Abstract
INTRODUCTION:
Lung cancer is among leading causes of death worldwide. Different histological types of the lung carcinoma show significant differences in behavior.
OBJECTIVES:
The aim of this study is to determine the distribution patterns of metastases of different lung cancer histological types in autopsied individuals.
METHODS:
Protocols from all autopsies performed at the Institute of Pathology from 2008 till 2014 were reviewed retrospectively, and information on individuals' age, sex, histological type of primary lung cancer, presence and location of metastases, and causes of death were recorded.
RESULTS:
More than 90% of the individuals with lung cancer metastases were older than 50 years (mean age: 64.5 ± 10.3), with two-fold male predominance. The most frequent histological type in both sexes was adenocarcinoma (48%). Although, in general, hematogenous metastases were mostly found in the liver and adrenal glands, various histological types of lung cancer show specific dissemination patterns. Metastases in adrenal glands derived mostly from adenocarcinoma and large-cell carcinoma. Metastases in the intestines most frequently originated from large-cell carcinoma (P = 0.01). Metastatic complications and bronchopneumonia were the most frequent causes of death.
CONCLUSIONS:
While, overall, the most frequent hematogenous metastases occur in the liver and adrenal glands, various histological types of lung cancer show specific dissemination patterns. Knowing distribution of metastases is essential for making algorithms of treatment, as well as for improving clinical assessment of the patients with unclear clinical findings and suspicion on occult primary lung cancer.
Keywords: Autopsy, distribution, histological type, lung cancer, metastases
Lung carcinoma is one of the leading cancer-related causes of death worldwide.[1] In Serbia, according to the data from the National Institute of Public Health “Dr. Milan Jovanović Batut,” Belgrade, lung carcinoma dominates and accounts for 25.8% of deaths due to malignant tumors in men and 7.9% (after breast and colorectal cancers) in women.[2] Heavy smokers have even 20-fold higher risk of lung cancer due to benzopyrene from tobacco and accumulated numerous genetic mutations in reserve cells.[3]
According to the World Health Organization, four main histological types of lung cancer are squamous cell carcinoma, adenocarcinoma, small-cell carcinoma, and large-cell carcinoma.[3] These histological types differ in biological behavior and prognosis, with the small-cell lung carcinoma (SCLC) often being contrasted with three remaining nonsmall-cell lung carcinomas (NSCLCs).[4,5] Lung cancer spreads both through lymph and bloodstream, and the metastases are responsible for many negative effects of this disease. Systemic effects of lung cancer's metastatic spreading can manifest as general weakness, cancer cachexia, weight loss and depletion of fat reserves, muscular atrophy, and malaise. These effects are related to tumor's secretion of a variety of cytokines that induce lipolysis and increased protein metabolism, reduce glucose uptake by the cells, and stimulate gluconeogenesis from the proteins.[6,7]
Prognosis of the disease is based on the TNM cancer staging system, where T1–T4 stands for primary tumor's spreading (increasing the tumor's size or invasive growth by direct penetration to the surrounding tissue), N0–N3 for dissemination to the regional lymph nodes, and an M0–M1 for absence or presence of distant metastases.[5,8] However, despite numerous studies investigating general mechanisms of tumors' dissemination,[9,10] epidemiology of cancer metastases is still insufficiently understood.[11] Moreover, it is useful for the clinical practice not only to explore the frequency of various histological types of the lung cancer but also tropism of the tumor cells of various histological types to different tissues and organs. By revealing differential dissemination patterns of different tumor types, it may be possible to assume the histological type of the lung cancer and its prognosis in a clinical context based on the specific distribution of the metastases.
Given that population-based cancer registries rarely record locations of metastases;[11] autopsy studies are indeed necessary to gain profound understanding of the distribution of metastatic deposits in different tumor types. In particular, autopsy reports are a relevant source of information of metastatic deposits of lung cancer that may have gone unnoticed clinically or erroneous clinical diagnoses, as well as of true causes of death.[12,13] Hence, the aim of the present study was to investigate the distribution of the metastases of the lung cancer in autopsy cases to determine the differential dissemination patterns of different histological types.
Methods
Protocols from all autopsies performed at the Institute of Pathology in the period from January 1, 2008, till December 31, 2014, were reviewed retrospectively. We recorded the information on individuals' age, sex, histological type of lung cancer, locations of lymphogenic and hematogenic metastases, as well as the cause of death. In addition, the survival time (time from clinical diagnosis to death), as well as the modes of applied anticancer treatment, were recorded where available.
Of the total number of autopsied individuals in this period, 175 (2.84%) with lung carcinoma presented with lymphogenous or hematogenous metastases. Histological type of the tumor was ascertained by hematoxylin and eosin staining, while specific immunohistochemical stainings were performed for clarification as necessary.
Statistical analysis was performed in a standard statistical software package (SPSS version 15.0, SPSS Inc., Chicago, IL, USA) and encompassed Student's t-test, ANOVA, Spearman's correlation, Chi-square, or Fisher's exact probability test. The statistical significance was set at the 0.05 level.
Results
Demographic characteristics and frequency of different histological types of primary lung cancer
Our autopsy study revealed that there were 175 individuals (2.84%) with lung cancer metastases. The average age of the autopsied individuals with lung cancer metastases was 64.5 ± 10.3 years (range: 25–89 years). There were 119 men (68%) and 56 women (32%) (P < 0.001). The mean age was 63.8 ± 10.7 years in men and 66.2 ± 9.4 years in women. There were no significant differences in age between men and women (P = 0.145). Yet, in both sexes, more than 90% of individuals showing lung cancer metastases were older than 50 [Figure 1a].
Figure 1.
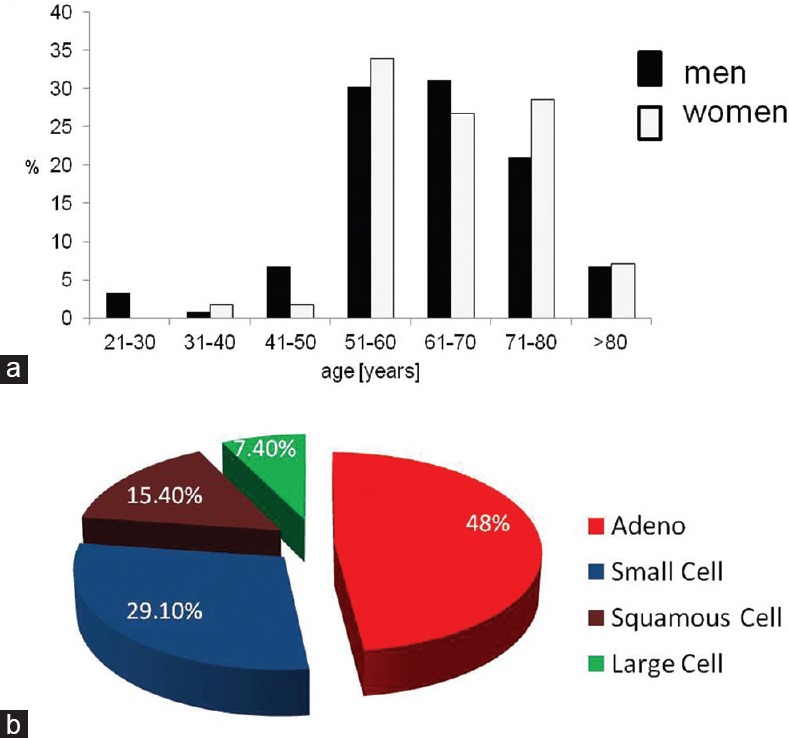
(a) Age distribution of the 175 autopsied individuals with lung cancer metastases. (b) Occurrence of various histological types of the primary lung carcinoma in 175 autopsied individuals with lung cancer metastases
Histological types of lung cancer showed a specific distribution in our sample (P < 0.001), with the most common histological type being adenocarcinoma (48%), followed by small-cell carcinoma (29.2%), squamous cell carcinoma (15.4%), and large-cell carcinoma (7.4%) [Figure 1b].
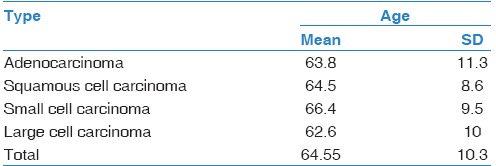
In men, adenocarcinoma (47.9%) was followed by small-cell carcinoma (27.7%), squamous cell carcinoma (16.8%), and large-cell carcinoma (7.6%). Likewise, adenocarcinoma was the most common histological type in women (48.2%), with slightly increased percentage of small-cell carcinoma (32.1%), followed by squamous cell (12.5%) and large-cell carcinoma (7.1%). There were no statistically significant differences in the distribution of histological types among the sexes (P = 0.878) [Table 1]. In addition, there were no differences in age between the individuals with different histological types of the lung cancer (ANOVA, in men: P = 0.655, in women: P = 0.872) [Table 2].
Table 1.
Distribution of histological types of lung cancer in relation to sex
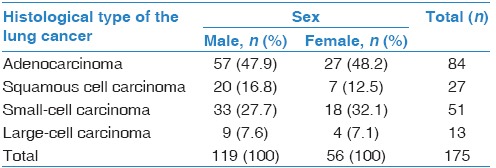
Table 2.
Age distributon in relation to histological type of the lung cancer
The time from clinical diagnosis of the lung cancer to death differed between SCLC and NSCLC (P = 0.019); namely, all individuals with SCLC died within 3 years from the diagnosis, whereas 25% of individuals with NSCLC lived more than 3 years after the clinical diagnosis. Considering four histological types of the lung cancer separately, it was evident that about 30% of individuals with adenocarcinoma or squamous cell carcinoma lived more than 3 years after clinical diagnosis, whereas no one with large-cell carcinoma or small-cell carcinoma survived 3 years (P = 0.02). In particular, large-cell carcinoma was fatal, with nearly 60% of individuals dying within the 1st year.
According to available clinical data, 17.4% of the individuals were subjected to surgical treatment (69% - adenocarcinoma, 19% - squamous cell carcinoma, and 12% - SCLC). Surgically treated individuals showed significantly improved survival time (P < 0.001): more than 70% of them lived more than 3 years, while nearly 45% survived more than 4 years. The best results were achieved in the case of adenocarcinoma: more than 80% survived more than 3 years, which was significantly higher than in nonoperated individuals (P < 0.001). All individuals with SCLC died within 3 years from the diagnosis. In addition, more than 90% of individuals received chemotherapy, either alone or combined with surgery (17.4%) or radiotherapy (9.8%).
Distribution of metastases of the primary lung carcinoma
Lymphogenous metastases were present in hilar lymph nodes in nearly half of the individuals (45.7%), followed by metastatic deposits in tracheobronchial (39.6%), paratracheal (20.6%), and paraaortic abdominal lymph nodes (11.4%). In 28 individuals, signs of these metastases were not observed. Distribution of individuals with lymphogenous metastases in relation to the tumor's type was concordant with the distribution of the histological types in the whole sample.
Lung cancer most commonly spread through bloodstream to the liver (34.3%) and adrenal glands (32.6%), followed by bones (14.9%) and other organs: central nervous system (CNS) (12%), kidney (10.9%), myocardium (9.1%), pancreas (5.1%), spleen (4%), stomach (2.3%) and small or large intestines (3.4%), thyroid gland (1.7%), and the ovary (0.6%). Age of the individuals with metastases located in the kidney, adrenal glands, myocardium, or CNS was about 5 years lower than in individuals in whom these locations were spared (i.e., on average 60 vs. 65 years; P = 0.04, P = 0.01, P = 0.04, and P = 0.02, respectively). However, the time from initial diagnosis to death was not significantly influenced, except in those with myocardial metastases who tended to die sooner than those without myocardial metastases (P = 0.06). Interestingly, all individuals with metastases in intestines died within the 1st year after clinical diagnosis of lung cancer (P = 0.023).
Distribution of metastases depending on histological type of the primary lung carcinoma
Figure 2 shows spatial distribution of the metastases in relation to the histological type of lung carcinoma. Division of the whole sample into two age groups (a group younger than 65 years and the group of elderly, i.e., 65 years and above) showed that the pattern of metastatic dissemination differed [Figure 3].
Figure 2.
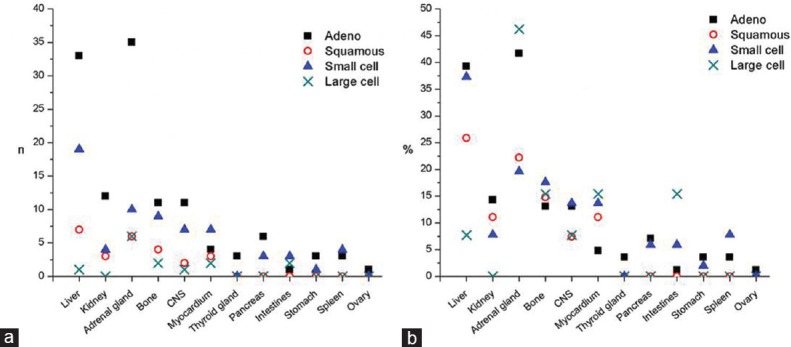
Distribution of the lung cancer metastases per histological type: (a) number (n) of individuals with a particular metastasis is shown to present the frequency of metastases from different tumor types in the particular anatomical location; (b) percentage (%) of individuals with a specific histological type of the tumor that demonstrated the particular metastases is shown to demonstrate the dissemination patterns of different histological types
Figure 3.
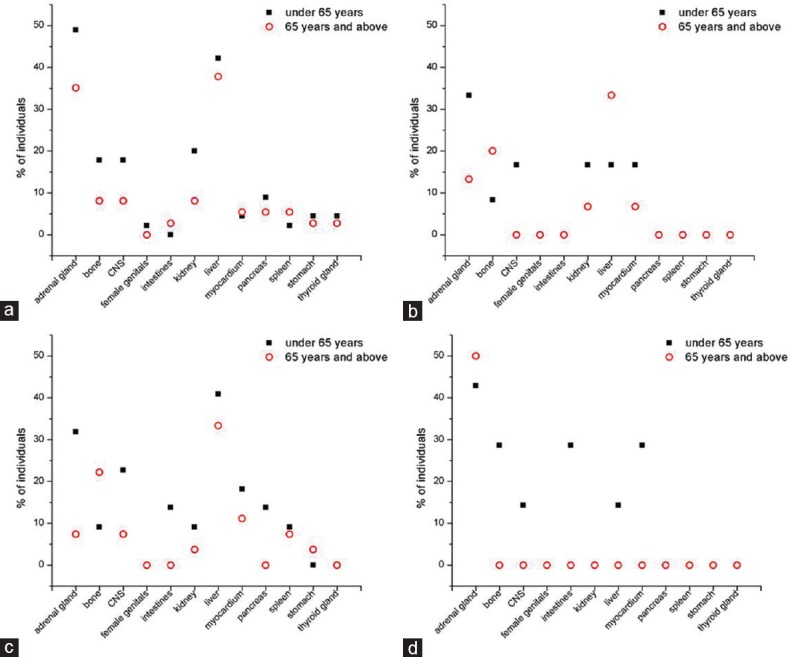
Distribution of the lung cancer metastases in relation to age group. (a) Adenocarcinoma, (b) squamous cell carcinoma, (c) small-cell carcinoma, and (d) large-cell carcinoma
Nonsmall-cell lung carcinoma
Observing the group of NSCLC, metastatic deposits were found most often in adrenal glands and the liver (37.9% and 33.1%, respectively). More than 80% of all adrenal metastases and nearly 70% of all liver metastases originated from NSCLC.
Distinguishing between different types of NSCLC revealed that each of them showed specific distribution of metastases. Adenocarcinoma most often disseminated to the liver and adrenal glands (each in more than 35% of individuals), while squamous cell carcinoma spread mostly to the liver and adrenal glands but in lower percentage (25.9% and 22.2% of individuals, respectively), commonly followed by bone (14.8%). More than 45% of individuals with large-cell carcinoma presented adrenal glands metastases, while other common metastatic locations included myocardium, intestines, and bone (each in 15% of individuals). Metastases in the intestines most frequently originated from the large-cell carcinoma and very rarely from squamous or adenocarcinoma (P = 0.041). Adrenal gland metastases were most likely to derive from adenocarcinoma (more than 60% of all adrenal metastases, P = 0.022) [Figure 2].
We further considered the distribution of metastases in individuals who died within the 1st year from the clinical diagnosis. Those with adenocarcinoma showed adrenal metastases in 54% of cases, followed by liver metastases (27%). Squamous cell carcinoma spread to the liver in 67% of cases who died during the 1st year from the diagnosis, as well as to adrenal glands and bone (33% each). Even 75% of the individuals with large-cell carcinoma who died during the 1st year had adrenal metastases, followed by intestinal and myocardial (50% each) or bone metastases (25% of individuals).
Small-cell lung carcinoma
Small-cell carcinoma's metastases were most commonly found in the liver (in more than 35% of individuals), followed by adrenal glands (nearly 20%), bone (17.6%), and CNS and myocardium (13.7% each). Of note, in individuals who died within the 1st year from the diagnosis, 33% had liver metastases, and 22% had intestinal metastases.
Mutual correlations between the presence of metastatic deposits in different anatomical locations in each histological type of the tumor
Nonsmall-cell lung carcinoma
In adenocarcinoma, the presence of liver metastases correlated with the presence of kidney and pancreatic metastases (ρ = 0.299, P = 0.006; ρ = 0.250, P = 0.022, respectively). Kidney metastases correlated with CNS metastases (ρ = 0.346, P = 0.001), while adrenal gland metastases correlated with thyroid metastatic deposits (ρ = 0.228, P = 0.037). Bone metastases correlated with pancreatic and gastric metastases (ρ = 0.303, P = 0.005; ρ = 0.306, P = 0.005). Pancreatic metastases also correlated with thyroid metastases (ρ = 0.445, P < 0.001), whereas intestinal metastases correlated with splenic metastases (ρ = 0.570, P < 0.001) [Figure 4].
Figure 4.
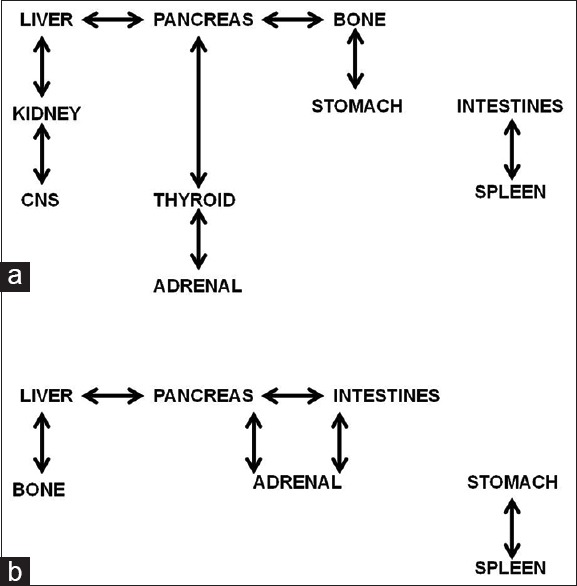
Mutual correlations in the presence of metastatic deposits between different anatomical locations. (a) Adenocarcinoma and (b) small-cell carcinoma
In squamous cell carcinoma, the presence of liver metastases was correlated with bone metastases (ρ = 0.467, P = 0.014), while renal metastases showed a strong relationship with adrenal metastases (ρ = 0.661, P < 0.001).
In large-cell carcinoma, metastases in liver were strongly correlated with the presence of bone metastases (ρ = 0.677, P = 0.011).
Small-cell lung carcinoma
In small-cell carcinoma, the presence of liver metastases moderately correlated with bone metastases (ρ = 0.282, P = 0.045) and pancreatic metastases (ρ = 0.324, P = 0.02). Adrenal metastases correlated with pancreatic and intestinal metastases (for both: ρ = 0.296, P = 0.035). Pancreatic metastases also correlated with intestinal metastases (ρ = 0.292, P = 0.038). Stomach metastases strongly correlated with spleen metastases (ρ = 0.485, P < 0.001) [Figure 4].
Causes of death
Causes of death noted in our sample were classified as metastatic complications, bronchopneumonia, acute respiratory distress syndrome, disseminated intravascular coagulation, hypovolemic shock, lung thromboembolism, pulmonary edema, and myocardial infarction. There was a statistically significant difference in frequency of individual causes of death (P < 0.001), with metastatic complications and bronchopneumonia being the most common in all tumor types [Figure 5]. There were no significant differences in cause of death between SCLC and NSCLC (P = 0.7). Yet, some patterns were noticeable: metastatic complications were the cause of death in nearly 60% of individuals with small-cell carcinoma and those with adenocarcinoma, while bronchopneumonia was responsible for from 15.4% of deaths (in the case of large-cell carcinoma) up to 33.3% (in the case of squamous cell carcinoma).
Figure 5.
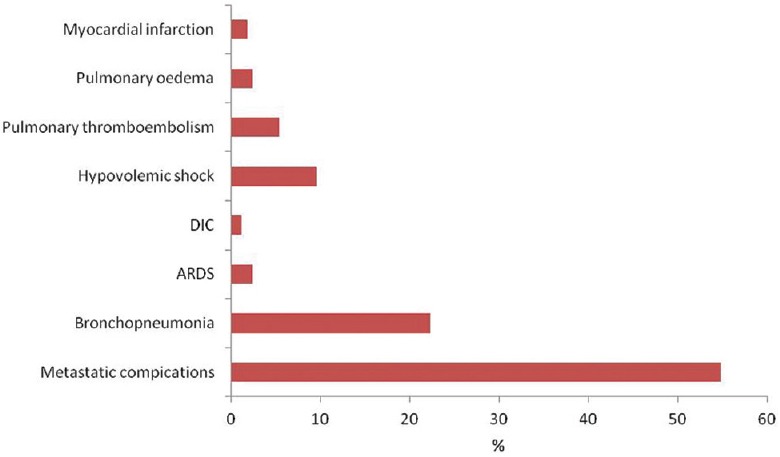
Causes of death of autopsied individuals with lung cancer metastases
Discussion
Here, we have shown on an autopsy dataset that lung adenocarcinoma is the most frequent histological type of the metastasizing primary lung cancer. This finding is in general agreement with previous worldwide reports showing that the incidence of different tumor types changed in recent years so that nowadays lung adenocarcinoma has overpowered squamous cell carcinoma.[13,14] Men are more often affected than women, and most individuals were older than 50, which is in rough agreement with other studies.[11,13] Lymphogenous metastases were most prevalent in hilar lymph nodes, whereas the most common localizations of the hematogenous metastases were in the liver, adrenal glands, and bone. Yet, our study revealed that dissemination pattern differed remarkably between the histological types of the primary tumor. Analysis of the distribution of the metastases revealed that those in small and large intestines were most commonly due to large-cell carcinoma and most rarely from squamous cell carcinoma or adenocarcinoma. On the other side, metastases in adrenal glands mostly arose from adenocarcinoma.
In our study, liver metastases were present in more than a third of individuals, out of which more than 30% originated from small-cell carcinoma. Extensive and diffuse metastases of small-cell carcinoma in the liver parenchyma were reported to potentially cause fulminant hepatic insufficiency (jaundice, coagulopathy, encephalopathy, and organs disfunction) with poor prognosis and often quick lethal outcome.[15] Accordingly, we found that 33% of the individuals with SCLC who died within the 1st year had liver metastases. Apart from small-cell carcinoma, we found that liver was also a frequent location of metastatic foci in other histological types, which is in agreement in the findings from the study of Riihimäki et al.;[11] however, our findings indicate large-cell carcinoma as an exception that rarely spreads to the liver.
Adrenal glands indeed are a frequent site of metastases of various cancers: breast cancer, lung cancer, renal carcinoma, melanoma, and lymphoma.[16] Following the initial diagnosis of nonsmall-cell lung cancer, metastases in adrenal glands were reported already in 10% of cases.[17] Furthermore, in advanced stages of the disease, nonsmall-cell lung cancer metastases are usually bilateral and appear in 40% of patients.[18] We found that in autopsied individuals, more than 60% of adrenal metastases originated from lung adenocarcinoma. In particular, more than 50% of individuals with adenocarcinoma who died during the 1st year from the diagnosis had adrenal metastases, suggesting them as an important metastatic spot related to poor survival. The explanation of this observation may be in serious complications of adrenal metastases, such as bleeding[19] or adrenal insufficiency due to the presence of a tumor, where reduced steroid hormones production may compromise diagnosis of the primary tumor.[20]
Our study revealed that bone was the third most common location of hematogenous metastases and that all the histological types of the lung cancer spread to bones. Sugiura et al. estimated the survival rates in the patients with bone metastases of the lung cancer at 9.7 months after the diagnosis.[21] Better prognosis was recorded in women, in individuals where adenocarcinoma was the histological type, in cases of solitary bone metastasis, as well as in the individuals without pathological fractures.[21]
About 25% of patients with lung carcinoma show brain metastases in advanced stages of the disease. Solitary brain metastases may clinically present with seizures, symptoms of the increased intracranial pressure, or other nonspecific symptoms even before diagnosing the lung cancer. These have a poor prognosis that strongly depends on the number of brain metastases, presence of metastases in other organs, as well as stage of the very lung cancer.[22] We showed that most cases of brain metastases occurred in individuals with adenocarcinoma that was also the most frequent types of lung cancer; yet, small-cell carcinoma presented the highest risk of brain metastases, which is in line with a previous report.[23] Our findings further showed that small-cell carcinoma was most likely to metastasize to the CNS in individuals below the age of 65 years (more than 20% of individuals); other histological types also metastasize to the brain more often in younger individuals.
Although metastases are generally rare in the heart, we found that in all histological types except adenocarcinoma more than 10% of individuals displayed heart metastases [Figure 2b]. Earlier reports that adenocarcinoma was the most common histological type among lung cancer metastases in the heart[24] could not be supported by our findings [Figure 2a]. Individuals with myocardial metastases tended to die sooner than those without myocardial metastases; particularly, 50% of individuals with large-cell carcinoma who died within the 1st year after clinical diagnosis showed cardiac metastases.
Other generally rare metastases, such as gastric, intestinal, and pancreatic metastases, were found in higher percentage than in previous studies,[25,26] which may suggest that they are just rarely diagnosed in clinical settings. Similar to an autopsy study by McNeill et al.,[26] intestinal metastases were most often from large-cell carcinoma, while adenocarcinoma and squamous cell carcinoma were rarely the sources of intestinal metastases. Interestingly, all individuals with metastases in intestines died within the 1st year after clinical diagnosis of lung cancer. One potential explanation may be related to the histological type that commonly spreads to the intestines (large-cell carcinoma).
In all histological types of the lung cancer, a tendency to either increased or similar metastatic dissemination was observed in younger versus elderly individuals in most of the anatomical locations [Figure 3]. Frequently, higher likelihood of metastasizing in younger individuals may not only signify more aggressive tumor forms but also age-dependent differential metabolic dynamics or different survival rates. In contrast to a previous study,[11] we demonstrated that bone metastases are the most common exception to this rule, given that they were more likely in the elderly than in younger individuals with squamous cell or small-cell carcinoma. This is, however, in general agreement with the previously reported age-dependent metastatic behavior of prostate, breast, and lung cancers.[27]
Analyses of mutual correlations between different metastatic locations in most histological types of the lung cancer revealed a relationship between hepatic and bone metastases (particularly strong in squamous and large-cell carcinoma). Apart from that, different histological types showed quite different correlations between metastases in various anatomical locations (intersite correlations). Namely, large-cell and squamous cell carcinoma presented few intersite correlations, whereas adenocarcinoma and small-cell carcinoma showed more developed pattern of mutual relationships between various metastases [Figure 4], which should be exploited in patient evaluation in clinical practice.
To better understand the tropism of particular cancer types for certain tissues or organs, systematic recording of the type-specific dissemination patterns should be performed. By remarkable decline of hospital autopsies in many countries,[28] valuable information for understanding the tumors' dissemination and improving clinical practice is lost. In contrast, our large autopsy study revealed differential dissemination patterns of different lung cancer types that should be further considered in clinical situations. While autopsies may not be sensitive to detect very small metastases, for example, in brain and bones, that could be detected antemortem by advanced clinical imaging tools, still autopsies provide unprecedented specificity with pathohistological confirmation of the exact tumor type and state-of-the-art determination of the cause of death.
Conclusion
In conclusion, different histological types showed differential patterns of hematogenous metastatic spreading. Understanding of distribution of the metastases in different histological types of lung cancer is important in planning the therapeutic algorithm as well as in the cases of unclear clinical presentation and suspicion on occult lung cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Batut MJ. Health Statistical Yearbook of the Republic of Serbia. Belgrade: Institute of Public Health of Serbia; 2012. [Last accessed on 2017 Feb 05]. Available from: http://www.batut.org.rs/download/publikacije/pub2012.pdf . [Google Scholar]
- 3.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669–92. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai H, Asamura H, Goya T, Eguchi K, Nakanishi Y, Sawabata N, et al. Survival differences by gender for resected non-small cell lung cancer: A retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J Thorac Oncol. 2010;5:1594–601. doi: 10.1097/JTO.0b013e3181f1923b. [DOI] [PubMed] [Google Scholar]
- 5.Asamura H, Goya T, Koshiishi Y, Eguchi K, Nakanishi Y, Sawabata N, et al. A Japanese Lung Cancer Registry study: Prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 6.Brašanac D. General pathology of tumors. In: Pathology. Belgrade: Libri Medicorum; 2003. [Google Scholar]
- 7.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 8.Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E., Jr The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol. 2012;4:128–34. doi: 10.4329/wjr.v4.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75–91. doi: 10.1007/s10555-016-9618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10(Suppl 1):S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Alfsen GC, Máhlen J. The value of autopsies for determining the cause of death. Tidsskr Nor Laegeforen. 2012;132:147–51. doi: 10.4045/tidsskr.11.0427. [DOI] [PubMed] [Google Scholar]
- 13.Morita T. A statistical study of lung cancer in the annual of pathological autopsy cases in Japan, from 1958 to 1997, with reference to time trends of lung cancer in the world. Jpn J Cancer Res. 2002;93:15–23. doi: 10.1111/j.1349-7006.2002.tb01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimi I, Ohshima A, Ajiki W, Tsukuma H, Sobue T. A comparison of trends in the incidence rate of lung cancer by histological type in the Osaka Cancer Registry, Japan and in the Surveillance, Epidemiology and End Results Program, USA. Jpn J Clin Oncol. 2003;33:98–104. doi: 10.1093/jjco/hyg019. [DOI] [PubMed] [Google Scholar]
- 15.McGuire BM, Cherwitz DL, Rabe KM, Ho SB. Small-cell carcinoma of the lung manifesting as acute hepatic failure. Mayo Clin Proc. 1997;72:133–9. doi: 10.4065/72.2.133. [DOI] [PubMed] [Google Scholar]
- 16.Brunt LM, Moley JF. Adrenal incidentaloma. World J Surg. 2001;25:905–13. doi: 10.1007/s00268-001-0029-0. [DOI] [PubMed] [Google Scholar]
- 17.Allard P, Yankaskas BC, Fletcher RH, Parker LA, Halvorsen RA., Jr Sensitivity and specificity of computed tomography for the detection of adrenal metastatic lesions among 91 autopsied lung cancer patients. Cancer. 1990;66:457–62. doi: 10.1002/1097-0142(19900801)66:3<457::aid-cncr2820660310>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Matthews MJ. Problems in morphology and behavior of bronchopulmonary malignant disease. In: Lung Cancer: Natural History, Prognosis and Therapy. New York: Academic Press; 1976. [Google Scholar]
- 19.Short S, Chaturvedi A, Leslie MD. Palliation of symptomatic adrenal gland metastases by radiotherapy. Clin Oncol (R Coll Radiol) 1996;8:387–9. doi: 10.1016/s0936-6555(96)80087-2. [DOI] [PubMed] [Google Scholar]
- 20.Karanikiotis C, Tentes AA, Markakidis S, Vafiadis K. Large bilateral adrenal metastases in non-small cell lung cancer. World J Surg Oncol. 2004;2:37. doi: 10.1186/1477-7819-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 2008;466:729–36. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrieta O, Villarreal-Garza C, Zamora J, Blake-Cerda M, de la Mata MD, Zavala DG, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011;6:166. doi: 10.1186/1748-717X-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagohashi K, Satoh H, Ishikawa H, Ohtsuka M, Sekizawa K. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol. 2003;20:25–8. doi: 10.1385/MO:20:1:25. [DOI] [PubMed] [Google Scholar]
- 24.Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990;65:1596–600. doi: 10.1002/1097-0142(19900401)65:7<1596::aid-cncr2820650724>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer. 1987;59:1486–9. doi: 10.1002/1097-0142(19870415)59:8<1486::aid-cncr2820590815>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Aebi M. Spinal metastases in the elderly. Eur Spine J. 2003;12(Suppl 2):S202–13. doi: 10.1007/s00586-003-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnbull A, Osborn M, Nicholas N. Hospital autopsy: Endangered or extinct? J Clin Pathol. 2015;68:601–4. doi: 10.1136/jclinpath-2014-202700. [DOI] [PMC free article] [PubMed] [Google Scholar]


