Abstract
BACKGROUND:
Although hypothyroidism has an insidious onset and relatively asymptomatic, exertional dyspnea and fatigue can be the presenting complaints.
OBJECTIVES:
The aim is to assess functional lung impairment in hypothyroid patients both at rest and during exercise.
METHODS:
A case-control study was carried out on 42 patients with newly diagnosed hypothyroidism and 12 control subjects. Hypothyroidism was diagnosed based on high value of thyroid stimulating hormone (TSH) ≥6 μIU/ml, and low value of free thyroxin (FT4) ≤0.8 ng/dl, both groups had chest X-ray, spirometry, diffusing capacity of the lungs for carbon monoxide (DLCO), arterial blood gases (ABGs) and symptom-limited exercise testing using treadmill.
RESULTS:
Both groups were comparable as regard age, sex, and body mass index. Although ABG and spirometry were within normal in both groups, forced vital capacity %, and forced expiratory flow (FEF25–75) % were significantly reduced in the hypothyroid group (P = 0.014, 0.000, respectively), DLCO significantly reduced in hypothyroidism (P = 0.005). As regard exercise testing parameters, maximum oxygen consumption %, minute ventilation, tidal volume, and oxygen pulse were significantly reduced in hypothyroidism (0.005, 0.000, 0.000, and 0.02 respectively). TSH significantly negatively correlated with forced expiratory volume in 1 s %, FEF25–75%, and DLCO while they significantly positively correlated with FT4.
CONCLUSION:
Even with the presence of normal chest X-ray, arterial blood gases, and spirometry in patients with hypothyroidism DLCO and exercise testing parameters can be significantly reduced.
Keywords: Arterial blood gases, exercise, hypothyroidism, resting pulmonary function
Thyroid hormones play a critical role in regulating the function of different systems of the human body including cardiovascular, respiratory, renal, homoeostasis, vascular tone, and central nervous system through their effect on metabolism.[1] Hypothyroidism is a common clinical problem with its prevalence in communities with deficient iodine 1%–2%, and it is ten times higher in women as compared to men.[2] Hypothyroidism has wide range of respiratory consequences ranging from mild dyspnea up to overt respiratory failure.[3] Dyspnea associated with hypothyroidism most probably related to a limited ventilator and cardiac reserve, in addition to decreased inspiratory and expiratory muscle strength.[4] Respiratory failure and difficult weaning from mechanical ventilation secondary to hypothyroidism are attributed to impaired ventilator derive to hypoxemia and hypercapnia in addition to respiratory muscle weakness.[5] Even in asymptomatic patients with hypothyroidism respiratory system can be dangerously and insidiously affected, many studies investigated the effect of hypothyroidism on pulmonary function and effect of hormone replacement therapy.[6,7] Some of these studies documented decreased pulmonary function in hypothyroidism which improved with hormonal replacement. In addition, a number of studies had investigated the effect of hypothyroidism on exercise capacity, and they documented impaired exercise tolerance.[8,9] We carried out the present study aiming to detect the effect of thyroid hormones deficiency in newly diagnosed patients on pulmonary functions both at rest and during maximal exercise testing. Also to detect if there is a correlation between the level of thyroid hormones and pulmonary function parameters.
Methods
This is a case-control study which took place between December 2015 and December 2016 in tertiary Hospital. Forty-two patients were recruited from the outpatient Endocrine Clinic; they were newly diagnosed with hypothyroidism and treatment naïve. Hypothyroid patients were diagnosed based on the presence of elevated serum thyroid stimulating hormone (TSH) levels, free thyroxin (FT4) values below the reference range (Diagnostic Products Corporation Kit criteria: TSH >6.0 μIU/mL, FT4 ≤0.8 ng/dl).[10] Twelve age- and sex-matched healthy volunteers also were included in the present study as a control group. The inclusion criteria were patients aged 20–50 years. Excluded patients include a history of smoking, systemic disease, any respiratory disorder or respiratory symptoms, the presence of goiter, pregnancy, use of drugs that could influence thyroid function, diagnosed cardiac diseases or systemic arterial hypertension, and the presence of any of contraindication of exercise testing.
Informed written consents were obtained both from patients and healthy volunteers; this study was approved by the Ethics Committee of Faculty of Medicine.
All participants were subjected to the following:
Complete medical history and physical examination and patients with respiratory or cardiac symptoms or signs or other systemic diseases were excluded from the study
Chest X-ray for the exclusion of any undiagnosed respiratory problem
Electrocardiogram for the exclusion of occult cardiac problem
Arterial blood gases (ABGs) on room air at rest were obtained from all participants by radial artery puncture and analyzed using blood gases analyzer (Rapid lab 850; CHIRON/Diagnostics; critical care systems) with the calculation of PaO2, SaO2, and PaCO2
Resting pulmonary function tests (PFTs), including spirometry and diffusing capacity of the lungs for carbon monoxide (DLCO) measured using single breath method using (D 97723; Zan 300, Oberthulba, Germany) where each of the following were measured (forced expiratory volume in 1 s [FEV1]/FVC, FVC%, FEV1%, forced expiratory flow [FEF25–75] %, and DLCO corrected for hemoglobin concentration)
Symptom-limited maximal exercise testing on treadmill using (Cosmed SrL, Quark PFTs ergo, P/N Co9035-12-99, made in Italy).
Gas exchange values and exercise parameters were collected using breath-by-breath methods. The following parameters were measured oxygen consumption (VO2), carbon dioxide output (VCO2), minute ventilation (VE), tidal volume (VT), respiratory rate (respiratory frequency), the anaerobic threshold, oxygen pulse (VO2/HR), and the breathing reserve (BR). The test was considered to be maximal when reaching any of the following a plateau of VO2, R (VCO2/VO2 respiratory quotient) ≥1.1, maximal heart rate (HR), or maximal dyspnea score according to Borg scale ≥9.[11,12] Protocol for exercise testing included four stages resting stage, warm up stage, exercise testing stage, and recovery stage during each stage systolic and diastolic blood pressure were measured. Different exercise testing parameters were recorded at peak VO2.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. IBM Corp., Armonk, NY) software. The results were expressed as means ± standard deviation or frequencies. Independent Student's t-test was done for comparison between patients and controls. Pearson's correlation analysis was used to evaluate the correlations between pulmonary function parameters and level thyroid hormones parameters in hypothyroid patients, P < 0.05 were considered statistically significant. Microsoft Excel was used for performing graphic illustrations.
Results
Our hypothyroid patients had neither respiratory complaint nor abnormal physical examination, and their chest X-rays were normal. As regard demographic data of our participants, there were no significant differences between hypothyroid patients and control group with respect to age, sex, and body mass index [Table 1]. TSH significantly increased in the hypothyroid group compared to healthy volunteer (P = 0.000*), on the other hand, FT4 significantly reduced in patients (P = 0.014*) [Table 2]. By comparing resting PFTs and ABG between hypothyroid patients and control group there were no significant differences as regard ABG, although FVC% was within normal range in both groups, it was significantly reduced in hypothyroid patients (P = 0.014*), also DLCO was significantly reduced in hypothyroid patients (P = 0.005*). Exercise testing parameters showed that each of VO2, VCO2, VE, VT, and VO2/HR were significantly reduced in hypothyroid patients while the BR, and HR reserve were significantly increased in hypothyroid patients [Tables 3 and 4]. FEV1%, and DLCO were significantly negatively correlated with TSH (r = −0.424; P = 0.039, r = −0.566; P = 0.004, respectively), while they were significantly positively correlated with FT4 (r = 0.584; P = 0.005, r = 0.457; P = 0.025, respectively) [Figure 1].
Table 1.
Demographic data of the study groups
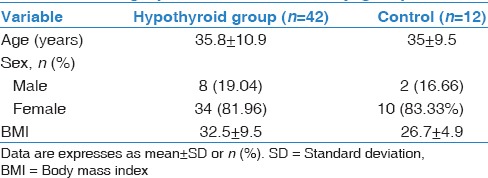
Table 2.
Laboratory data of controls and hypothyroid patients
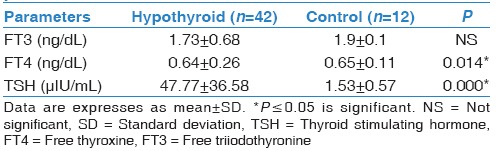
Table 3.
Resting pulmonary function test and arterial blood gases in hypothyroid and control group
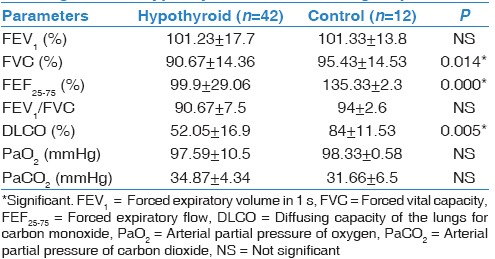
Table 4.
Exercise testing parameters in hypothyroid and control group
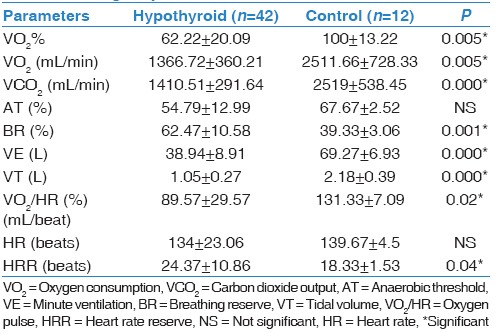
Figure 1.
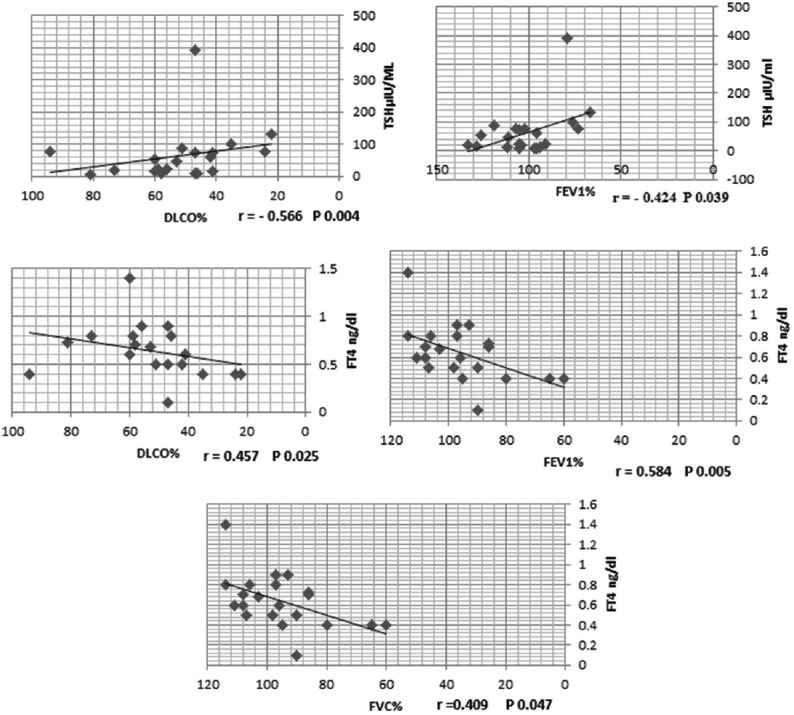
Correlations between thyroid hormones level and pulmonary function parameters
Discussion
It is uncommon for hypothyroid patients to be first presented with respiratory manifestations.[13] Hypothyroidism usually has an insidious onset and usually asymptomatic.[13] These findings in agree with our finding while studying forty-two newly diagnosed hypothyroid patients we observed that they had no respiratory symptoms, their chest X-ray were normal, and their ABG were normal and comparable to control group. On performing spirometry and DLCO we observed that FVC%, FEF25–75%, and DLCO significantly reduced in hypothyroid patients compared to control group (P = 0.014*, 0.000*, 0.005* respectively). Roel et al.[14] in accordance with our results they observed that both FVC and FEV1 reduced in hypothyroid patients compared to control, but only FVC was significantly reduced. Also each of Valjevac et al.[15], and Cakmak et al.[6] reported that FVC, FEV1, FEF25%–75% significantly reduced in hypothyroid patients compared to control group, moreover Cakmak et al.[6] also observed that DLCO was significantly reduced in hypothyroid patients. In addition, Bassi et al.[16] while studying the effect of thyroid hormone replacement on respiratory function in hypothyroid women, they observed that FEV1, FVC, and FEF significantly reduced in untreated hypothyroid women compared to euthyroid controls and treated hypothyroid patients. Bassi et al.[16] explained these changes by alveolar hypoventilation secondary to respiratory muscle weakness, depressed respiratory center, limitation of neuromuscular transmission as a result of low FT4, and also decreased lung elasticity and increased work of breathing. van Tuyl et al. and Husain and Kumar[17,18] also reported that reduced lung compliance and reduced surfactant phospholipid, phosphatidylglycerol, and phosphatidic acid in addition to reduced lung elasticity as a result of mucopolysaccharide deposition in the lung lead to reduced ventilatory function of the lungs. Furthermore, Roel et al.[14] attributed spirometric changes in hypothyroid patients to the reduced level of FT4 and subsequent hypoventilation and weakness of inspiratory muscle. By performing correlation study, we observed that FEV1%, and DLCO were significantly negatively correlated with TSH (r = −0.424; P = 0.039, r = −0.566; P = 0.004, respectively), whereas they were significantly positively correlated with FT4 (r = 0.584; P = 0.005, r = 0.457; P = 0.025, respectively) Figure 1. Roel et al.[14] supported our results; they observed a negative and positive correlation between spirometric parameters and each of TSH, and FT4, respectively. In addition, Bassi et al.[16] observed significant negative correlations between TSH and FEV1% in untreated hypothyroid patients. As regard exercise capacity we observed impaired exercise capacity in hypothyroid patients with significantly low each of the following, VO2, VCO2, VO2/HR, VE, and VT. Many studies had supported our results,[8,19] Biondi et al.[19] demonstrated that hypothyroid patients under levothyroxine treatment had impaired exercise capacity, in addition, Caraccio et al.[8] while studying “muscle metabolism and exercise tolerance in subclinical hypothyroidism: a controlled trial of levothyroxine” observed significant exercise intolerance in hypothyroid patients secondary to Hashimoto's thyroiditis compared to control individuals. Ittermann et al.[20] disagree with our results they documented that thyroid dysfunction not related to either pulmonary function or to cardiopulmonary exercise capacity. This discrepancy between this study and our study may be attributed to that we had studied a group of patients with overt hypothyroidism, meanwhile Ittermann et al.[20] studied a population-based sample, so this discrepancy is related to different study populations. In addition, Mainenti et al.[21] observed improvements in VE, VO2 and HR at submaximal intensity of exercise in treated hypothyroid patients compared to untreated subjects, and so they may support our results.
Thyroid hormones are determinants of the metabolic and contractile phenotype of skeletal muscles, and so they modulate the function of mitochondria.[22] Thyroid hormones activate production of adenosine triphosphate with subsequent increasing mitochondrial respiration, so reduction of these hormones associated with impaired efficiency of oxidative phosphorylation. Changes in mitochondrial metabolism in hypothyroidism resulting changes in structure and biochemistry of skeletal,[23] which may play a role in exercise intolerance in these patients.
Several studies reported delayed VO2 recovery after exercise in patients with hypothyroidism compared to healthy controls, where VO2 kinetics was 23% slower in hypothyroidism group compared to the control group. This in turn resulting in greater metabolic cost to perform repeated tasks and significant impairment of daily living tasks.[24,25,26,27]
Conclusion
Hypothyroidism has a significant effect both in resting PFTs and exercise testing parameters even in its early stages, so we recommend early intervention in cases of hypothyroidism with strict observation of pulmonary function and exercise tolerance.
The strength of the study is that our patients were newly diagnosed with overt hypothyroidism with nil thyroid replacement therapy, our patients had no other comorbidities, and hence pulmonary exercise effects were solely related to low thyroid hormones level.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank a lot technicians of pulmonary function lab because of their hard work during our research.
References
- 1.Stathatos N, Wartofsky L. Perioperative management of patients with hypothyroidism. Endocrinol Metab Clin North Am. 2003;32:503–18. doi: 10.1016/s0889-8529(03)00007-0. [DOI] [PubMed] [Google Scholar]
- 2.Vanderpump MP. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, editors. Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia: J.B. Lippincott-Raven; 2005. pp. 398–496. [Google Scholar]
- 3.McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: A PreCIS database study. Thyroid. 2011;21:837–43. doi: 10.1089/thy.2010.0298. [DOI] [PubMed] [Google Scholar]
- 4.Ingbar DH. The Thyroid. 7th ed. Philadelphia: Lipponcott-Raven; 1996. The respiratory system in hypothyroidism; pp. 807–8. [Google Scholar]
- 5.Datta D, Scalise P. Hypothyroidism and failure to wean in patients receiving prolonged mechanical ventilation at a regional weaning center. Chest. 2004;126:1307–12. doi: 10.1378/chest.126.4.1307. [DOI] [PubMed] [Google Scholar]
- 6.Cakmak G, Saler T, Saglam ZA, Yenigün M, Demir T. Spirometry in patients with clinical and subclinical hypothyroidism. Tuberk Toraks. 2007;55:266–70. [PubMed] [Google Scholar]
- 7.Akha O, Kashi Z, Poor AS, Zadeh ZT, Zakeri HR. Evaluation of levothyroxine effect on pulmonary function in hypothyroidism. J Mazandaran Univ Med Sci. 2008;18:1–6. [Google Scholar]
- 8.Caraccio N, Natali A, Sironi A, Baldi S, Frascerra S, Dardano A, et al. Muscle metabolism and exercise tolerance in subclinical hypothyroidism: A controlled trial of levothyroxine. J Clin Endocrinol Metab. 2005;90:4057–62. doi: 10.1210/jc.2004-2344. [DOI] [PubMed] [Google Scholar]
- 9.Fontana M, Passino C, Poletti R, Zyw L, Prontera C, Scarlattini M, et al. Low triiodothyronine and exercise capacity in heart failure. Int J Cardiol. 2012;154:153–7. doi: 10.1016/j.ijcard.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200–35. doi: 10.1089/thy.2012.0205. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 12.Mador MJ, Rodis A, Magalang UJ. Reproducibility of Borg scale measurements of dyspnea during exercise in patients with COPD. Chest. 1995;107:1590–7. doi: 10.1378/chest.107.6.1590. [DOI] [PubMed] [Google Scholar]
- 13.Braverman LE, Utiger RD. Introduction to hypothyroidism. In: Braverman LE, Utiger RD, Werner SC, Ingbar SH, editors. The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 697–9. [Google Scholar]
- 14.Roel S, Punyabati O, Prasad L, Salam R, Ningshen K, Shimray AJ, et al. Assessment of functional lung impairment in hypothyroidism. IOSR J Dent Med Sci. 2014;13:4–7. [Google Scholar]
- 15.Valjevac S, Hadzovic-Dzuvo A, Valjevac A, Kucukalic-Selimovic E, Lepara O. Assessment of lung dysfunction with spirometry in patients with thyroid disorders. Acta Inform Med. 2011;19:16–8. [Google Scholar]
- 16.Bassi R, Dhillon SK, Sharma S, Sharma A, Tapdiya M. Effect of thyroid hormone replacement on respiratory functions tests in hypothyroid women. Pak J Physiol. 2012;8:20–3. [Google Scholar]
- 17.van Tuyl M, Blommaart PE, de Boer PA, Wert SE, Ruijter JM, Islam S, et al. Prenatal exposure to thyroid hormone is necessary for normal postnatal development of murine heart and lungs. Dev Biol. 2004;272:104–17. doi: 10.1016/j.ydbio.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Husain AN, Kumar V. Robbins and Katran Pathologic Basis of Disease. 7th ed. Philadelphia: The W.B. Saunders Company; 1999. The lung; pp. 711–72. [Google Scholar]
- 19.Biondi B, Fazio S, Cuocolo A, Sabatini D, Nicolai E, Lombardi G, et al. Impaired cardiac reserve and exercise capacity in patients receiving long-term thyrotropin suppressive therapy with levothyroxine. J Clin Endocrinol Metab. 1996;81:4224–8. doi: 10.1210/jcem.81.12.8954019. [DOI] [PubMed] [Google Scholar]
- 20.Ittermann T, Gläser S, Ewert R, Felix S, Völzke H, Dörr M. Serum thyroid-stimulating hormone levels are not associated with exercise capacity and lung function parameters in two population-based studies. BMC Pulm Med. 2014;14:145. doi: 10.1186/1471-2466-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mainenti MR, Vigário PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest. 2009;32:470–3. doi: 10.1007/BF03346488. [DOI] [PubMed] [Google Scholar]
- 22.Simonides WS, van Hardeveld C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid. 2008;18:205–16. doi: 10.1089/thy.2007.0256. [DOI] [PubMed] [Google Scholar]
- 23.Khushu S, Rana P, Sekhri T, Sripathy G, Tripathi RP. Bio-energetic impairment in human calf muscle in thyroid disorders: A 31P MRS study. Magn Reson Imaging. 2010;28:683–9. doi: 10.1016/j.mri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto T, Kanazawa H, Hirata K, Yoshikawa J. Evaluation of oxygen uptake kinetics and oxygen kinetics of peripheral skeletal muscle during recovery from exercise in patients with chronic obstructive pulmonary disease. Clin Physiol Funct Imaging. 2003;23:257–62. doi: 10.1046/j.1475-097x.2003.00500.x. [DOI] [PubMed] [Google Scholar]
- 25.Belardinelli R, Barstow TJ, Nguyen P, Wasserman K. Skeletal muscle oxygenation and oxygen uptake kinetics following constant work rate exercise in chronic congestive heart failure. Am J Cardiol. 1997;80:1319–24. doi: 10.1016/s0002-9149(97)00672-3. [DOI] [PubMed] [Google Scholar]
- 26.Nanas S, Sakellariou D, Kapsimalakou S, Dimopoulos S, Tassiou A, Tasoulis A, et al. Heart rate recovery and oxygen kinetics after exercise in obstructive sleep apnea syndrome. Clin Cardiol. 2010;33:46–51. doi: 10.1002/clc.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen-Solal A, Laperche T, Morvan D, Geneves M, Caviezel B, Gourgon R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure. Analysis with gas exchange measurements and NMR spectroscopy. Circulation. 1995;91:2924–32. doi: 10.1161/01.cir.91.12.2924. [DOI] [PubMed] [Google Scholar]


