fact, combining fluoroscopy with the conventional flexible bronchoscopy since the 70's and gradually adding modern guidance techniques, permitted physicians to increase their diagnostic yield from <20% to >70% nowadays.
Electromagnetic navigation bronchoscopy
Bronchoscopic electromagnetic navigation is using similar technology to the global positioning system used to navigate cars. This novel technology surpasses the VBN by incorporating VB images with an electromagnetic navigation system. Here the 3D reconstructed image is superimposed on the real anatomy of the patient through magnetic field created around the patient and a sensor device detecting the location and the orientation within this magnetic field.
A recently published meta-analysis of 17 studies of 1,106 patients with PPLs showed that the pooled sensitivity of the electromagnetic navigation bronchoscopy (ENB) is 82%.[36] Some of the included studies in this meta-analysis used additional techniques to improve the diagnostic yield such as rapid on-site evaluation, fluoroscopy and radial probe EBUS. The diagnostic yield in studies where ENB used as a sole navigational modality ranged from 62.5% to 77.4%. The material obtained with this technique has been proven adequate for molecular assessment and mutation tracking.
The best evidence available with head to head comparison of ENB to RP-EBUS comes from a randomized trial where the diagnostic yield was 59% and 69%, respectively, whereas combining both modalities increased the yield to 88%.[30] The diagnostic yield of ENB drops significantly if the lesion is located at the lower lobes.[30,36,37]
In Eberhardt's study, the diagnostic yield of ENB for lower lobes lesions was only 29%. It is important to mention that 35.9% of the lesions in ENB arm were in the lower lobes, which explains the low overall diagnostic yield. Decreased diagnostic yield of the ENB in lower lobes is explained by the navigation error due to diaphragmatic movement during breathing which is one of the major limitations of the method and is estimated to change the lesion's position by 17.6 mm in average.[38] Consequently, intravenous general anesthesia and muscle relaxation has been advocated by the majority of the authors so far leading to higher cost and increased logistic requirements.[36] Nevertheless, the very high implementation and maintenance cost of this modality remains the main obstacle for a wider spread of this method worldwide.
The cumulative diagnostic accuracy of various combinations of bronchoscopic and guiding modalities is presented in Table 2.
Trans-parenchymal nodule access
Early in the year 2014, the bronchoscopic trans-paranchymal nodule access (B-TPNA) was described. The rational of this novel technique is to combine the advantages of the transthoracic approach of being able to directly reach the peripheral lesion independent of the airways anatomy while maintaining the safety profile of the bronchoscopic modalities. The feasibility and safety of this new technique was confirmed on animal models.[39]
B-TPNA is performed under general anesthesia; the described technique includes uploading CT images with special specifications (0.75-mm slice thickness and 0.5-mm overlap) to the B-TPNA software (Broncus Medical, Inc.) which can be integrated to the VBN system. The B-TPNA software generates a vessel free, straight path from an assigned point of entry (POE) in a central airway directly to the targeted lesion. The operator then uploads the preplanned procedure to the VBN system which guides the bronchoscopist while tunneling his path to the peripheral lesion. In the study, the authors used a balloon to create an opening at POE and then tunnels were created using a styleted-sheath under fused CT scan/fluoroscopic guidance. Once the sheath is placed in the targeted lesion, TBB are performed using a standard biopsy forceps.[39] Recent study on canine models shows that diagnostic yield reaches 90.3%.[40] 28 samples out of 31 were positive in the simulated sub-centimetric pulmonary nodules. Neither significant bleeding nor pneumothorax, were reported.
Conclusion
Respiratory medicine community is expecting to witness a rise in the incidence of peripheral pulmonary nodules due to newly implemented chest CT screening programs. Early diagnosis of such nodules is crucial as it substantially impacts the disease outcome. Selection between transthoracic and bronchoscopic approaches should be based on clinical and radiological features for each individual patient. Comprehensive knowledge of the available techniques and factors affecting their diagnostic yield and the possible complications is necessary to weigh the risk and benefits. The presence of high risk situations such as emphysema or bleeding diathesis should prevent transthoracic approach whereas the presence of “bronchus sign” or a fluoroscopically visible lesion should prompt a thorough bronchoscopic approach with combined modalities.
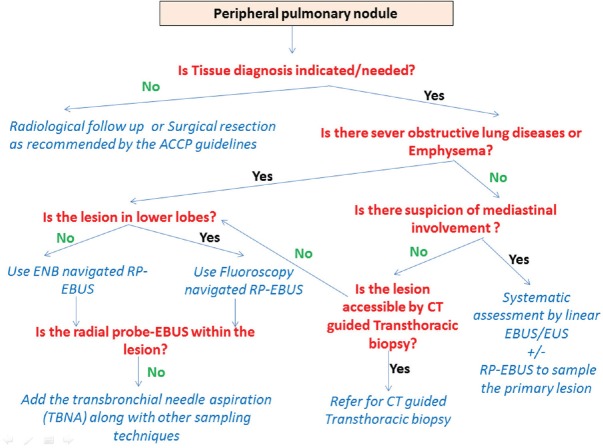
In our practice, we use a structured algorithm to target the broncoscopically occult peripheral pulmonary nodules according the presence or absence of several characteristics [Figure 5].
Figure 5.
Suggested algorithm to approach peripheral pulmonary nodules based on risk and benefits of the available techniques
Contemporary medicine's ethical commitment of providing the best available treatments for patients equally means to provide early and firm diagnosis using the best available modalities. Where facilities and expertise are available, combination of diagnostic techniques should be the standard of care in the peripherally located nodules, as various studies have proven that techniques combination increases the diagnostic yield without increasing their complication rate.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Cancer Society. Cancer facts and figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.World Health Organization. Cancer. Fact Sheet Number 297. [Last updated on 2017 Feb]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/
- 3.Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, et al. The IASLC Lung Cancer Staging Project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 4.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kauczor HU, Bonomo L, Gaga M, Nackaerts K, Peled N, Prokop M, et al. ESR/ERS white paper on lung cancer screening. Eur Respir J. 2015;46:28–39. doi: 10.1183/09031936.00033015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–65S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 7.Vansteenkiste J, De Ruysscher D, Eberhardt WE, Lim E, Senan S, Felip E, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi89–98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 8.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer. Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines? Chest. 2013;143(5 Suppl):e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swensen SJ, Silverstein, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–55. [PubMed] [Google Scholar]
- 10.Wang Y, Jiang F, Tan X, Tian P. CT-guided percutaneous transthoracic needle biopsy for paramediastinal and nonparamediastinal lung lesions: Diagnostic yield and complications in 1484 patients. Medicine (Baltimore) 2016;95:e4460. doi: 10.1097/MD.0000000000004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: Incidence and risk factors. AJR Am J Roentgenol. 1999;172:1049–53. doi: 10.2214/ajr.172.4.10587145. [DOI] [PubMed] [Google Scholar]
- 12.DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis. 2015;7(Suppl 4):S304–16. doi: 10.3978/j.issn.2072-1439.2015.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawabata N, Ohta M, Maeda H. Fine-needle aspiration cytologic technique for lung cancer has a high potential of malignant cell spread through the tract. Chest. 2000;118:936–9. doi: 10.1378/chest.118.4.936. [DOI] [PubMed] [Google Scholar]
- 14.Turaka A, Parsons RB. A rare complication of a CT-guided biopsy. J Thorac Oncol. 2011;6:1438. doi: 10.1097/JTO.0b013e318220907e. [DOI] [PubMed] [Google Scholar]
- 15.Yarmus LB, Arias S, Feller-Kopman D, Semaan R, Wang KP, Frimpong B, et al. Electromagnetic navigation transthoracic needle aspiration for the diagnosis of pulmonary nodules: A safety and feasibility pilot study. J Thorac Dis. 2016;8:186–94. doi: 10.3978/j.issn.2072-1439.2016.01.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grippi MA, Elias JA, Kotloff RM, Pack AI, Fishman JA, Senior RM. Fishman's Pulmonary Diseases and Disorders. 5th ed. New York: McGraw-Hill Education; 2015. [Google Scholar]
- 17.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117:1049–54. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 18.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: Systematic review and meta-analysis. Eur Respir J. 2011;37:902–10. doi: 10.1183/09031936.00075310. [DOI] [PubMed] [Google Scholar]
- 19.Mak VH, Johnston ID, Hetzel MR, Grubb C. Value of washings and brushings at fibreoptic bronchoscopy in the diagnosis of lung cancer. Thorax. 1990;45:373–6. doi: 10.1136/thx.45.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavala DC. Diagnostic fiberoptic bronchoscopy: Techniques and results of biopsy in 600 patients. Chest. 1975;68:12–9. doi: 10.1378/chest.68.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Popovich J, Jr, Kvale PA, Eichenhorn MS, Radke JR, Ohorodnik JM, Fine G. Diagnostic accuracy of multiple biopsies from flexible fiberoptic bronchoscopy. A comparison of central versus peripheral carcinoma. Am Rev Respir Dis. 1982;125:521–3. doi: 10.1164/arrd.1982.125.5.521. [DOI] [PubMed] [Google Scholar]
- 22.Meisinger QC, Stahl CM, Andre MP, Kinney TB, Newton IG. Radiation protection for the fluoroscopy operator and staff. AJR Am J Roentgenol. 2016;207:745–54. doi: 10.2214/AJR.16.16556. [DOI] [PubMed] [Google Scholar]
- 23.Anantham D, Koh MS, Ernst A. Endobronchial ultrasound. Respir Med. 2009;103:1406–14. doi: 10.1016/j.rmed.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Ernst A, Herth FJ. Principles and Practice of Interventional Pulmonology. New York: Springer; 2013. [Google Scholar]
- 25.Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20:972–4. doi: 10.1183/09031936.02.00032001. [DOI] [PubMed] [Google Scholar]
- 26.Paone G, Nicastri E, Lucantoni G, Dello Iacono R, Battistoni P, D'Angeli AL, et al. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest. 2005;128:3551–7. doi: 10.1378/chest.128.5.3551. [DOI] [PubMed] [Google Scholar]
- 27.Gasparini S, Ferretti M, Secchi EB, Baldelli S, Zuccatosta L, Gusella P. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses. Experience with 1,027 consecutive cases. Chest. 1995;108:131–7. doi: 10.1378/chest.108.1.131. [DOI] [PubMed] [Google Scholar]
- 28.Yamada N, Yamazaki K, Kurimoto N, Asahina H, Kikuchi E, Shinagawa N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007;132:603–8. doi: 10.1378/chest.07-0637. [DOI] [PubMed] [Google Scholar]
- 29.Chao TY, Chien MT, Lie CH, Chung YH, Wang JL, Lin MC. Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: A randomized trial. Chest. 2009;136:229–36. doi: 10.1378/chest.08-0577. [DOI] [PubMed] [Google Scholar]
- 30.Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: A randomized controlled trial. Am J Respir Crit Care Med. 2007;176:36–41. doi: 10.1164/rccm.200612-1866OC. [DOI] [PubMed] [Google Scholar]
- 31.Casutt A, Prella M, Beigelman-Aubry C, Fitting JW, Nicod L, Koutsokera A, et al. Fluoroscopic-guided radial endobronchial ultrasound without guide sheath for peripheral pulmonary lesions: A safe and efficient combination. Arch Bronconeumol. 2015;51:338–43. doi: 10.1016/j.arbres.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Ishida T, Asano F, Yamazaki K, Shinagawa N, Oizumi S, Moriya H, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: A randomised trial. Thorax. 2011;66:1072–7. doi: 10.1136/thx.2010.145490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franzen D, Diacon AH, Freitag L, Schubert PT, Wright CA, Schuurmans MM. Ultrathin bronchoscopy for solitary pulmonary lesions in a region endemic for tuberculosis: A randomised pilot trial. BMC Pulm Med. 2016;16:62. doi: 10.1186/s12890-016-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asano F, Shinagawa N, Ishida T, Shindoh J, Anzai M, Tsuzuku A, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med. 2013;188:327–33. doi: 10.1164/rccm.201211-2104OC. [DOI] [PubMed] [Google Scholar]
- 35.Oki M, Saka H, Ando M, Asano F, Kurimoto N, Morita K, et al. Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions. A randomized trial. Am J Respir Crit Care Med. 2015;192:468–76. doi: 10.1164/rccm.201502-0205OC. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Chen S, Dong X, Lei P. Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J Thorac Dis. 2015;7:799–809. doi: 10.3978/j.issn.2072-1439.2015.04.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: A systematic review and meta-analysis. Respiration. 2014;87:165–76. doi: 10.1159/000355710. [DOI] [PubMed] [Google Scholar]
- 38.Chen A, Pastis N, Furukawa B, Silvestri GA. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest. 2015;147:1275–81. doi: 10.1378/chest.14-1425. [DOI] [PubMed] [Google Scholar]
- 39.Silvestri GA, Herth FJ, Keast T, Rai L, Gibbs J, Wibowo H, et al. Feasibility and safety of bronchoscopic transparenchymal nodule access in canines: A new real-time image-guided approach to lung lesions. Chest. 2014;145:833–8. doi: 10.1378/chest.13-1971. [DOI] [PubMed] [Google Scholar]
- 40.Sterman DH, Keast T, Rai L, Gibbs J, Wibowo H, Draper J, et al. High yield of bronchoscopic transparenchymal nodule access real-time image-guided sampling in a novel model of small pulmonary nodules in canines. Chest. 2015;147:700–7. doi: 10.1378/chest.14-0724. [DOI] [PubMed] [Google Scholar]