Summary
Objective
To describe the phenomenology of monitored Sudden Unexpected Death in Epilepsy (SUDEP) occurring in the inter-ictal period where death occurs without a seizure preceding it.
Methods
We report a case series of monitored definite and probable SUDEP where no electroclinical evidence of underlying seizures was found preceding death.
Results
Three patients (2 definite and 1 probable) suffered SUDEP. They had a typical high SUDEP-risk profile with longstanding intractable epilepsy and frequent generalized tonic-clonic seizures (GTCS). All patients had varying patterns of respiratory and bradyarrhythmic cardiac dysfunction with profound EEG suppression. In two patients, patterns of cardio-respiratory failure were similar to those seen in some patients in the Mortality in Epilepsy Monitoring Units Study (MORTEMUS).
Significance
Sudden Unexpected Deaths in Epilepsy (SUDEP) almost always occur postictally, after GTCS and less commonly after a partial seizure. Monitored SUDEP or near-SUDEP cases without a seizure have not yet been reported in literature. When non-monitored SUDEP occurs in an ambulatory setting without an overt seizure, the absence of EEG information prevents the exclusion of a subtle seizure. These cases confirm the existence of non-seizure SUDEP; such deaths may not be prevented by seizure detection based devices. SUDEP risk in epilepsy patients may constitute a spectrum of susceptibility wherein some are relatively immune, death occurs in others with frequent GTCS with one ultimately proving fatal, while in others still, death may occur even in the absence of a seizure. We emphasize the heterogeneity of SUDEP phenomena.
Keywords: SUDEP, non-seizure SUDEP, near-SUDEP, EEG suppression
Introduction
Circumstantial, 1–3 witnessed 4 and monitored evidences 5; 6 suggest that Sudden Unexpected Death in Epilepsy (SUDEP) almost always occurs postictally, after a generalized tonic-clonic seizure (GTCS). 7 Monitored SUDEP or near-SUDEP cases without a seizure have not been reported in literature. Where non-monitored SUDEP has occurred in the community without an overt seizure, 2 the likelihood of electrographic seizures implicated in the agonal pathway is likely substantial. 7 We present a case series of monitored definite and probable SUDEP where there is no clinical or electroencephalographic evidence of underlying seizures.
Patients and Methods
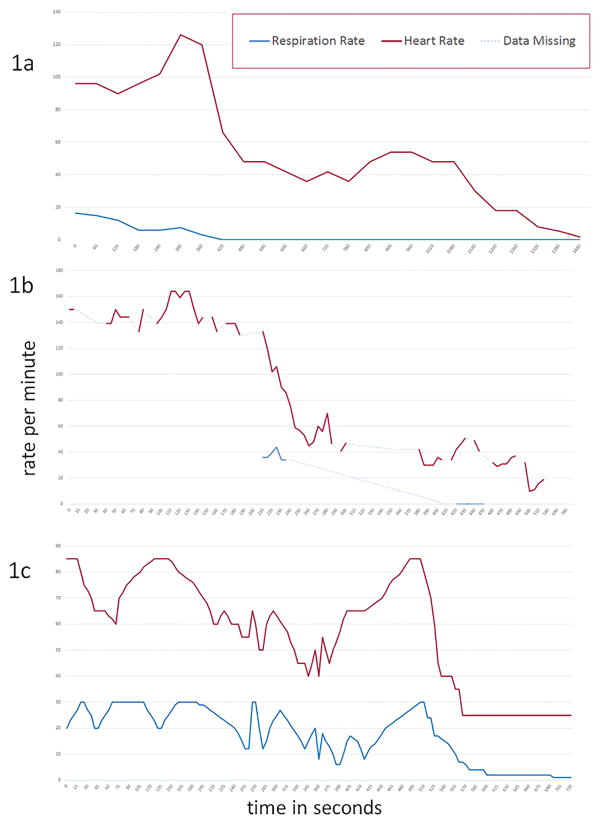
Monitored sudden unexpected death events in patients with epilepsy without apparent accompanying clinical and/or electrographic seizures were retrospectively ascertained from epilepsy monitoring units contributing data to the Center for SUDEP Research, a National Institute for Neurological Disorders and Stroke funded Center Without Walls. 8 In each case, good quality video EEG (VEEG) and single channel EKG were recorded during the terminal event. In Case 2, the patient was out of camera view for much of the event. EEG was analyzed using standard 10–20 electrode acquisition and bipolar montages in two cases and intracranial EEG in one. EEG suppression was defined as generalized background suppression of EEG, allowing for muscle, electrode or movement artifact. A stringent, 10 microvolt criterion was not used for suppression since one of the patients had intracranial EEG recording (Case 2). Using the same methodology as in the landmark MORTEMUS study, 5 we used video and movement artifact patterns to determine respiratory rates. In Case 2, the absence of reliable video data is represented in the missing data points seen in figure 1b.
Figure 1.
Sequence of cardiac, respiratory and EEG events in Case 1 (Figure 1a), Case 2 (Figure 1b) and Case 3 (Figure 1c) are shown. (CPR=cardiopulmonary resuscitation).
Results
Case 1
History
This 33 year old, right handed woman had focal epilepsy since the age of 8 years, with frequent complex partial seizures, right hand myoclonic jerks and GTCS. Seizures persisted despite numerous antiepileptic drugs (AEDs) and vagal nerve stimulation. Her only epilepsy risk factor was mild head injury. Left frontal lobe epilepsy was suspected based on semiology and interictal EEG spikes in the frontal regions, consistently maximum on the left. Ictal VEEG monitoring failed to lateralize seizure onsets. Structural (MRI) and functional (PET, SPECT) brain imaging were non-contributory. Carotid amobarbital (WADA) test confirmed left hemisphere language dominance. An invasive EEG study was undertaken to identify a resectable focus as the patient continued to suffer frequent, disabling seizures (multiple GTCS monthly) despite treatment with Felbamate, Phenobarbital, Lacosamide and Phenytoin. She had no known cardiac or pulmonary disease.
Invasive EEG recording with bilateral subdural strips in the fronto-temporal regions found independent left frontal and synchronous bifrontal discharges, some with a slight left frontal lead. Numerous clinical (behavioral arrest) and electrographic events suggested multifocal localization related epilepsy. Resective surgery was not considered feasible. Three years later, VEEG monitoring was performed after status epilepticus. AEDs were not withdrawn. Seven 20–60 second subclinical electrographic seizures were recorded, revealing bifrontal 3 Hz polyspike and wave complexes with left frontal maxima. Because of the severity of her condition, a second invasive recording with bilateral subdural strips was performed immediately following anterior 2/3rd callosotomy. Independent electrographic seizures restricted to either frontal region, or bifrontal discharges with a significantly greater left frontal maxima, were captured. The electrodes were removed without resection. Two CT head scans, two days apart, showed frontal craniotomy defects and postsurgical changes. A small bifrontal pneumocephalus in the first scan was reduced in the second. Resection cavity in the genu of the corpus callosum, extending to the anterior cingulate gyrus, was seen, with a small area of hypodensity consistent with ischemic edema in the adjacent frontal lobes.
Premortem hospital course
On day 11 after removal of subdural electrodes, due to persistent headaches and somnolence she was transferred back to the epilepsy monitoring unit from the rehabilitation unit to exclude subclinical seizures. She was prescribed buspirone 15 mg BID, clonazepam 0.5 mg qhs, lamotrigine 25 mg qd, felbamate 900 mg q6, fluoxetine 40 mg qhs, lacosamide 150 mg q8, levetiracetam 1000am and 1500pm, phenobarbital 45 mg q8, dexamethasone 3 mg q8 and butalbital/acetaminophen/caffeine 1–2 tabs q4 as needed. Fentanyl 25mcg q2 IV PRN was given intermittently for severe headache. Frequent left frontal (Fp1/F3/F7) maximum spike/polyspike and slow-wave discharges were seen. Subclinical seizures occurred several times per hour, with spike/polyspike and slow wave (Fp1/F3/F7) bursts of 20–40 seconds, during wakefulness without any behavioral correlates or disruption of behavioral activity. Metabolic studies were normal. On day three of scalp EEG monitoring, SUDEP occurred; Table 1 describes the sequence of events.
Table 1.
Progression of clinical and electrophysiological events in Case 1.
| Time | EEG | EKG | Respiration | Clinical |
|---|---|---|---|---|
| 21.32.04 | Drowsy, posterior dominant rhythm 7–8 Hz. | 105–110 beats per minute (bpm) | 16 breaths per minute (brpm) | Baseline, patient at rest in sitting position, right arm elevated |
| 21.35.01 | ~10 second burst of 1 Hz left fronto-central spikes followed by diffuse slowing of mixed ~1 Hz delta and low voltage theta throughout. | 110–120 bpm | 7brpm | Right arm slumps across chest; possible loss of postural tone. |
| 21:36:11 | Partial recovery, faster delta and theta activity | 128bpm | 3brpm | Patient immobile |
| 21:36:18 | Transition to generalized EEG suppression | 120bpm | 3brpm | Patient immobile |
| 21:37.00 | Marked generalized EEG suppression (Figure 2) | 120bpm to 50bpm within 120 seconds | 0brpm | Patient immobile |
| 21:39:28 | Marked generalized EEG suppression | 50bpm | 0brpm | Nurse injects fentanyl, earlier requested by patient. She remains until 21:40:00. |
| 21:40:10–21:41:55 | Marked generalized EEG suppression | 40 bpm | 0brpm | Patient immobile, does not respond to attempts to rouse |
| 21:42:18 | Marked generalized EEG suppression | 48 bpm | 0brpm | Patient immobile |
| 21:49:00 | Marked generalized EEG suppression | 20–25bpm, Occasional P waves without QRS | 0brpm | Patient immobile |
| 21:52:28, | Marked generalized EEG suppression | 6 bpm | 0brpm | Patient immobile |
| 21:53:49 | Marked generalized EEG suppression | Asystole | 0brpm | Patient immobile |
| 21:53:29; | Marked generalized EEG suppression | Asystole | 0brpm | EEG tech attempts to arouse patient. Calls nursing staff. |
| 21:54:29 | Marked generalized EEG suppression | Asystole | 0brpm | Nursing staff attempt to rouse patient with sternal rubs. Code is called. |
| 21:59:59 | Marked generalized EEG suppression | Asystole | 0brpm | Chest compressions began |
| 22:07:03, | Marked generalized EEG suppression | Sinus rhythm | Resuscitation continues |
Post-resuscitation course and death
The patient was transferred to the neuro intensive care unit and treated with hypothermia with continuous EEG monitoring. The EEG remained markedly suppressed until 08:21 am the following morning. This was followed by a burst suppression pattern that evolved to bursts of polyphasic epileptic discharges, slightly more prominent on the left indicating severe hypoxic ischemic brain damage. Three days post-arrest, ventilator support was withdrawn with family consent, given the poor prognosis for meaningful recovery. An autopsy revealed no anatomical or toxicological cause for death other than hypoxic ischemic brain injury.
Case 2
History
This 36 year-old right-handed man developed seizures at age 8 years, after undergoing a cystic cerebellar tumor resection, complicated by aseptic meningitis. He had mainly nocturnal, secondarily GTCS (4/month), occurring in clusters of one to four seizures at 45 to 120 minute intervals. Medically refractory to multiple medications, he took lamotrigine, zonisamide, and phenytoin at the time of death. He had suffered deep venous thrombosis, treated with Plavix and discontinued 4 months prior to hospital admission. His family history was negative for seizures. He had no cardiac or pulmonary disease.
MRI brain scans showed a midline cerebellar cystic lesion, and mild right temporal lobe atrophy. PET scan revealed right temporal and right cerebellar hypometabolism. EEG showed left and right sphenoidal sharp waves, and complex partial seizures EEG onset in the left sphenoidal electrode. Discordant imaging and scalp EEG data led to an invasive evaluation with bilateral subdural strip and depth electrodes (6 subdural electrodes over frontal, frontoparietal, and temporal regions on each side and 3 depth electrodes in each temporal lobe in the hippocampus and parahippocampal gyrus). He tolerated the procedure well and underwent a routine post-operative head CT on Day 1 of admission, subsequent MRI brain imaging on Day 2, and another head CT on Day 5. All studies revealed stable bilateral intracranial extra-axial air along the convexity with some mass effect but without midline shift, and a small amount of blood in the ventricles.
Premortem hospital course and death
He had 3 GTCS on Day 6 (ictal onsets right temporal ×2 and right frontal ×1). On Days 6–9, he had intermittent low grade fevers up to 100.3 F and on day 9, up to 101.1 F. CSF examination showed no white blood cells. Peripheral complete blood counts, urinalysis, metabolic panel, blood and CSF cultures were normal. Bilateral lower extremity ultrasound testing revealed no deep venous thrombosis. On day 9, he went to the bathroom to urinate, was heard falling off the toilet and found unresponsive (“Event 1”, see table for details). This was followed closely by a second episode of unresponsiveness (“Event 2”, see table for details) from which he did not recover. His family declined autopsy.
Case 3
History
The patient was a 50 year old right handed man with left temporal lobe epilepsy due to hippocampal sclerosis since childhood, with nocturnal complex partial seizures secondarily generalizing at least twice a month. He had a history of a childhood febrile convulsion at age 6 months. He was both medically (> ten medications) and surgically (left temporal lobectomy at age 32 years after subdural electrode study, vagal nerve stimulator in-situ at age 45 years) refractory. After 5 years of post-surgical seizure freedom, his habitual seizures returned. Multiple epilepsy monitoring unit admissions (including one the previous year) recorded rare left temporal sharp waves (maximum at F7) but no seizures. Six years previously, a habitual seizure had been recorded on video EEG, characterized by hypermotor features, including pelvic thrusting, screaming and grabbing his throat with his left hand. The ictal EEG showed diffuse left hemisphere beta maximum in the temporal chain, evolving into repetitive spiking in the left frontal region. He was hypertensive but had no known cardiac or pulmonary disease.
Premortem hospital course and death
He was admitted to hospital through the emergency department with a witnessed, prolonged daytime GTCS requiring lorazepam 2mg and a repeat dose of 2 mg by paramedics. On arrival in the ED, he was hypoxic with shallow breaths. He was loaded with levetiracetam 1gm and then 1gm BID, additional to his daily medication (felbamate 3000mg, valproic acid 500mg, lamotrigine 175mg, lisinopril 20mg, ramipril 5mg, Prilosec 40 mg, quetiapine 25mg and simvastatin 20mg). CT scan showed prior left temporal lobectomy. He was intubated, sedated with propofol and admitted to the intensive care unit for a day, following which he was moved to the epilepsy monitoring unit. Chest X-ray showed possible right lower lobe consolidation from presumed aspiration pneumonia, treated with intravenous piperacillin/tazobactam. Video EEG monitoring over the ensuing 4 days showed normal posterior background and a left temporal breach rhythm. No epileptiform abnormalities or electrographic seizures were recorded.
On Day 5, he had a coughing episode following which he developed respiratory distress and then terminal cardio-respiratory failure (Table 3). In 25 minutes of cardio-pulmonary resuscitation (CPR), he suffered two cycles of asystole, followed by one cycle of pulseless electrical activity, followed by four cycles of ventricular tachycardia requiring DC shock. After initial resuscitation, the patient was transferred to the intensive care unit and ventilated. The patient’s condition deteriorated further and he died on Day 3 of readmission to the ICU. Heart, kidneys, corneas and skin were removed for organ donation. Autopsy of the remaining organs revealed no anatomical or toxicological cause of death; lung parenchyma was normal (despite premortem X-ray findings). There was no evidence of pulmonary embolism.
Table 3.
Progression of clinical and electrophysiological events in Case 3.
| Time | EEG | EKG | Respiration | Clinical |
|---|---|---|---|---|
| 17:30:02 | Baseline. Intermittent left temporal theta slowing. | 75bpm | Coughs every 2–3 seconds | Patient sitting in bed. Has coughing attack lasting until 17:32:30 |
| 17:32:30 | Baseline | 85bpm | 25 brpm | Mumbling. Appears agitated as if struggling to breath. |
| 17:32:42 | Diffuse mixed delta-theta slowing | 75bpm | 30 breaths per min. Fast and shallow, prominent movement of sternocleidomastoids | Appears agitated, struggles weakly to breathe |
| 17:33:41 | Marked generalized EEG suppression ( abrupt onset) | 60bpm | 25 breaths per min. shallow breathing, prominent movement of sternocleidomastoids | Immobile |
| 17:34:15 | Marked generalized EEG suppression | 55bpm | 15 breaths per min. shallow breathing | Immobile |
| 17:34:22 | EEG picks up in amplitude into generalized delta slowing | 55bpm | 15 breaths per min. shallow breathing | Immobile, moaning heard with breaths |
| 17:34:29 | Mixture of marked generalized EEG suppression and generalized delta slowing | 60bpm | 30 breaths per min. gradually decreases to 15 breaths per min. | Immobile, moaning heard with breaths. Nurse enters, assumes patient is seizing. Administers O2. |
| 17:35:21 | Marked generalized EEG suppression | 55 bpm | 15–20 breaths per min., shallow breathing | Immobile, unresponsive |
| 17:36:40 | EEG picks up in amplitude into generalized delta slowing | 65–70 bpm | 25 breaths per min., shallow breathing | Patient suddenly becomes agitated, moaning and moving arms. Two right facial twitches seen. |
| 17:37:30 | Marked generalized EEG suppression | 70–85 bpm | 25–30 breath per minute, shallow breathing | Immobile, unresponsive despite suction and O2. Ativan 2mg at 17:38:20. |
| 17:41:30 | Marked generalized EEG suppression | Slows to 20 bpm | Breathing appears to stop | Immobile, unresponsive |
| 17:44:50 | Marked generalized EEG suppression | 20bpm | Respiratory arrest | CPR started |
DISCUSSION
Deaths in epilepsy patients occurring more than one hour after a known terminal event, interrupted by temporarily successful CPR, are classified as near-SUDEP. 9 Since two of the three cases presented succumbed in this fashion after what were clearly terminal events that would have ended in immediate death without CPR, we refer to all three cases as SUDEP (two definite and one probable). They suggest that non-seizure SUDEP can occur and further emphasize the heterogeneity of SUDEP phenomenology. SUDEP usually occurs after GTCS. 7 Prior reported video EEG monitored SUDEP deaths have followed GTCS and all previously monitored near SUDEP cases were preceded by either GTCS or partial seizures. 5; 7 SUDEPs witnessed by family without an overt or habitual seizure in 50% of pediatric cases, 2 suggests that SUDEP can occur outside the peri-ictal period, although the absence of EEG monitoring in ambulatory settings renders this speculative. In a series of witnessed SUDEP, three of 15 cases did not have overt seizures preceding death. However, one of the three likely had at least an aura (shouted “I’m going to have a seizure” before collapsing), one had suffered a seizure five minutes before and one had circumstantial evidence of a preceding, unwitnessed seizure (confusion, incontinence). 4 In another study, 21 of 24 (88%) patients had evidence of an agonal seizure but seizures in the remaining three cases were not reported, 1 an observation common to all studies where direct or indirect evidence of a premortem seizure is lacking in some patients.3; 10–14
The failure to record electrographic seizures in our three patients immediately prior to respiratory and cardiac arrest does not exclude hidden seizures that may have been causally implicated in death, but renders them highly unlikely. All three patients were on their full antiepileptic medications at the time of death and there was no increase in inter-ictal EEG abnormalities preceding death. This scenario excludes GTCS, the main SUDEP seizure type as the proximate cause, since these have characteristic clinical, EEG and EMG signatures that were absent in all cases. Habitual clinical partial seizures, which are far less common in SUDEP, 5 were also not seen. Further, two cases had unilateral or bilateral cranial skull defects due to prior surgery (the third case had infratentorial cranial surgery), creating a defect that allows greater sensitivity to record cortical EEG activity. The likelihood of entirely new (non-habitual) seizure types, involving extremely focal cortical areas (less than 10cm2 15), but sufficient to cause death, is implausible. It therefore seems reasonable to conclude that in all three cases, evidence supports cardio-respiratory collapse occurring without preceding seizures.
The factors that initiated death in these individuals remain unknown. All three patients were typical of high SUDEP risk cases with longstanding epilepsy and frequent GTCS. Our data excludes a primary cardiac cause since neither tachyarrhythmias (ventricular tachycardia, fibrillation, torsades des pointes) nor asystole initiated the fatal cascade. In MORTEMUS, no tachyarrhythmic deaths occurred, and indeed, such occurrences in SUDEP/near-SUDEP reports are extremely rare. Peri-ictal tachyarrhythmias have occurred in three near-SUDEP cases16, one probable SUDEP, 17 and one possible SUDEP with significant ischemic heart disease.18 The association of long QT genes with epilepsy, 19 and the occurrence of sudden cardiac deaths in epilepsy patients, 20 raise the possibility of fatal cardiac arrhythmias occurring independently of epileptic seizures and may account for the over-representation of LQT genes found in un-monitored SUDEP. 21; 22 However, all three patients had reliable EKG recordings and none had any ventricular tacharrhythmias prior to cardio-respiratory arrest and cardiopulmonary resuscitation. Case 3 had four cycles of post CPR ventricular tachycardia which likely resulted from the CPR itself but did not initially trigger the fatal cascade.
A primary brainstem cause initially affecting respiration is suggested by the highly abnormal breathing patterns, and EEG suppression seen in our patients. In Case 1, there appeared to be sudden loss of postural tone with sudden halving of respiratory rate, without accompanying bradycardia, and diffuse EEG slowing. In the next 70 seconds, respiration dropped to 3 breaths per minute and then breathing stopped after another 49 seconds. Onsets of terminal apnea, profound bradycardia (120/minute to 50/minute within 20 seconds), and marked EEG suppression were simultaneous. This suggests failure of cortical function as well pontomedullary mechanisms responsible for cardio-respiratory homeostasis. Profound hypotension alone is a possible 23 scenario although the abrupt high amplitude delta slowing that precedes EEG flattening in such patients, was absent in this case. 24 Without a cortical seizure, an initial brainstem mechanism in the fatal cascade is probable, such as the spreading depression initiated by dorsal medullary application of potassium chloride microinjection in Kv1.1 SUDEP mouse models. 25 The absence of a seizure trigger for spreading depression is consistent with the spontaneous onset of such a phenomenon in many patients with migraine with aura and familial hemiplegic migraine, where attacks may occur without an identifiable trigger. Although fentanyl was administered, this was after apnea developed, suggesting that this drug was not primarily responsible for the fatal events. Earlier injections had not caused similar difficulties.
In Case 2, the absence of video rendered analysis difficult although the pattern of events was similar to some of the MORTEMUS cases; initial tachypnea and tachycardia was followed by progressive bradycardia and apnea. Peripheral pulses were weak suggesting possible hypotension. Autopsy was not carried out and although alternative cardio-pulmonary explanations for death cannot be entirely excluded, we considered any setting for pulmonary embolism (no deep vein thrombosis, negative lower limb Doppler studies), myocardial infarction, raised intracranial pressure or cerebrovascular events unlikely. In Case 3, respiratory distress appeared to start with a bout of coughing, without any apparent structural cause revealed by autopsy. Recurrent bradypnea, EEG suppression and bradycardia appeared to roughly coincide, similar to some MORTEMUS patients although in the latter, there was no recovery of EEG suppression. Lorazepam 2mg was given to the patient 50 seconds after terminal EEG suppression onset. Although this could have contributed to respiratory depression, it is extremely unlikely to have caused bradycardia or EEG suppression, both of which are more likely to be causally linked to hypopnea. The coughing and initially severe respiratory distress may have represented laryngospasm, a rare occurrence in seizures. 26 However, neither inspiratory stridor nor cyanosis were observed; this phenomenon had never occurred before and there were no ictal EEG changes.
Sudden deaths in adults are usually cardiac or seizure related. Co-incidental sudden death unrelated to epilepsy is a possibility although our patients had the “classic” SUDEP phenotype and this scenario is therefore relatively unlikely. All three patients were Caucasian, deaths took place during evening hours (while awake), none were prone, and no arrhythmias were seen. SCN5A related sudden unexpected nocturnal death syndrome is therefore an unlikely explanation. In all cases, although terminal events included varying patterns of cardiac and respiratory dysfunction, their triggers are unclear and epileptic seizures cannot be considered as a contributory factor. Many patients with frequent GTCS do not suffer SUDEP and it is hypothesized that fatal risk is conferred by structural and/or functional brainstem abnormalities, underpinned by genetic predisposition, all coming to a head in the aftermath of a critical event – the seizure. 8 SUDEP risk in epilepsy patients may however, constitute a spectrum of susceptibility wherein some are relatively immune, death occurs in others with frequent GTCS with one ultimately proving fatal, while in others still, death may occur even in the absence of a seizure. Such non-seizure deaths are likely to constitute a minority of SUDEP cases, and importantly, are unlikely to be detected by motion detecting seizure alarms; apnea detectors may have an advantage in such cases.
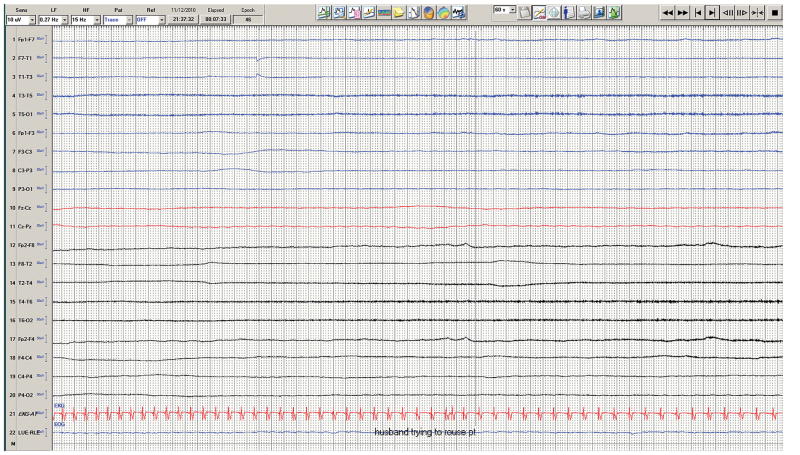
Figure 2.
Sixty second record showing marked generalized EEG suppression and progressive bradycardia in Case 1.
Table 2.
Progression of clinical and electrophysiological events in Case 2.
| Time | EEG (intracranial) | EKG | Respiration | Clinical (out of camera range; audio only) |
|---|---|---|---|---|
| Event 1 | ||||
| 9.32.04 | Baseline | 132bpm | N/A | No complaints, asks to go to the bathroom; Registered Nurse (RN) observes patient sit on toilet (for safety) |
| 19:44:36 | Abrupt theta-delta, progressed to diffuse delta | 120bpm, later obscured by artifact | N/A | Patient heard falling in bathroom at 19:44:50. RN assumes seizure |
| 19:44:50 | Suppression | 84bpm | N/A | Unresponsive |
| 19:45:05 | Diffuse delta-theta, with intermittent generalized suppression, then diffuse theta | 84bpm | N/A | Patient initially unable to follow commands, moving, some verbalization |
| 19.45.52 | Diffuse theta | 84bpm | Deep breathing at 42 brpm heard briefly | Following commands |
| 19:49:10 | Return to baseline | Heart rate 144 BPM | N/A | Asks to lie down; asks for water; complains of left leg numbness. Noted to be cold and clammy. Denied shortness of breath or headache. |
| Event 2 | ||||
| 20:00:17 – 20:02:00 | Baseline | 144bpm | N/A | Patient feels cold to touch; reliable BP unobtainable. Patient states he feels “a little light and hot” |
| 20:02:16 | Baseline | 144bpm | N/A | Patient feels “clammy and wet”; RN asks patient to stay still. Conversing. |
| 20:02:47 – 20:03:00 | Transition to diffuse theta slowing | 132bpm | 42brpm | RN trying to help patient sit up |
| 20:03:33 – 20:03:56 | Diffusely slow (theta/delta) – progresses to diffuse delta | 102bpm | 42brpm, moaning | RN encourages patient to get up from floor with assistance. Patient feels hot, drinks water. |
| 20:03:57 | Marked generalized EEG suppression | 60bpm, then progressive bradycardia and idioventricular rhythm | N/A | Unresponsive; RN assumes seizure |
| 20:08:37 | Marked generalized EEG suppression | Intermittent ventricular complexes | 2brpm Face briefly seen on video |
Unresponsive. RN assessing patient, weak pulse noted. RN administers O2 via mask/manual ventilation (video/audio available at this point) |
| 20:09:00 – 20:13:00 | No data | No data | No data | Cardiopulmonary resuscitation (CPR) begun at the end of this this period |
Key Points.
SUDEP can occur without a preceding seizure
Such deaths are not primarily cardiac
Progressive cardio-respiratory compromise and EEG suppression characterize deaths
Non-seizure SUDEP patients have a similar risk profile to other SUDEP patients
SUDEP is a heterogeneous phenomenon
Acknowledgments
This work is supported by NIH grants U01-NS090405, U01-NS090407, U01-NS090405 (SDL, MN, OD, BZ, NL), U01 NS090407, U01 NS090415, R01 EB 018308, R01 MH 094480 and CDC # U48 DP 005008-01S4 (OD).
Footnotes
Ethical Publication Statement: ‘We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.’
Disclosures: None of the authors has any conflict of interest to disclose.
References
- 1.Nashef L, Garner S, Sander JW, et al. Circumstances of death in sudden death in epilepsy: interviews of bereaved relatives. J Neurol Neurosurg Psychiatry. 1998;64:349–352. doi: 10.1136/jnnp.64.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donner EJ, Smith CR, Snead OC., 3rd Sudden unexplained death in children with epilepsy. Neurology. 2001;57:430–434. doi: 10.1212/wnl.57.3.430. [DOI] [PubMed] [Google Scholar]
- 3.Kloster R, Engelskjon T. Sudden unexpected death in epilepsy (SUDEP): a clinical perspective and a search for risk factors. J Neurol Neurosurg Psychiatry. 1999;67:439–444. doi: 10.1136/jnnp.67.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langan Y, Nashef L, Sander JW. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry. 2000;68:211–213. doi: 10.1136/jnnp.68.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 6.Hewertson J, Poets CF, Samuels MP, et al. Epileptic seizure-induced hypoxemia in infants with apparent life-threatening events. Pediatrics. 1994;94:148–156. [PubMed] [Google Scholar]
- 7.Devinsky O. Sudden, unexpected death in epilepsy. N Engl J Med. 2011;365:1801–1811. doi: 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]
- 8.Lhatoo S, Noebels J, Whittemore V. Sudden unexpected death in epilepsy: Identifying risk and preventing mortality. Epilepsia. 2015;56:1700–1706. doi: 10.1111/epi.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nashef L, So EL, Ryvlin P, et al. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012;53:227–233. doi: 10.1111/j.1528-1167.2011.03358.x. [DOI] [PubMed] [Google Scholar]
- 10.Nashef L, Fish DR, Sander JW, et al. Incidence of sudden unexpected death in an adult outpatient cohort with epilepsy at a tertiary referral centre. J Neurol Neurosurg Psychiatry. 1995;58:462–464. doi: 10.1136/jnnp.58.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klenerman P, Sander JW, Shorvon SD. Mortality in patients with epilepsy: a study of patients in long term residential care. J Neurol Neurosurg Psychiatry. 1993;56:149–152. doi: 10.1136/jnnp.56.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leestma JE, Annegers JF, Brodie MJ, et al. Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia. 1997;38:47–55. doi: 10.1111/j.1528-1157.1997.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 13.Earnest MP, Thomas GE, Eden RA, et al. The sudden unexplained death syndrome in epilepsy: demographic, clinical, and postmortem features. Epilepsia. 1992;33:310–316. doi: 10.1111/j.1528-1157.1992.tb02321.x. [DOI] [PubMed] [Google Scholar]
- 14.Leestma JE, Walczak T, Hughes JR, et al. A prospective study on sudden unexpected death in epilepsy. Ann Neurol. 1989;26:195–203. doi: 10.1002/ana.410260203. [DOI] [PubMed] [Google Scholar]
- 15.Tao JX, Ray A, Hawes-Ebersole S, et al. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46:669–676. doi: 10.1111/j.1528-1167.2005.11404.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Lende M, Surges R, Sander JW, et al. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry. 2016;87:69–74. doi: 10.1136/jnnp-2015-310559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nei M, Ho RT, Abou-Khalil BW, et al. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia. 2004;45:338–345. doi: 10.1111/j.0013-9580.2004.05503.x. [DOI] [PubMed] [Google Scholar]
- 18.Dasheiff RM. Sudden unexpected death in epilepsy: a series from an epilepsy surgery program and speculation on the relationship to sudden cardiac death. J Clin Neurophysiol. 1991;8:216–222. [PubMed] [Google Scholar]
- 19.Johnson JN, Hofman N, Haglund CM, et al. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology. 2009;72:224–231. doi: 10.1212/01.wnl.0000335760.02995.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamberts RJ, Blom MT, Wassenaar M, et al. Sudden cardiac arrest in people with epilepsy in the community: Circumstances and risk factors. Neurology. 2015;85:212–218. doi: 10.1212/WNL.0000000000001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagnall RD, Crompton DE, Petrovski S, et al. Exome-based analysis of cardiac arrhythmia, respiratory control and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol. 2015 doi: 10.1002/ana.24596. [DOI] [PubMed] [Google Scholar]
- 22.Leu C, Balestrini S, Maher B, et al. Genome-wide Polygenic Burden of Rare Deleterious Variants in Sudden Unexpected Death in Epilepsy. Ebiomedicine. 2015;2:1063–1070. doi: 10.1016/j.ebiom.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozorgi A, Chung S, Kaffashi F, et al. Significant postictal hypotension: expanding the spectrum of seizure-induced autonomic dysregulation. Epilepsia. 2013;54:e127–130. doi: 10.1111/epi.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Dijk JG, Thijs RD, van Zwet E, et al. The semiology of tilt-induced reflex syncope in relation to electroencephalographic changes. Brain. 2014;137:576–585. doi: 10.1093/brain/awt332. [DOI] [PubMed] [Google Scholar]
- 25.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med. 2015;7:282ra246. doi: 10.1126/scitranslmed.aaa4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavee J, Morris H., 3rd Severe postictal laryngospasm as a potential mechanism for sudden unexpected death in epilepsy: a near-miss in an EMU. Epilepsia. 2008;49:2113–2117. doi: 10.1111/j.1528-1167.2008.01781.x. [DOI] [PubMed] [Google Scholar]