Abstract
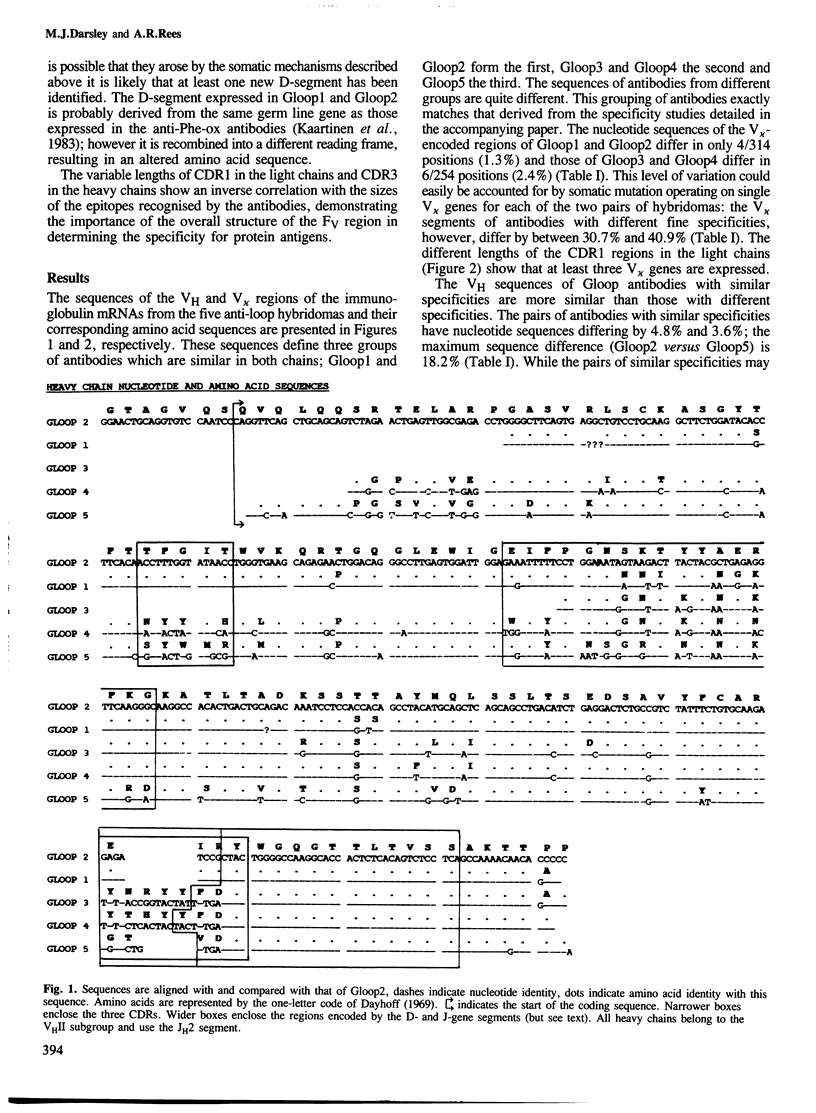
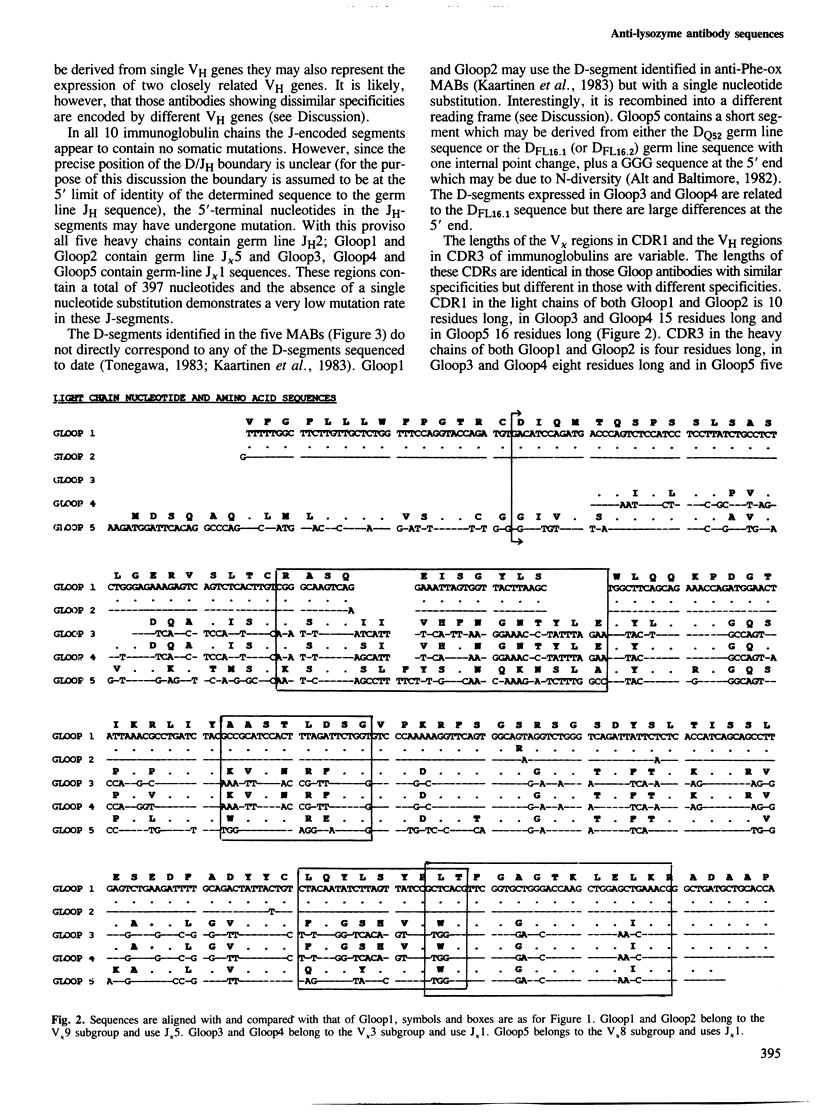
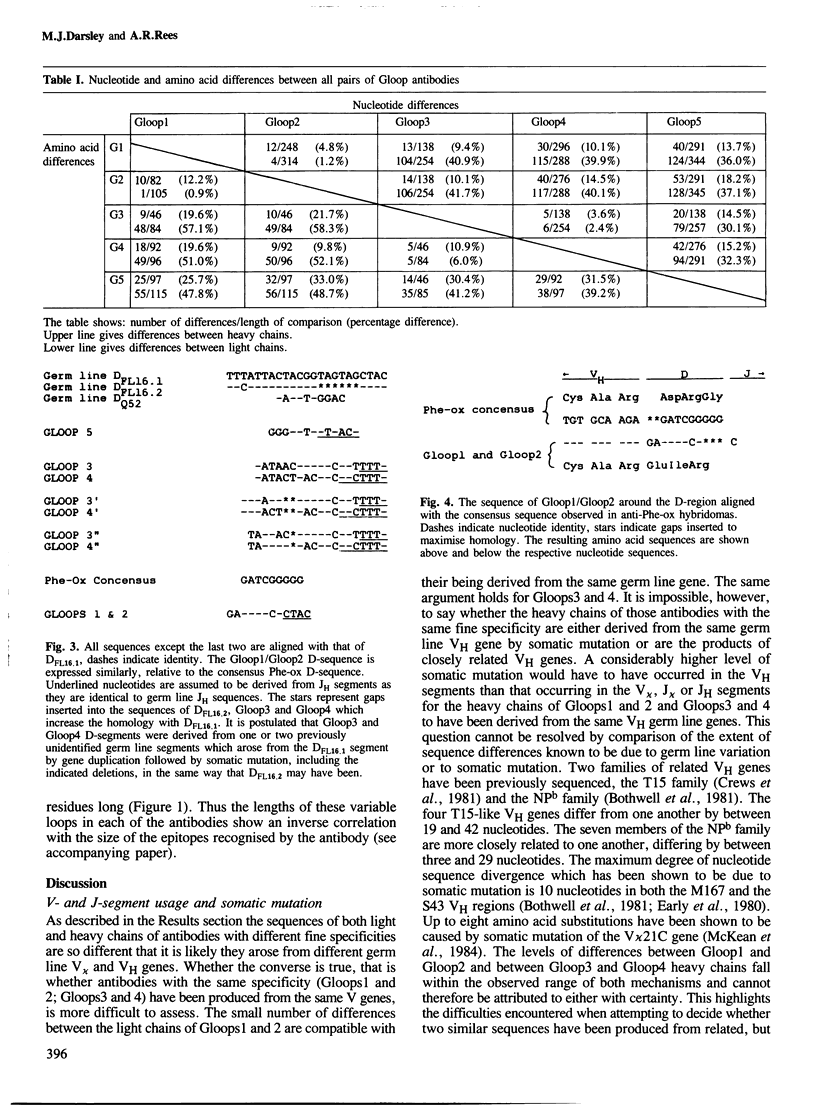
The nucleotide sequences of the heavy and light chain immunoglobulin mRNAs derived from five hybridomas (Gloop 1-5) secreting IgGs specific for the loop region of hen egg lysozyme were determined. These monoclonal antibodies recognise three distinct but overlapping epitopes within the loop region. The sequences of two pairs of antibodies with indistinguishable fine specificities were similar in both chains whereas the sequences of antibodies of non-identical specificities were very different. It is proposed that the D-segments expressed in two of the antibodies (Gloop3 and Gloop4) are the products of one, or perhaps two, previously unidentified germ line D-genes. Gloop1 and Gloop2 use a D-segment previously identified in antibodies specific for the hapten 2-phenyloxazolone; however it is recombined in a different reading frame in the anti-lysozyme antibodies, producing a different amino acid sequence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Markham A. F., Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. 1983 Jul 28-Aug 3Nature. 304(5924):320–324. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner E. C., Capra J. D. VH families in the antibody response to p-azophenylarsonate: correlation between serology and amino acid sequence. J Immunol. 1982 Jul;129(1):193–199. [PubMed] [Google Scholar]
- Padlan E. A., Davies D. R., Pecht I., Givol D., Wright C. Model-building studies of antigen-binding sites: the hapten-binding site of mopc-315. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):627–637. doi: 10.1101/sqb.1977.041.01.072. [DOI] [PubMed] [Google Scholar]
- Pollok B. A., Kearney J. F., Vakil M., Perry R. P. A biological consequence of variation in the site of D-JH gene rearrangement. 1984 Sep 27-Oct 3Nature. 311(5984):376–379. doi: 10.1038/311376a0. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Giusti A. M., Cook W. D., Scharff M. D. Single amino acid substitution altering antigen-binding specificity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1979–1983. doi: 10.1073/pnas.79.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Heller M. Somatic diversification of immunoglobulins. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2162–2166. doi: 10.1073/pnas.81.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N. H., Klinman N. R. The B-cell clonotype repertoire. Adv Immunol. 1978;26:255–337. doi: 10.1016/s0065-2776(08)60232-1. [DOI] [PubMed] [Google Scholar]
- Smith-Gill S. J., Mainhart C. R., Lavoie T. B., Rudikoff S., Potter M. VL-VH expression by monoclonal antibodies recognizing avian lysozyme. J Immunol. 1984 Feb;132(2):963–967. [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Alexander H., Houghten R. A., Olson A. J., Lerner R. A., Hendrickson W. A. The reactivity of anti-peptide antibodies is a function of the atomic mobility of sites in a protein. Nature. 1984 Nov 8;312(5990):127–134. doi: 10.1038/312127a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]


