Summary
An area of active research in the field of cardiac gene therapy aims to achieve high transfection efficiency without eliciting immune or inflammatory reactions. Nanomedicine offers an attractive alternative to traditional viral delivery vehicles because nanoparticle technology can enable safer and more controlled delivery of therapeutic agents. Here we describe the use of lipidoid nanoparticles for delivery of modified mRNA (modRNA) to the myocardium in vivo, with a focus on rodent models that represent a first step toward preclinical studies. Three major procedures are discussed in this chapter: 1) preparation of lipid modRNA nanoparticles, 2) intramyocardial delivery of the lipid modRNA nanoparticles by direct injection with an open chest technique in rats, and 3) intracoronary delivery of the lipid modRNA nanoparticles with open chest and temporary aortic cross clamping in rats.
Keywords: lipid nanoparticles, modified mRNA, lipidoid, intramyocardial injection, intracoronary, injection
1. Introduction
Nanotechnology is a multidisciplinary field that uses principles from chemistry, biology, physics and engineering to design, fabricate and study nanoparticle structures, defined as having at least one dimension in the 1–300 nanometer range. For biomedical applications, nanoparticles made using a variety of formulations, including polymers or lipid-based components, have been used to encapsulate drugs, DNA and RNA [1, 2]. Lipid nanoparticles are attractive as non-viral transfection agents, allowing delivery of genetic material with temporally controlled release at high transfection rates. DNA and RNA carried in lipid nanoparticles also exhibit lower degradation rates and higher likelihood of cellular uptake compared to free nucleotides in solution[3]. In addition, the use of synthetic modified mRNA (modRNA) incorporating pseudouridine instead of uridine results in a lower degree of translation inhibition [4]. We have recently combined lipid-based nanoparticles and modified mRNA technologies, and demonstrated highly efficient delivery and expression in myocardium of small and large animal models (Fig. 1) [5].
Figure 1.
Schematic of preparation of FLNPs/modRNA. A) An epoxide-derived lipidoid (mixed in ethanol with stabilizers (DSPC, cholesterol, PEG-DMG), is added to pseudouridine-modified mRNA (dissolved in citrate buffer) resulting in synthesis of FLNPs. B) Representative immunofluorescence microscopy image of rat heart (10µm cryosection) 20 hours after intramyocardial injection of FLNPs/ modRNA (10µg of eGFP modRNA), showing GFP positive cells (green), nuclei stained with DAPI. Arrows point to the epicardium. Scale bar=100µm.
To help make this technology accessible to other investigators, herein we describe lipid-based nanoparticles containing modRNA using a custom formulation consisting of an epoxide-derived lipidoid (mixed in ethanol with the stabilizers DSPC, cholesterol and PEG-DMG), added to modified mRNA (dissolved in citrate buffer) resulting in synthesis of formulated lipid nanoparticles (FLNPs) via nanoprecipitation. The nanoparticles are then purified by dialysis at 4°C for 3 hours. After the dialysis period, this synthesis process renders formulated lipidoid/modRNA nanoparticles (FLNP/modRNA) that can be maintained at 4°C and remain stable for at least 14 days from the time of synthesis; this stability is clearly advantageous for future preclinical studies, since it allows for storage and transport of the nanoparticle solution to the facility where it would be required.
We also describe two different routes of delivery to the heart, which are applicable for rat in vivo studies: 1) intramyocardial injection using a common open chest technique, and 2) intracoronary injection using temporary aortic cross clamping in an open-chest preparation. Both have proven to be effective delivery methods for virus-associated gene therapies to the heart [6], and recently we showed their use for delivery of eGFP FLNP/modRNA [5]. There are variations of the cross-clamping technique, ranging from clamping the aorta alone, aorta and pulmonary artery, and superior and inferior vena cava [6–11]. Of particular interest is the intracoronary delivery route, for although it requires an invasive procedure for small animal applications as described here, it serves to evaluate a method of delivery that involves only a minimally invasive procedure by percutaneous catheter-based intracoronary delivery in large animals and humans, which is appropriate in preclinical and translational studies.
2. Materials
2.1 Formulated lipidoid mRNA nanoparticles
- Lipids (see Note 1). Prepare 10 mg/mL ethanol solutions of the 4 lipids listed below:
- epoxide-derived lipidoid reagent, termed C14-113 lipidoid (MW 541), as synthesized in Love et al. [12]. Store at 4°C.
- distearoyl phosphatidylcholine (DSPC) (MW 790.15) (Avanti Polar Lipids). Store at −20°C.
- Cholesterol (MW 386.66). Store at −20°C.
- polyethylene glycol (PEG)-lipid conjugate (PEG-DMG) (MW 2555) (14:0 PEG2000 PE) (Avanti Polar Lipids). Store at −20°C.
eGFP modRNA (1 mg/mL in water) (see Note 2).
10 mM sodium citrate buffer (pH 3.0), sterile-filtered.
1X phosphate-buffered saline (PBS, pH 7.4), sterile-filtered.
Ethanol 100%.
Deionized water.
Microcentrifuge tubes (sterile, DNAse/RNAse free).
Barrier pipette tips (sterile, DNAse/RNAse free).
Slide-A-Lyzer Dialysis Cassette 20K MWCO.
Sterile disposable syringes (1mL).
Hypodermic needles (18 G - 21G, at least 1.5” long).
Large glass beaker, 4L.
Stir bar.
Stir plate (placed in cold room at 4°C).
Aluminum foil (to cover glass beaker during dialysis).
Permanent ink marker.
2.2 Intramyocardial delivery of lipid mRNA nanoparticles
Drugs: Ketamine, xylazine, buprenorphine.
Electric razor (animal hair clipper).
16G or 18G IV catheter.
Mechanical ventilator.
Heating pad.
Adhesive tape (1–2” wide).
Gooseneck lamp-microscope illuminator.
Stereo microscope
Surgical instruments (sterile): Micro-Adson forceps, Hemostat clamps, Extra Fine Bonn scissors, Alm retractor, Castroviejo needle holder, Halsey needle holder, scalpel blade and handle.
Sutures: Silk 6-0 (tapered needle), 4-0, Nylon 5-0.
Gauze pads (2”×2”).
Cotton-tipped applicators.
2.3 Intracoronary delivery of lipid mRNA nanoparticles
Same as in 2.2 above, and in addition include the following:
24G × ¾” IV catheter.
Atraumatic clamp.
3. Methods
3.1 Calculation of required materials
Calculate the required quantities of each component, following steps 1–6.
- Establish the total amount of µg of mRNA that you plan to use for injection(s). Based on that, the total formulation volume (µl) is calculated for a final mRNA concentration of 0.1mg/ml.
- Next, calculate the “required” volume of the organic lipid mixture (µl) and the aqueous mRNA mixture volume (µl) using the following formulas (based on the 1:3 volumetric mixing ratio of the organic lipid mixture to aqueous mRNA solution):
- Calculate the required volume of each of the organic lipid mixture components: C14-113 lipidoid, DSPC, Cholesterol and PEG-DMG at a working molar ratio of 50:10:38.5:1.5 and a working concentration of 4mg/ml, 1.168mg/ml, 2.201 mg/ml and 0.567mg/ml respectively; starting with a stock concentration of 10mg/ml for each of the components (see “Lipids” in Materials). Substitute the component in square brackets by either lipidoid, DSPC, Cholesterol or PEG-DMG.
- Calculate the required ethanol volume on which the lipidoid components will be diluted.
-
Calculate “scale up” volumes of each organic lipid mixture component. It is important to always make more organic lipid mixture than what is required so that at the time of synthesis sufficient lipid solution is available (see Note 3). A scale-up factor of 2 is generally adequate; it can be adjusted as needed.
The above calculations serve to specify the minimum stock quantities required from each of the components of the organic lipid mixture.
- Based on a 10:1 mass ratio of C14-113 lipidoid to mRNA, and the 1:3 volumetric mixing ratio of the organic lipid mixture to aqueous mRNA solution, dilute the stock modRNA in citrate buffer as needed. A stock concentration of 1 mg/ml is ideal; if substantially below this concentration, mRNA can be concentrated by the standard protocol of nucleic acid precipitation and centrifugation (using an RNAse-free mixture of sodium acetate buffer and ethanol). For a working concentration of 4 mg/ml of lipidoid, a working mRNA concentration of 0.133 mg/ml is needed, that is, 4 mg/ml lipidoid / (10 mg lipidoid / 1 mg mRNA) × (1 ml lipidoid / 3 ml mRNA). Using the given stock mRNA concentration, the aqueous mRNA mixture volume, and the working mRNA concentration, calculate the stock mRNA volume and citrate buffer volume required for the dilution as follows.
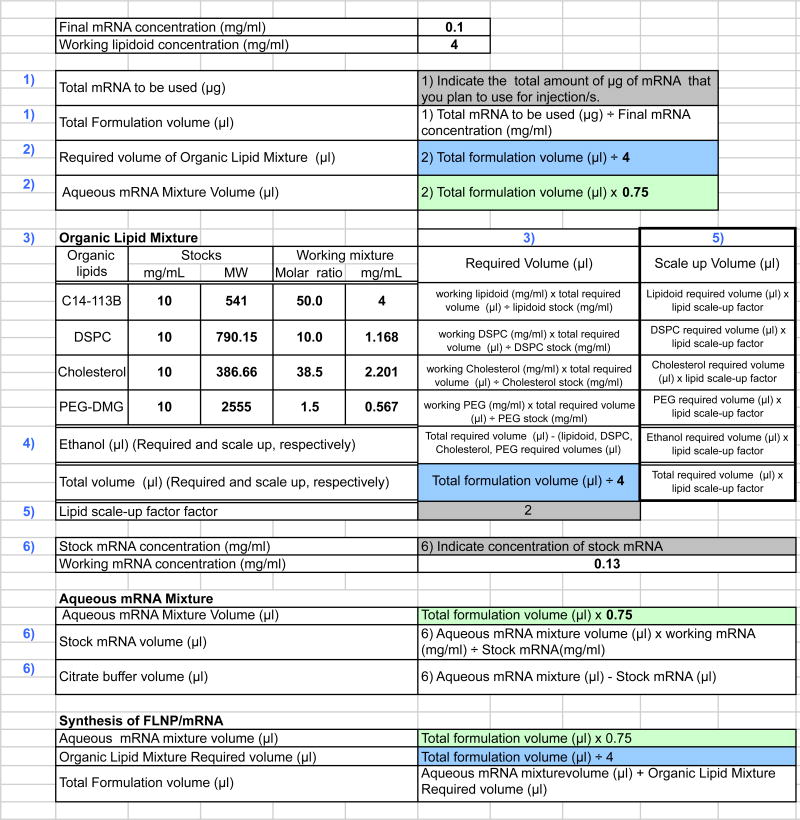
To organize the equations and easily perform the calculations each time they are needed, we recommend creating an electronic spreadsheet using the values and formulas shown in Figure 2. An example of the results from these calculations is shown in Figure 3.
Figure 2.
Formulas and values for calculation of required quantities of each component for preparation of formulated lipidoid modRNA nanoparticles. Grey box: The input in these cells is determined by the investigator according to the planned experiments. Blue box: The output in this box is the required volume of organic lipid mixture in microliters. Green box: The output in this box is the aqueous mRNA mixture volume in microliters.
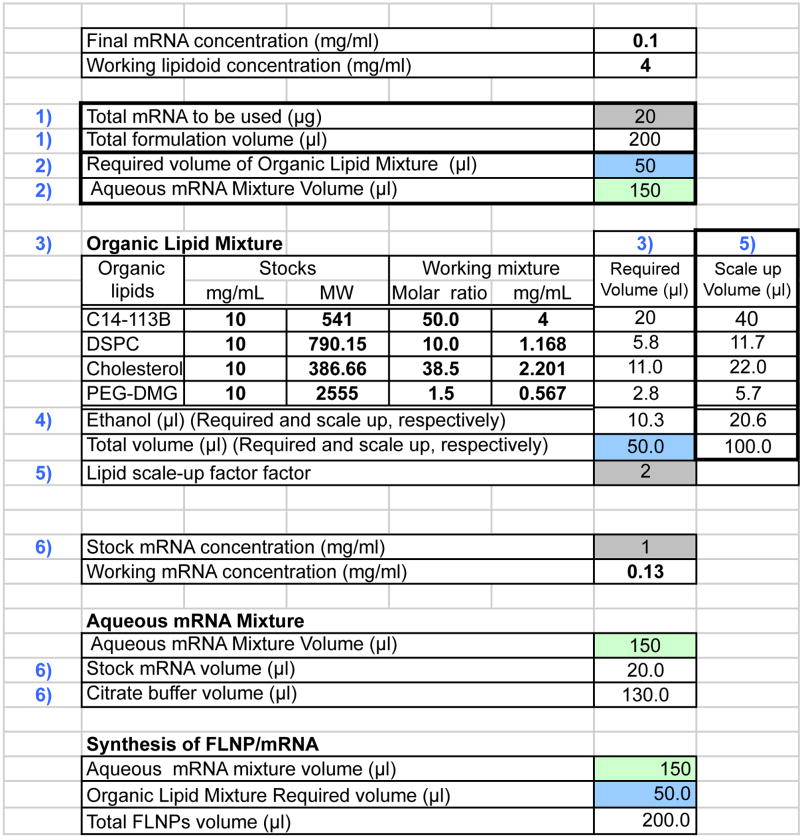
Figure 3.
Example of calculation of required quantities of each component for preparation of formulated lipidoid modRNA nanoparticles. Color coding same as figure 2.
3.2 Preparation of formulated lipidoid modRNA nanoparticles
Bring all stocks of the required components to room temperature (lipidoid, DSPC, PEG, and cholesterol) and make sure they are well dissolved prior to use (see Note 4).
Prepare the organic lipid mixture. In a microcentrifuge tube, first add the “scale up” volume of ethanol, then add the “scale up” volumes of each of the 4 lipid components in the following order: lipidoid, DSPC, cholesterol and PEG-DMG. Mix by pipetting several times after adding each component.
Prepare the aqueous mRNA mixture. In a microcentrifuge tube, first add the citrate buffer, and then add the mRNA. Mix by pipetting several times.
Synthesize formulated lipid nanoparticles/mRNA (FLNP/mRNA). Add the “required volume” of the organic lipid mixture to the tube containing the aqueous mRNA mixture (1:3 volume/volume organic lipid: aqueous mRNA). Mix by repeated pipetting for 15 seconds.
Leave undisturbed for 10 min at room temperature to allow for self-assembly of FLNPs.
In a large glass beaker, add 3 L of 1X PBS and a magnetic stir bar. Pre-wet the dialysis cassette membrane by immersion of the dialysis cassette in the beaker for at least 2 min. Remove dialysis cassette from the beaker and gently tap to remove excess fluid (see Note 5). Cover the top of the beaker with aluminum foil. Bring the beaker with the PBS and stir bar to a cold room (4°C) and place on a magnetic stir plate (see Note 6).
Dilute the FLNP/mRNA solution by adding 1X PBS as needed and pipet to mix.
Collect the sample from the microcentrifuge tube (FLNP/mRNA solution) into a 1-mL syringe with needle, leaving a small amount of air in the syringe.
Mark one corner port of the dialysis cassette with a permanent ink marker. Penetrate the gasket through a syringe guide port at the marked corner and inject the sample into the dialysis cassette while holding it upright. Use caution, as inserting the needle too far into the sample chamber may puncture the membrane (see Note 7).
Place the dialysis cassette in the beaker with PBS on a stir plate. Let it float vertically and set the stir plate to a low setting (gentle stirring). Dialyze overnight (or for at least 3 hours) at 4°C (see Note 8). For dialysis volumes lower than 750µl there is no need to exchange the PBS dialysis buffer. For larger volumes, replace the dialysis buffer with fresh 1× PBS once after the first 2 hours.
Remove the dialysis cassette form the beaker. Penetrate gasket with a new syringe and needle through the unused syringe guide port (the one that is not marked) and slowly inject air into the chamber to the maximum allowed volume to separate the membranes, which helps prevent needle puncture of the cassette membrane. With the needle in place, turn the cassette upside down so that the needle is at the bottom, and as sample collects near the port withdraw sample into the syringe.
Transfer the solution containing dialyzed FLNP/mRNA from the syringe into a microcentrifuge tube. Measure the new volume (post dialysis volume) (See Note 9) and adjust the concentration accordingly to calculate the volume/dose to be injected. Maintain the formulation at 4°C until ready for use (See Note 10).
3.2 Intramyocardial delivery of the lipid mRNA nanoparticles by direct injection in rats
Calculate in advance of the procedure the total volume (µl) of FLNP/modRNA required for delivering the desired full dose of mRNA (µg). As described below, the total volume will be delivered in multiple injections.
Anesthetize the rat by intraperitoneal injection of ketamine/zylaxine (60–80 mg/kg and 5mg/kg, respectively). Shave the chest.
Once the animal is sedated, cannulate the trachea with a 16G or 18G (1 ¼”-2” length) IV catheter, (See Note 11) and connect to a mechanical ventilator providing room air (70–90 breaths/min) (See Note 12). Place animal in right lateral position, and secure the animal by the limbs to the operating board with adhesive tape. Use a heating pad to maintain a body temperature of 37°C. Wipe the chest wall with povidone-iodine solution.
Perform a left thoracotomy between the fourth and fifth ribs. Place a self-retaining retractor in the fourth intercostal space to stretch open the thorax. Incise the pericardium to expose the heart.
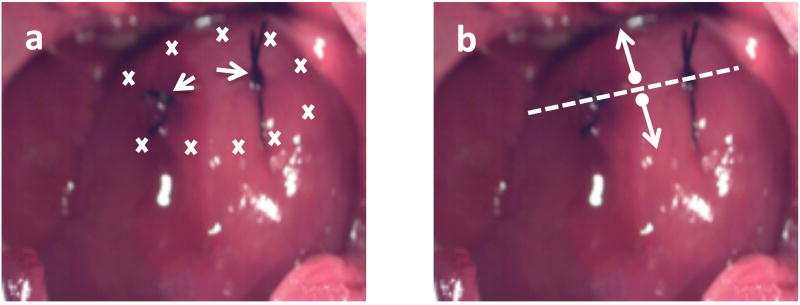
Thread two 6-0 silk sutures (with taper point needle) on the exposed left ventricular free wall of the heart and tie them loosely (Fig. 4A)(See Note 13). These sutures are to be placed superficially, avoiding the coronary vessels, and will serve as reference markers at the time of injection and also during subsequent tissue harvest (See Note 14).
Load the total amount of FLNP/modRNA to be delivered into a 1-mL syringe with a 25G ⅝” needle. Change needle to a 30G ½”-1” needle. Approach the heart surface with the needle at a shallow angle (30–45°) relative to the epicardial surface, advance the needle along the free wall of the heart (See Note 15). Inject the solution as you retract the needle. Distribute the injections on the anterior, apical and lateral regions of the heart to the left and right of the silk sutures placed as reference, administering 40–100µl per injection site (See Note 16).
To close, first bring the rib cage together with interrupted sutures, and then the muscular and fascia layer with running sutures. Lastly suture close the skin (See Note 17).
Allow the animal to recover while being maintained connected to the ventilator until the animal is able to breath spontaneously, without signs of labored breathing.
For analgesia, administer buprenorphine 0.1–0.5 mg/kg subcutaneous, twice a day for 3 days.
Figure 4.
Direct intramyocardial injection. A) Image of rat heart with schematic of intramyocardial injection sites (marks) on either side of the silk sutures. Arrows point to silk sutures loosely tied used as landmarks; and B) schematic illustrates the lengthwise cut (dashed line) through the 6-0 silk sutures placed as landmarks, splitting the heart in two to use one half for histology and the other for RNA extraction.
3.4 Intracoronary delivery of the formulated lipid mRNA nanoparticles by intraventricular injection with temporary aortic cross clamping in rats
Anesthetize and prep the animal as described in previous section on intramyocardial injection (steps 1–3), with the exception that the rat is placed in a supine rather than lateral position.
Make a skin incision (approximately 1.5-cm long) on the chest along the midline; continue the incision through the subcutaneous tissue. The heart can be approached through a mid sternotomy, or alternatively a left distal parasternal approach (See Note 18).
Incise the pericardium to expose the heart. Apply a cephalad retraction to the thymus and identify the aorta and pulmonary artery.
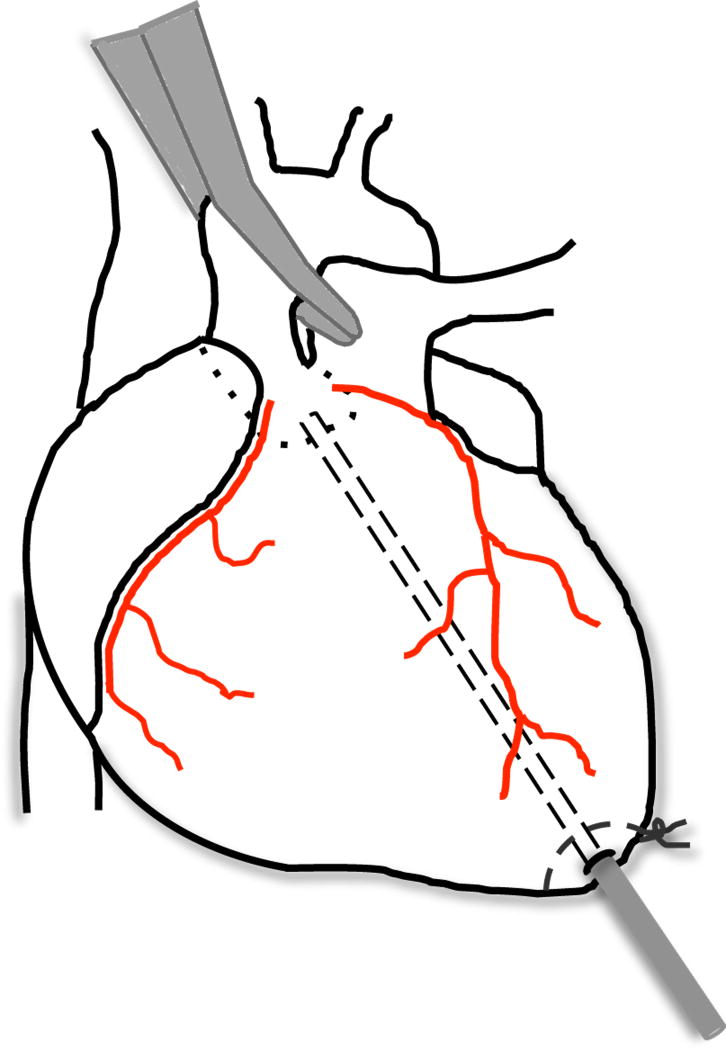
Partially exteriorize the heart by gently advancing a cotton tipped applicator from the apex towards the underside of the heart (See Note 19). Place a 6-0 silk suture (with taper point needle) at the apex as a purse string, without tying it. Introduce a 24G × ¾” catheter through the left ventricular apex at the center of the purse string. Immediately upon gaining access to the left ventricle, remove the needle, watch for blood return and connect the catheter to a 1-mL syringe containing the FLNP/modRNA solution to be injected.
Advance the catheter to the aortic root; it is likely that the catheter will be visualized below the surface of the aortic wall as it advances into the ascending aorta. Proceed cautiously to avoid puncturing the wall of the aorta. Then retract the catheter slightly to leave the tip of the catheter at the base of the aortic root.
Place an atraumatic clamp (a padded clamp can be used) across the ascending aorta just distal to the aortic root (Fig. 5) (See Note 20). Inject the FLNP/modRNA solution into the aortic root while the heart is pumping against a closed system, this allows the solution to circulate down the coronary arteries and perfuse the heart without direct manipulation of the coronaries (See Note 21). After 30 seconds of aortic cross clamping, release the clamp (See Note 22).
Withdraw the catheter and immediately tie the purse string suture on the apex of the heart to seal the catheter puncture site. Apply a cotton tipped applicator to the puncture site to aid stopping of bleeding.
Close the chest by first bringing together the edges of the sternum, and then the muscular and fascia layer in the midline. Lastly, suture close the skin layer.
Release the animal from the restrains while maintaining connected to the ventilator until the animal is able to breath spontaneously, without signs of labored breathing.
For analgesia administer buprenorphine 0.1–0.5 mg/kg subcutaneous, twice a day for 3 days (See Note 23).
Figure 5.
Intracoronary injection. Schematic of rat heart depicting intracoronary injection with aortic cross clamping.
Footnotes
Weigh the dried lipids into tared vials and add ethanol. Use a vortex-mixer to ensure complete dissolution. If the lipids do not dissolve, heat to 37°C until the solution becomes transparent.
Aliquot and store at −70°C. For use, thaw the required aliquot(s) of mRNA on ice. Always wear gloves and use RNAse-free tips and tubes when handling mRNA to avoid RNA degradation. We strongly recommend the use of modified mRNA, which incorporates both pseudouridine and 5-methylcytidine modified nucleotides, reducing the innate immune response to RNA [13–15].
When mixing the lipid components, make a volume larger than the minimum required; we recommend a 200% increase to ensure a sufficient amount of the required organic lipid mixture to add to the aqueous mRNA mixture (step 4 of Preparation of lipidoid modRNA nanoparticles). It also helps to increase the working volumes when the required minimum of any of the components is too small (e.g., less than 1µl).
When lipids are brought to room temperature, some particulates of the lipids will be visible. To dissolve the lipids properly for use, place in water bath at 37°C while shaking for 1–2 minutes and then vortex intermittently. Check that the lipids are well dissolved; no particles should be visible. Repeat as needed.
The beaker containing the PBS can be prepared beforehand, prior to bringing lipids to room temperature, but the best time to pre-wet the dialysis cassette membrane is during the 10-minute incubation period for FLNP self-assembly.
To avoid malfunction of electronics due to condensation, it is recommended that the stir plate be brought to the cold room at least 24 hours prior to its use.
Slowly advance the needle into the dialysis cassette cavity so that the sharp tip of the needle is barely visible. Inserting the needle too far into the cassette chamber may puncture the dialysis membrane and render it useless. Inject approximately half of the sample. Withdraw some air from the cassette by pulling back on the syringe piston and then inject remaining sample. With the needle in the cassette cavity, withdraw remaining air to compress the chamber windows to allow maximum contact of the sample with membrane surface area, maximizing the dialysis efficiency. Always use caution to prevent the needle from contacting the membrane.
Dialysis is required for 3 hours but it can be extended to overnight, as convenient for the scheduled experiments, e.g., timing of the surgical procedures for injection of the FLNP/modRNA. The longer period of dialysis is not detrimental to the formulation.
To measure the post-dialysis volume, collect the entire volume of the sample into the 1-mL syringe and measure the volume according to the graduation marks on the syringe.
Successful transfection of myocardium in vivo was achieved when the FLNP/modRNA was injected within the first 24hours after preparation. However, in vitro tests showed that the formulation remains stable and retains activity for at least 14 days when stored at 4°C [5].
Prepare the catheter prior to use by cutting off the sharp end of needle, a wire cutter is the ideal tool, and exercise caution to avoid personal injury when cutting the needle tip.
Ventilator settings must be adjusted per individual animal, with respiratory rate 70–90 breaths/min and inspiration time 0.4–0.6 seconds.
Reference markers are required when performing the procedure in a sham animal to later identify the sites of nanoparticle injection; such landmarks may not be required when injecting the nanoparticles into the heart of an animal with a clearly defined ischemic region, e.g. post LAD ligation.
The procedure of organ harvest must be carried out expeditiously to avoid tissue degradation. Label tubes and cryomolds beforehand. To process heart tissue from animals that received direct intramyocardial injection, harvest the heart and rinse in PBS (avoid perfusion with fixative), cut the heart in half lengthwise through the 6-0 silk sutures placed as landmarks (Fig. 4B). One half, to be used for RNA extraction, should be cut into ≤1mm3 pieces, collect into a DNAse/RNAse free microcentrifuge tube and store at −80°C until ready for processing. The other half is to be used for histology; place tissue sample in cryomold, embed in OCT, place on dry ice, and once the OCT freezes store at −80°C until ready for cryosectioning. Apply fixative to each cryosection prior to staining.
As you advance the needle the bevel should be parallel to the surface of the heart to avoid puncturing through the full thickness of the myocardium and into the ventricular chamber.
The recommended maximum volume at any single injection point is 100µl.
The recommended suture sizes are 3-0 to 4-0, and 5-0 for skin layer. For the type of suture material, use slowly absorbed or nonabsorbable suture material, avoid using silk for skin closure.
For the parasternal approach, cut off the most distal two ribs from their attachment to the left side of the sternum, and then advancing proximally, cut off the next two ribs on the right side of the sternum, and lastly cut through the sternum diagonally. Place a self-retaining retractor to bring apart the distal part of the sectioned sternum towards the right and the proximal part of the sternum to the left.
Applying external pressure on the right side of the thorax can also help to exteriorize the heart.
The procedure that includes clamping of the pulmonary artery along with aorta is expected to result in a wider distribution of particles throughout the myocardium [6].
While one person alone without assistants performs most rodent surgeries, it is recommended that for this particular technique one person takes control of the clamp on the aorta while an assistant applies the injection. Once sufficient proficiency is acquired with the technique it may be performed by one person alone.
It is expected that during the cross clamping the heart rate will decrease, but it should recover to baseline within 30 seconds of clamp release. The general recommendation for a maximum volume to be delivered is to not exceed 500µl due to risk of pulmonary edema [8]; we have delivered up to 600µl in the rat without overt compromise of heart or lung function and with animal survival for at least 2 weeks, which was the longest time point at which the animals were euthanized [5].
It is expected that recovery from the sternotomy will induce greater discomfort for the animal than the left thoracotomy used for the direct injection, thus requiring adjustment of buprenorphine dosage for appropriate analgesia.
References
- 1.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 3.Mashaghi S, Jadidi T, Koenderink G, Mashaghi A. Lipid nanotechnology. Int J Mol Sci. 2013;14:4242–4282. doi: 10.3390/ijms14024242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson BR, et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010;38:5884–5892. doi: 10.1093/nar/gkq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull IC, et al. Myocardial Delivery of Lipidoid Nanoparticle Carrying modRNA Induces Rapid and Transient Expression. Mol Ther. 2015 doi: 10.1038/mt.2015.193. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Monte F, Hajjar RJ. Efficient viral gene transfer to rodent hearts in vivo. Methods Mol Biol. 2003;219:179–193. doi: 10.1385/1-59259-350-x:179. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar RJ, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kho C, et al. Efficient Viral Gene Transfer to Rat Hearts In Vivo. Nature Protocol Exchange/Community Contributed. 2011 doi: 10.1038/protex.2011.256. [DOI] [Google Scholar]
- 9.Chen J, et al. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakata S, et al. Preservation of mechanical and energetic function after adenoviral gene transfer in normal rat hearts. Clin Exp Pharmacol Physiol. 2007;34:1300–1306. doi: 10.1111/j.1440-1681.2007.04742.x. [DOI] [PubMed] [Google Scholar]
- 11.Hajjar RJ, del Monte F, Matsui T, Rosenzweig A. Prospects for gene therapy for heart failure. Circ Res. 2000;86:616–621. doi: 10.1161/01.res.86.6.616. [DOI] [PubMed] [Google Scholar]
- 12.Love KT, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kormann MS, et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol. 2011;29:154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol Ther. 2013;21:358–367. doi: 10.1038/mt.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]