Abstract
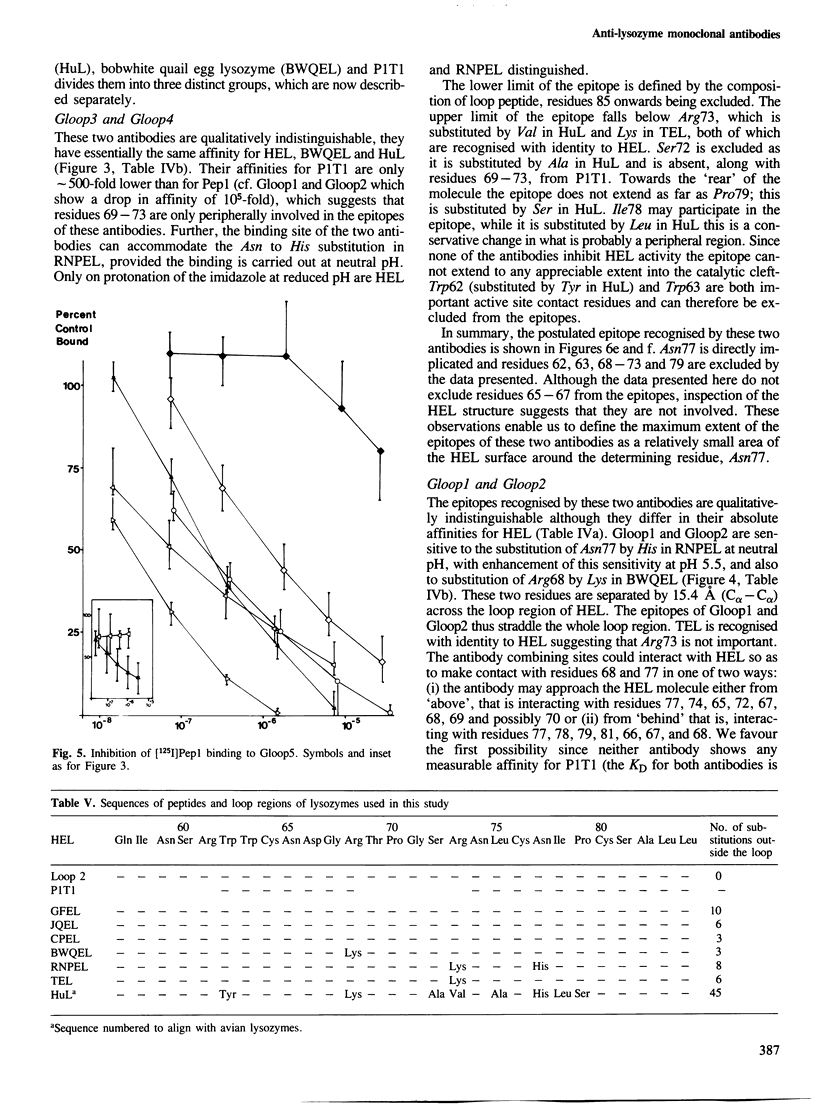
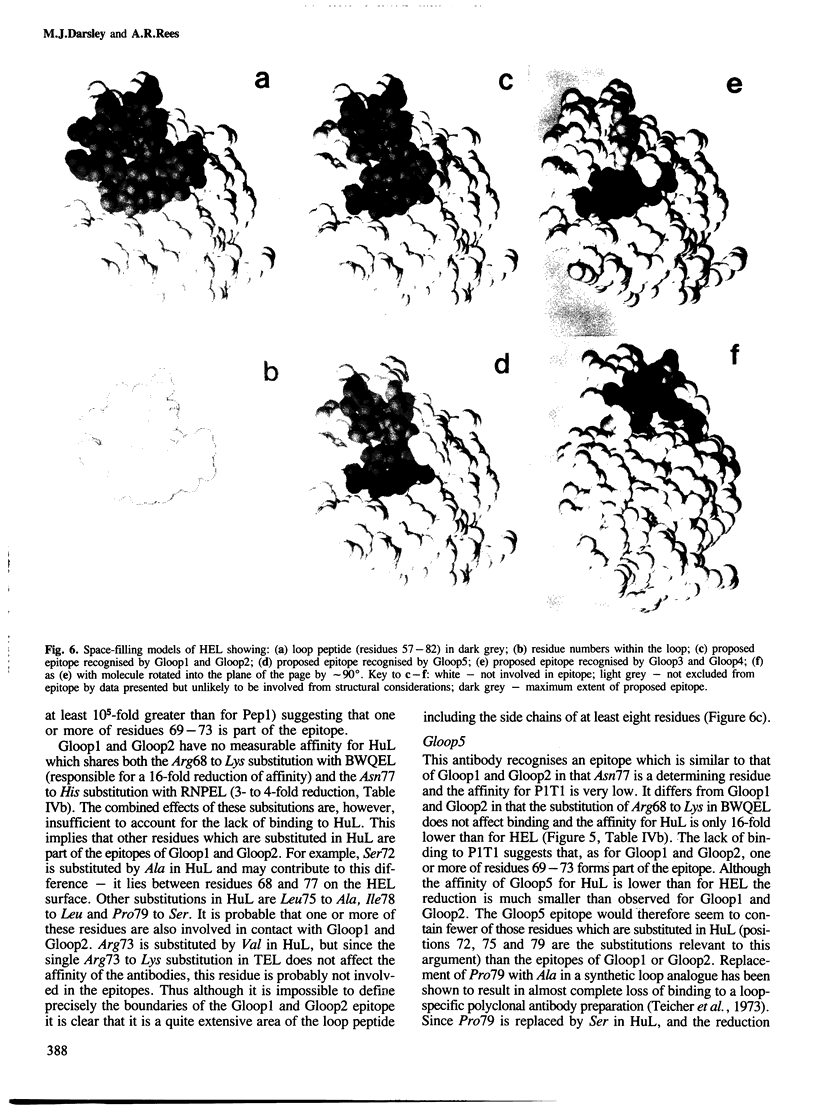
Five monoclonal antibodies specific for the loop region of hen egg lysozyme were prepared by immunisation with a synthetic conjugate of a proteolytic fragment of lysozyme coupled to bovine serum albumin. Their fine specificities were investigated using a panel of variant lysozymes and peptide fragments of lysozyme in a quantitative radio-immunoassay procedure. Knowledge of the structure of hen lysozyme to high resolution and the use of computer graphics enables the localisation of the epitopes recognised by the antibodies with some precision. The antibodies were shown to define three distinct, overlapping epitopes within what was previously considered to be a single antigenic site. These results are discussed in relation to current ideas of the antigenic nature of proteins and other recent studies in which anti-protein antibodies have been elicited by immunisation with small peptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CROCKER C. Réactions colorées spécifiques de l'arginine et de la tyrosine réalisées après chromatographie sur papier. Biochim Biophys Acta. 1952 Dec;9(6):704–705. doi: 10.1016/0006-3002(52)90236-9. [DOI] [PubMed] [Google Scholar]
- Arnon R., Sela M. Antibodies to a unique region in lysozyme provoked by a synthetic antigen conjugate. Proc Natl Acad Sci U S A. 1969 Jan;62(1):163–170. doi: 10.1073/pnas.62.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artymiuk P. J., Blake C. C. Refinement of human lysozyme at 1.5 A resolution analysis of non-bonded and hydrogen-bond interactions. J Mol Biol. 1981 Nov 15;152(4):737–762. doi: 10.1016/0022-2836(81)90125-x. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Habeeb A. F., Ando K. Enzymic and immunochemical properties of lysozyme. VII. Location of all the antigenic reactive regions. A new approach to study immunochemistry of tight proteins. Biochim Biophys Acta. 1973 Mar 23;303(1):203–209. [PubMed] [Google Scholar]
- Atassi M. Z., Kazim A. L., Sakata S. High yield coupling of peptides to protein carriers. Biochim Biophys Acta. 1981 Sep 29;670(2):300–302. doi: 10.1016/0005-2795(81)90025-8. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Lee C. L. The precise and entire antigenic structure of native lysozyme. Biochem J. 1978 May 1;171(2):429–434. doi: 10.1042/bj1710429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Buckenmeyer G. K., Hicks G., Gurd F. R., Feldmann R. J., Minna J. Topographic antigenic determinants recognized by monoclonal antibodies to sperm whale myoglobin. J Biol Chem. 1982 Mar 25;257(6):3189–3198. [PubMed] [Google Scholar]
- Blake C. C., Koenig D. F., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature. 1965 May 22;206(4986):757–761. doi: 10.1038/206757a0. [DOI] [PubMed] [Google Scholar]
- Bott R., Sarma R. Crystal structure of turkey egg-white lysozyme: results of the molecular replacement method at 5 A resolution. J Mol Biol. 1976 Oct 5;106(4):1037–1046. doi: 10.1016/0022-2836(76)90351-x. [DOI] [PubMed] [Google Scholar]
- CANFIELD R. E., LIU A. K. THE DISULFIDE BONDS OF EGG WHITE LYSOZYME (MURAMIDASE). J Biol Chem. 1965 May;240:1997–2002. [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- East I. J., Hurrell J. G., Todd P. E., Leach S. J. Antigenic specificity of monoclonal antibodies to human myoglobin. J Biol Chem. 1982 Mar 25;257(6):3199–3202. [PubMed] [Google Scholar]
- Feldmann R. J., Potter M., Glaudemans C. P. A hypothetical space-filling model of the V-regions of the galactan-binding myeloma immunoglobulin J539. Mol Immunol. 1981 Aug;18(8):683–698. doi: 10.1016/0161-5890(81)90060-2. [DOI] [PubMed] [Google Scholar]
- Fjuio H., Imanishi M., Nishioka K., Amano T. Antigenic structures of hen egg white lysozyme. II. Significance of the N-and C-terminal region as an antigenic site. Biken J. 1968 Sep;11(3):207–218. [PubMed] [Google Scholar]
- Fujio H., Imanishi M., Nishioka K., Amano T. Proof of independency of two antigenic sites in egg white lysozyme. Biken J. 1968 Sep;11(3):219–223. [PubMed] [Google Scholar]
- Geiger B., Arnon R. Immunogenicity and antigenic specificity of the loop fragment of lysozyme. Eur J Immunol. 1974 Sep;4(9):632–634. doi: 10.1002/eji.1830040912. [DOI] [PubMed] [Google Scholar]
- Gettins P., Pótter M., Leatherbarrow R. J., Dwek R. A. A combined proton and phosphorus-31 nuclear magnetic resonance investigation of the combining site of M603, a phosphocholine-binding myeloma protein. Biochemistry. 1982 Sep 28;21(20):4927–4931. doi: 10.1021/bi00263a015. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Fujio H., Kondo K., Dohi Y., Hirayama A., Takagaki Y., Kosaki G., Amano T. A monoclonal antibody specific for a distinct region of hen egg-white lysozyme. Mol Immunol. 1982 Apr;19(4):619–630. doi: 10.1016/0161-5890(82)90231-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Jackson W. R., Dwek R. A. Role of tyrosines in the combining site of the dinitrophenyl-binding IgA myeloma M315: specific nitration and high-resolution hydrogen-1 nuclear magnetic resonance studies. Biochemistry. 1982 Oct 12;21(21):5124–5129. doi: 10.1021/bi00264a003. [DOI] [PubMed] [Google Scholar]
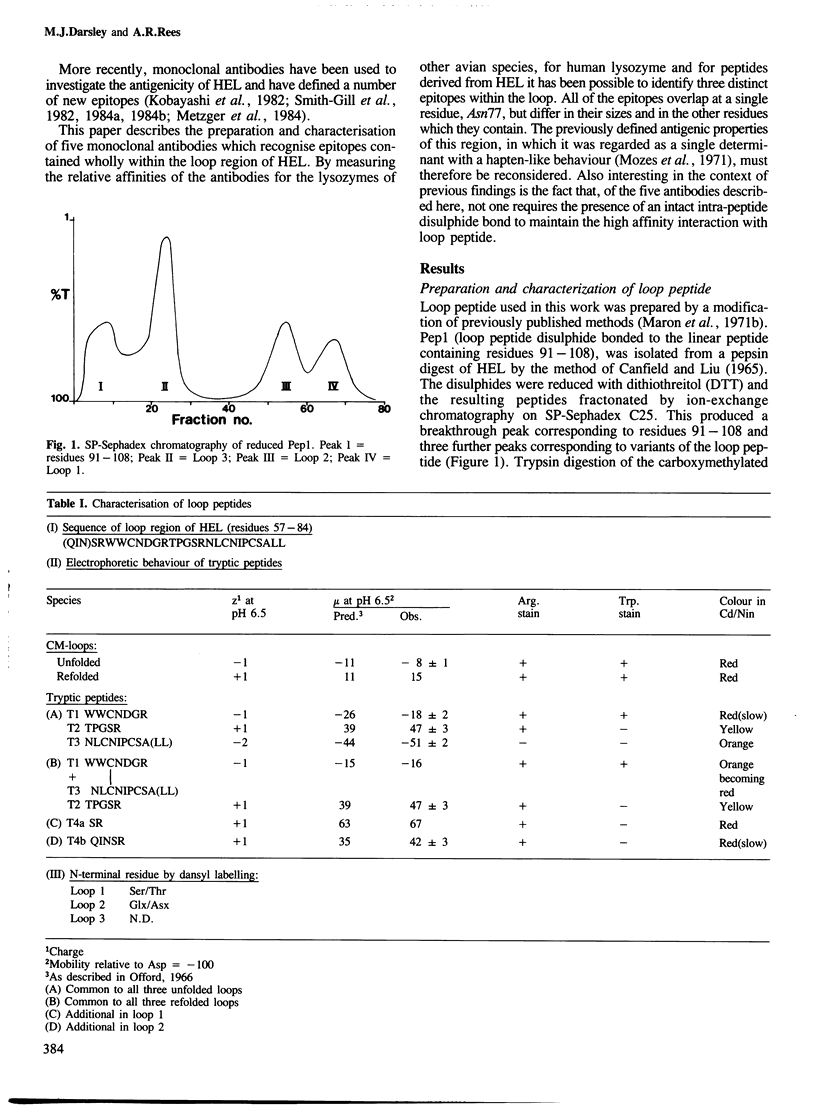
- Maron E., Arnon R., Bonavida B. Sequential appearance of antibodies directed against different antigenic determinants of hen egg-white lysozyme. Eur J Immunol. 1971 Jun;1(3):181–185. doi: 10.1002/eji.1830010307. [DOI] [PubMed] [Google Scholar]
- Maron E., Shiozawa C., Arnon R., Sela M. Chemical and immunological characterization of a unique antigenic region in lysozyme. Biochemistry. 1971 Mar 2;10(5):763–771. doi: 10.1021/bi00781a007. [DOI] [PubMed] [Google Scholar]
- Matthyssens G. E., Giebens G., Kanarek L. Structure and antigenicity of hen egg-white lysozyme fragments. Study of region 57-107. Eur J Biochem. 1974 Apr 1;43(2):353–362. doi: 10.1111/j.1432-1033.1974.tb03420.x. [DOI] [PubMed] [Google Scholar]
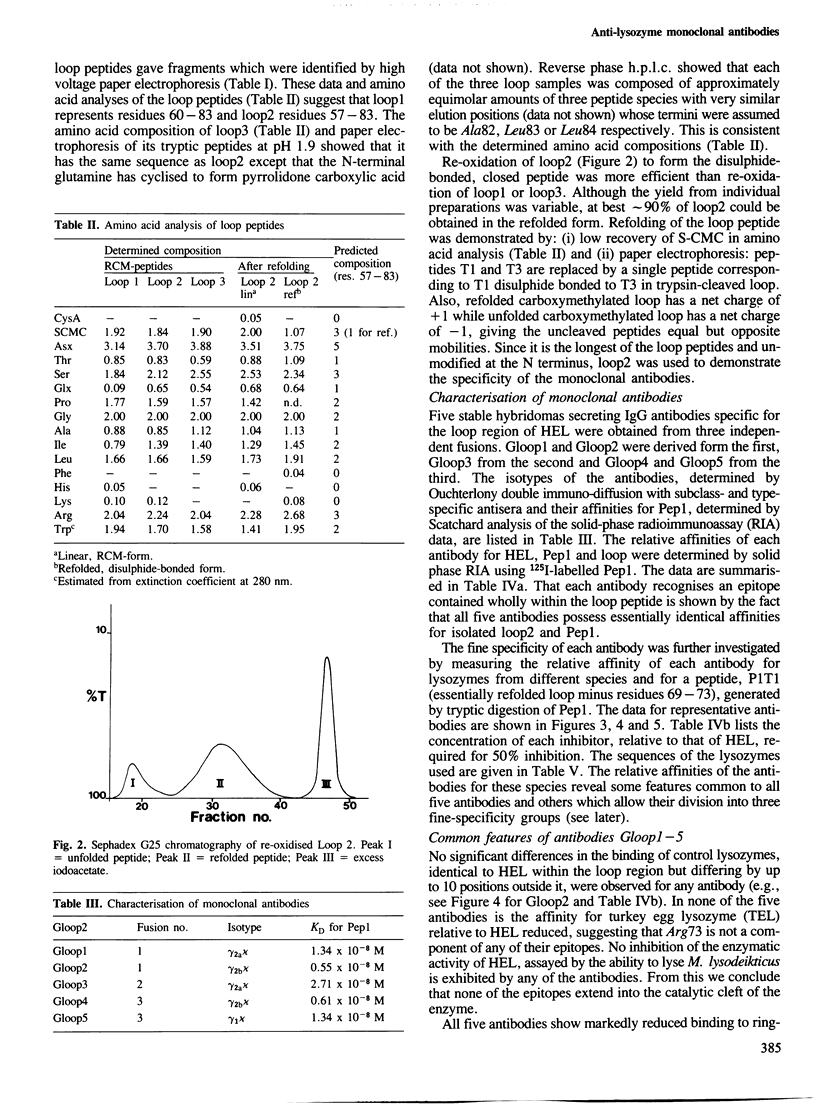
- Metzger D. W., Ch'ng L. K., Miller A., Sercarz E. E. The expressed lysozyme-specific B cell repertoire. I. Heterogeneity in the monoclonal anti-hen egg white lysozyme specificity repertoire, and its difference from the in situ repertoire. Eur J Immunol. 1984 Jan;14(1):87–93. doi: 10.1002/eji.1830140116. [DOI] [PubMed] [Google Scholar]
- Mozes E., Maron E., Arnon R., Sela M. [Strain-dependent differences in the specificity of antibody responses toward lysozyme]. J Immunol. 1971 Mar;106(3):862–864. [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Davies D. R., Pecht I., Givol D., Wright C. Model-building studies of antigen-binding sites: the hapten-binding site of mopc-315. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):627–637. doi: 10.1101/sqb.1977.041.01.072. [DOI] [PubMed] [Google Scholar]
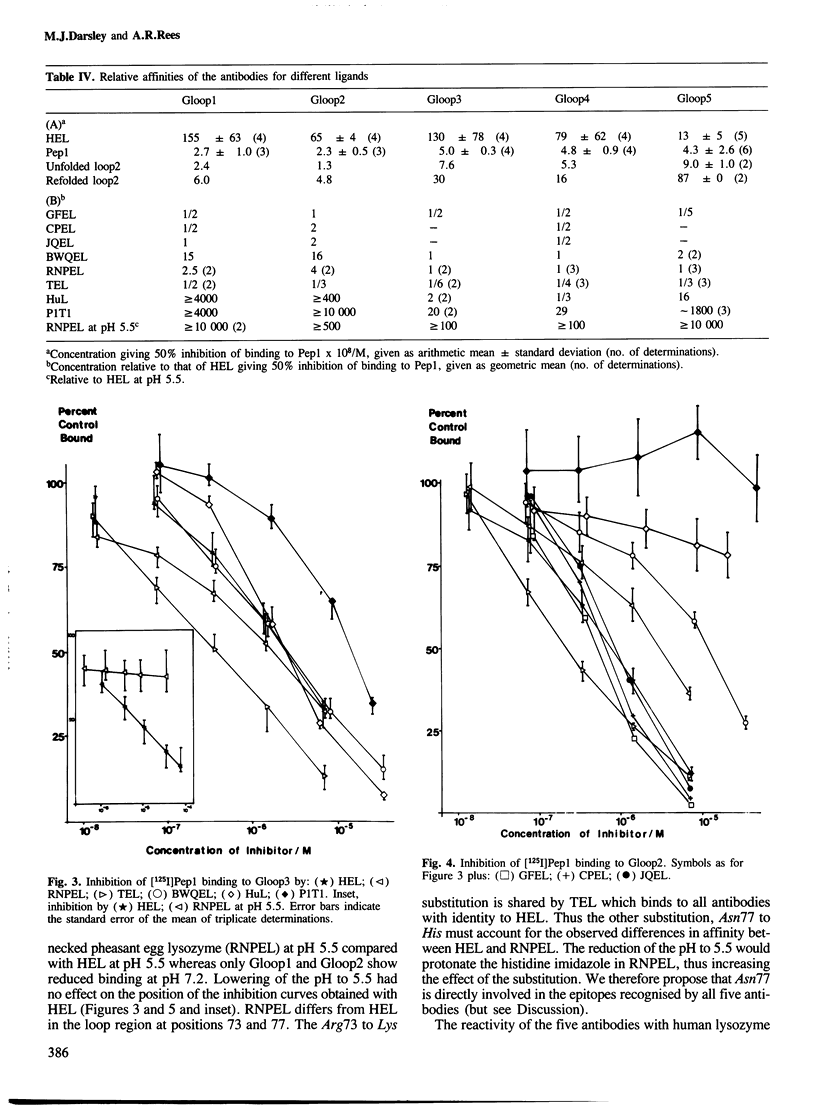
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- REDDI K. K., KODICEK E. Metabolism of nicotinic acid and related compounds in man and rat. Biochem J. 1953 Jan;53(2):286–294. doi: 10.1042/bj0530286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul F. A., Amzel L. M., Poljak R. J. Preliminary refinement and structural analysis of the Fab fragment from human immunoglobulin new at 2.0 A resolution. J Biol Chem. 1978 Jan 25;253(2):585–597. [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Gill S. J., Lavoie T. B., Mainhart C. R. Antigenic regions defined by monoclonal antibodies correspond to structural domains of avian lysozyme. J Immunol. 1984 Jul;133(1):384–393. [PubMed] [Google Scholar]
- Smith-Gill S. J., Mainhart C. R., Lavoie T. B., Rudikoff S., Potter M. VL-VH expression by monoclonal antibodies recognizing avian lysozyme. J Immunol. 1984 Feb;132(2):963–967. [PubMed] [Google Scholar]
- Smith-Gill S. J., Wilson A. C., Potter M., Prager E. M., Feldmann R. J., Mainhart C. R. Mapping the antigenic epitope for a monoclonal antibody against lysozyme. J Immunol. 1982 Jan;128(1):314–322. [PubMed] [Google Scholar]
- Teicher E., Maron E., Arnon R. The role of specific amino acid residues in the antigenic reactivity of the loop peptide of lysozyme. Immunochemistry. 1973 Apr;10(4):265–271. doi: 10.1016/0019-2791(73)90204-8. [DOI] [PubMed] [Google Scholar]