Abstract
Purpose
To enable future studies of retinal pigment epithelium (RPE) fate in the macular atrophy occurring in eyes with neovascular AMD (nvAMD), we determined how RPE morphology changes across the transition from health to atrophy in donor eyes with nvAMD.
Method
In RPE-Bruch’s membrane flat mounts of 5 nvAMD eyes the terminations of organized RPE cytoskeleton and autofluorescent material were compared. In high-resolution histological sections of 27 nvAMD eyes, RPE phenotypes were assessed at ±500 and ±100 µm from the descent of the external limiting membrane (ELM) towards Bruch’s membrane. Thicknesses of RPE, basal laminar deposit (BLamD), and RPE+BLamD were determined. Shapes of the ELM descent were recorded.
Results
Approaching the ELM descent, the percentage of different RPE phenotypes (Zanzottera, IOVS 2015) and the thickness of RPE, BLamD, and RPE + BLamD each stayed roughly constant. When compared to a separately described cohort of eyes with geographic atrophy, eyes with nvAMD were more likely to have RPE dysmorphia that did not worsen towards the atrophy border, thinner BLamD overall (3.25 ± 3.46 µm vs 7.99 ± 7.49 µm for geographic atrophy), and a higher proportion of oblique ELM descents (47.9% vs 31.9%).
Conclusion
The distribution of RPE phenotypes at the transition to macular atrophy in eyes with nvAMD differs from that in primary geographic atrophy, likely reflecting greater photoreceptor loss and the effects of exudation in nvAMD. This distribution, the shape of ELM descents, and thickness profiles may be useful metrics in clinical studies of macular atrophy utilizing optical coherence tomography and fundus autofluorescence.
Keywords: Age-related macular degeneration, retinal pigment epithelium, neovascularization, histology, optical coherence tomography, autofluorescence, macular atrophy
Précis
Morphological phenotypes of the retinal pigment epithelium (RPE) distribute across the transition to atrophy in neovascular age-related macular degeneration in a manner different from primary geographic atrophy, as described separately. Thickness of RPE-basal laminar deposit may be useful as a metric to distinguish macular atrophy from geographic atrophy by optical coherence tomography.
Introduction
Intravitreally injected anti-vascular endothelial growth factor (VEGF) agents help preserve vision in many eyes with neovascular complications of age-related macular degeneration (nvAMD).1, 2 Ten years of clinical anti-VEGF experience has shown that atrophy occurring in eyes receiving long-term therapy is associated with worse visual outcomes.3 The term “macular atrophy” has been proposed to describe the degeneration of the retinal pigment epithelium (RPE) and outer retina seen in the context of nvAMD, and to distinguish this form of atrophy from the primary degeneration of non-neovascular AMD known as “geographic atrophy” (GA).
In early trials of anti-VEGF agents, visual acuity often declined even as the effects of neovascularization were blunted4. Analyses of trial data suggested that continuous monthly treatment (vs pro re nata) was associated with a higher risk of incident atrophy and faster atrophy progression over 1–2 years5–8. This effect varies with the neovascularization subtype8–12 and may depend on the specific anti-VEGF agent used8. In contrast, when followed 5 years or more, vision loss may progress more slowly in eyes receiving a continuous vs pro re nata anti-VEGF regimen2. Interestingly, in some continuously dosed eyes with type 1 neovascularization, atrophy grows slower than that occurring in a fellow eye with non-neovascular AMD10, 13. Concern regarding a possible deleterious effect of anti-VEGF treatment on atrophy progression is further heightened because neutralization or deletion of RPE-derived VEGF in mouse models results in choriocapillary endothelium dedifferentiation and atrophy14–16. Because good vision in nvAMD is highly dependent on RPE survival,9, 17–21a current question of clinical importance is how to best monitor RPE health in these patients.
The advent of intravitreal anti-VEGF therapy coincided with the availability of spectral domain optical coherence tomography (SDOCT) to guide treatment decisions based on cross-sectional chorioretinal anatomy. In addition, fundus autofluorescence (FAF) is informative for monitoring RPE health and metabolism by enabling detection of autofluorescence attributable to lipofuscin and melanolipofuscin, long-lasting intracellular residual bodies rich in bisretinoid derivatives of vitamin A. As assessed with FAF, RPE atrophy is inferred in nvAMD from the loss of homogenous and contiguous signal, in concert with vision loss9, 17–21. Further, discontinuities in the RPE layer of treated nvAMD eyes are visible via polarization sensitive OCT, which reveals RPE cell bodies specifically.22 A challenge to understanding macular atrophy is that standardized definitions for RPE atrophy after anti-VEGF therapy are not yet available.23 Thus opinions vary about whether macular atrophy in nvAMD eyes undergoing anti-VEGF therapy is the same8 or different3–5, 21 from primary GA, with evidence tipping towards different24. The most detailed descriptions3, 19, 21 focus on clinical features such as earlier age of onset, more central macular involvement, outward direction of spread, and lack of sharply demarcated borders in nvAMD relative to GA.
The interpretation of macular atrophy in clinical imaging would benefit from a detailed histopathologic description of RPE in human eyes with nvAMD. Previous clinicopathologic correlations for nvAMD (reviewed by Curcio et al25) focused on which aspect of RPE contacted neovascular membranes26 with little attention to cells at atrophy margins. A notable exception is Sarks and associates19 who reported that fibrovascular scars lack a hyperpigmented border clinically and have alternating RPE attenuation and hyperpigmentation histologically. These findings contrasted with the hyperpigmented border and RPE cells of irregular shape and pigmentation seen in GA27. Using high-resolution histology, we recently developed a system of RPE morphologic phenotypes and determined that many are visible by SDOCT28, 29. In eyes with GA30, we found that the RPE layer thickens near the border of atrophy, due to a progressive dysmorphia that also helps explain variable FAF at the margin of atrophy31. Here we use these same methods to investigate RPE morphology and thickness in the transition to atrophy in eyes with nvAMD. We find distinct differences between macular atrophy occurring with nvAMD and GA that may provide a basis for new metrics in future clinical studies utilizing SDOCT and FAF as core imaging technologies.
Methods
RPE flat mounts of nvAMD eyes were studied to assess the relationship of histological autofluorescence (AF) and cytoskeleton at the termination of the intact RPE layer, as done for GA.30, 32 In other nvAMD eyes, sub-micrometer histological cross-sections through the fovea and superior perifovea were studied to assess phenotypes of RPE morphology, as done for GA.28–30 Tissues came from a bio-repository of short post-mortem (<6 hr) eyes assembled for AMD research from donors to the Alabama Eye Bank 1995–2009. More information on all eyes is available (Supplementary material28). Studies were approved by institutional review at the University of Alabama at Birmingham and adhered to the Tenets of the Declaration of Helsinki.
Clinical histories were not available. AMD maculopathy status was determined at the time of accession using stereo color photographs and internal examination using a dissecting microscope.33 Eyes used in this study had atrophic areas detectable by inspection of the post-mortem fundus. Maculopathy was further checked at the time of histologic preparation (2011–2013) using ex vivo multimodal imaging including SDOCT and FAF (Spectralis, Heidelberg Engineering, Heidelberg, Germany).34 Atrophy in one nvAMD eye used for cross-sectional analysis, revealed by ex vivo color photography and FAF imaging, is shown in Figure 1A–C.
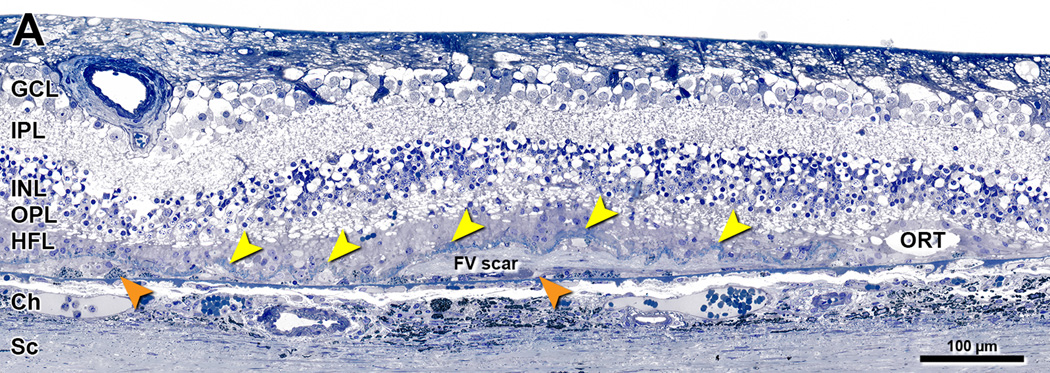
Figure 1. Ex vivo imaging of a donor eye with neovascular AMD.
96-year-old white woman. A. Color photograph shows a large atrophic area with scalloped edges, especially on the temporal side. Nasal to the fovea the retina is thickened with two areas of black intraretinal pigment corresponding to ‘melanotic’ cells of RPE origin.29 Green line indicates the approximate position of the B-scan in panel D. Ruby bead65 is 1 mm in diameter. B. Red-free photograph shows choroidal vessels through thinned retina and dark areas representing the black pigment in panel A. C. 488 nm autofluorescence shows atrophy that is multilobular on superior, nasal, and inferior aspects, and small hypoautofluorescent spots superior to the optic nerve head. In this thinned retina, autofluorescence is associated with walls of large retinal vessels. Black pigment is hypoautofluorescent. D, E. B-scan and corresponding submicrometer histologic section through the fovea. Red arrowheads delimit an area with sub-retinal fibrovascular membrane, as determined by inspection of the histology at higher magnification. No sub-RPE fibrovascular membranes were detected. Yellow arrowheads 1 and 2 delimit an area of continuous RPE. At the right of arrowhead 3 is also continuous RPE. ELM descents near arrowheads 1 and 3 met criteria for inclusion in the border analysis. An ELM descent near arrowhead 2 was not included, because it was ≤500 µm from subretinal fibrovascular scar. Teal arrowheads show groups of ‘dissociated’ RPE atop thick basal laminar deposit (stained blue).37 This deposit extends across the atrophic area and is particularly reflective in the peripapillary area (to the left of arrowhead 1) where it also contains neovessels66.
Flat-mounts
Of 35 RPE-Bruch’s membrane flat-mounts previously prepared from 35 Caucasian donors32, 5 nvAMD tissues were revisited. To enable definition of RPE cytoskeleton, F-actin was labeled with Alexa 647 conjugated phalloidin (Life Technologies, Grand Island, NY, USA). To illustrate RPE cell melanosome content and to illustrate F-actin and lipofuscin/melanolipofuscin distribution, bright field and fluorescence imaging, respectively, was performed using a confocal microscope (BX51, Olympus, Center Valley PA USA) at predefined locations and settings, as described35, plus additional images in areas affected by atrophy.
Cross-sectional histology and analysis
From 82 AMD eyes prepared for sub-micrometer epoxy sections stained with toluidine blue as described,36, 37 39 nvAMD eyes of 39 donors were identified by fibrovascular scar in the sub-RPE or sub-retinal space in the presence of basal linear deposit or drusen, as determined by histology (Figure 1D–E). These eyes also had histologically confirmed atrophy, often visible by ex vivo SDOCT (Figure 1D–E). In areas of RPE atrophy, a boundary between subretinal and sub-RPE compartments was defined by basal laminar deposit (BLamD) (Figure 2), which persisted across the atrophic area in continuity with BLamD at the atrophy margin.37, 38 If BLamD was absent, then the neurosensory retina contacted Bruch’s membrane directly and for the purpose of analysis was considered equivalent to the subretinal compartment. The sub-RPE compartment was located between BLamD and inner collagenous layer of Bruch’s membrane. If BLamD was absent, then there was no sub-RPE compartment. Because we did not have clinical histories, and we did not exhaustively section entire maculas, we cannot state definitively that these eyes lacked variations and/or sequelae of neovascularization such as vessels that originated in one compartment and penetrated another, quiescent sub-RPE vessels, chorioretinal anastomoses, multilayered pigment epithelial detachments, or RPE tears.
Figure 2. Definitions of layers and compartments within atrophic area of neovascular AMD.
80-year-old woman. Submicrometer epoxy section, toluidine blue stain. GCL, ganglion cell layer; IPL, inner plexiform layer; INL= Inner nuclear layer; OPL, outer plexiform layer; HFL, Henle fiber layer; Ch, choroid; Sc, sclera; FV scar, fibrovascular scar; ORT, outer retinal tubulation. Persistent basal laminar deposits (BLamD) (also called outer retinal corrugations in SDOCT37), yellow arrowheads. ‘Subducted’ cells of hypothesized RPE origin, external to scar and resting on Bruch’s membrane29, orange arrowheads. Photoreceptors have degenerated, and HFL contacts BLamD. In the analysis of atrophic areas lacking a continuous RPE layer, BLamD divided retinal and (obliterated) subretinal compartments from the sub-RPE compartment38. The sub-RPE compartment in these eyes may be empty or may contain scar, neovascularization, blood, fluid, cells, and/or basal linear deposit, an extracellular lipid-rich lesion equivalent to soft drusen.
As recently done for eyes with GA30, we annotated RPE morphology and measured RPE and BLamD thickness at atrophy borders in two sections, one through the foveal center and another at 2 mm superior to the foveal center. The border of atrophy was defined as the descent of the external limiting membrane (ELM) towards Bruch’s membrane.30 As described30, 39, the descent of the ELM is comprised of reactive Müller cell processes extending outward to approach Bruch’s membrane in areas of photoreceptor loss. An ELM descent was analyzed if it was at least 500 µm from the termination of subretinal fibrovascular scar. Sub-RPE scar in these locations was not an exclusion criterion. At 100 µm and 500 µm on either side of the ELM descent, we annotated RPE morphology and measured RPE and basal laminar deposit (BLamD) thickness, for a total of 4 assessments per transition, 2 in the atrophic area, with positive distances (+100 and +500 µm) and 2 in the non-atrophic area, with negative distances (−500 and −100 µm), so that progression moves like a time-line from left (non-atrophic) to right (atrophic) in all figures. This sampling pattern, centered around a defined point, is the same as our independent analysis of eyes with GA30 and differs from our previous publications in which samples were spaced systematically across the whole macula.28, 29 Annotations were recorded in a custom database (Filemaker, Adobe, San Jose CA) while using a 60× numerical aperture = 1.4 objective and CCD camera to display tissue images at 1900× on a monitor. We used the nomenclature for RPE morphology as defined by Zanzottera et al28, 29 and applied in analyzing GA30. In brief, cells containing spindle-shaped melanosomes and lipofuscin apposed to a basal lamina or BLamD were considered RPE. Cells in other layers or spaces containing those organelles or plausibly derived from such cells, were considered RPE-derived. These included two phenotypes found only in nvAMD eyes29: ‘entombed’ within disciform scars thought to arise from ‘bilaminar’ RPE and ‘melanotic’, with spherical melanosomes visible funduscopically as black pigment inside scars. The percentage of each RPE phenotype was referenced to the total number of samples with RPE (also differing from our previous studies28, 29). The shape of ELM descent was recorded as curved, reflected, oblique, or indeterminate, as described30. The percentage of (curved + reflected) / (curved + reflected + oblique – indeterminate) was calculated for nvAMD and also for the separately reported GA eyes, so that they could be compared.
We compared mean RPE thickness for each phenotype at −500 µm and −100 µm and mean BLamD thickness at ±500 µm and ±100 µm. Per previous convention28, 29, only cells in the RPE layer (and not pigmented cells out of the layer) were measured. RPE thicknesses were available for all RPE morphologies except ‘atrophy with BLamD’, ‘atrophy without BLamD’, and ‘dissociated’ RPE. Generalized estimating equations were used to test if the mean thickness of RPE (or BLamD) at each location was different from zero. A pooled variance was used so that a p-value could be generated even if only one observation was included in one of the groups. A p-value <0.05 was considered statistically significant.
Results
In flat-mounts we compared the termination of intact RPE layer, signified by orderly cytoskeleton delimiting polygonal cells, to the distribution of AF attributable to lipofuscin and melanolipofuscin (Figure 3). A sharp co-termination of cytoskeleton and RPE AF is apparent in some locations (Figure 3, row A1–4), but not all. AF material does not necessarily respect the termination of RPE cytoskeleton and can extend beyond it (Figure 3, row B1–4). This AF material represents RPE cells and cellular fragments, as supported by simultaneous appearance of melanosomes (Figure 3, column 4). Thus the atrophy border in nvAMD is not sharply defined in the RPE layer itself, just like in GA30, justifying the use of the ELM descent as a reference point for analyzing the sequence of RPE degeneration.
Figure 3. Variation in RPE autofluorescence and cytoskeleton in neovascular AMD.
Images are from a 93-year-old female donor. Column 1 demonstrates that RPE cells at the border of atrophy show either intact or disrupted cytoskeleton. In the atrophic zone, cells lose f-actin cytoskeleton, and condensed f-actin tangles form from remaining f-actin fragments. Column 2 shows brightly AF lipofuscin/ melanolipofuscin granules, either within isolated cells or in extracellular granule aggregates shed into basal laminar deposit32. Column 3 superimposes images from columns 1 and 2 to show the relationship of AF material to the termination of organized cytoskeleton. Column 4 shows bright field images to confirm that cells and cellular fragments also have melanosomes. Row A shows coterminous AF and organized cytoskeleton, with a clear boundary between intact RPE and atrophy. Row B shows AF stopping short of the end of organized cytoskeleton as well as extending into the atrophic area. Scale bars: 50 µm.
We analyzed cross-sectional histology in 27 nv-AMD eyes of 27 donors meeting our criteria (16 women, 11 men, age 84.8 ± 7.3 years). Of these 27 eyes, 21 had subretinal fibrovascular scar, 23 had sub-RPE fibrovascular scar, 1 had subretinal hemorrhage, 2 had subretinal fibrin, and 1 had a serous pigment epithelium detachment34. We analyzed 39 sections (18 central, 21 superior), 70 transitions, and 194 assessment locations (45 at −500 µm, 51 at −100 µm, 50 at +100 µm, 48 at +500 µm). The number and pattern of assessment locations differed among eyes, because atrophic areas extended off section edges or were less than 1000 µm wide.
The distribution of RPE morphologies with respect to the ELM descent30 in cross-sectional histology of nvAMD eyes is illustrated in Figures 4 and 5, which are high-magnification and panoramic views, respectively, of the same four nvAMD eyes. Figures 4A,B,C and 5A,B,C show three nvAMD eyes with sub-RPE fibrovascular scar meeting criteria for inclusion in this analysis. A typical eye (Figures 4A, 5A) has ‘sloughed’ RPE and shed cone inner segments40 outside the atrophy. An atypical eye (Figures 4B, 5B) has cells with spherical melanosomes (‘melanotic’)29 in the continuous RPE layer and in sub-RPE scar. Figures 4C, 5C show a continuous RPE layer with some ‘sloughed’ RPE, with degeneration of the overlying photoreceptors. Figures 4D, 5D show an eye that did not meet criteria due to a sub-retinal fibrovascular scar and fibrin located within 500 µm of the ELM descent. Of the illustrated criterion-meeting nvAMD eyes, those in Figure 4A,B and 5A,B have oblique ELM descents, and the eye in Figures 4C,5C has a curved ELM descent. At 51 transitions to atrophy, 47.4% of ELM descents were curved, 7.8% were reflected, 59.0% were oblique, and 5.9% were indeterminate. In two eyes (Figures 4A,C, 5A,C), the outer nuclear layer ended coterminously with the ELM descent. In two eyes (Figures 4B,D, 5B,D) the outer nuclear layer extended beyond the ELM descent into the atrophic area. In one eye (Figure 4C, 5C) an island of photoreceptors survived with a few RPE cells over a fibrovascular scar.
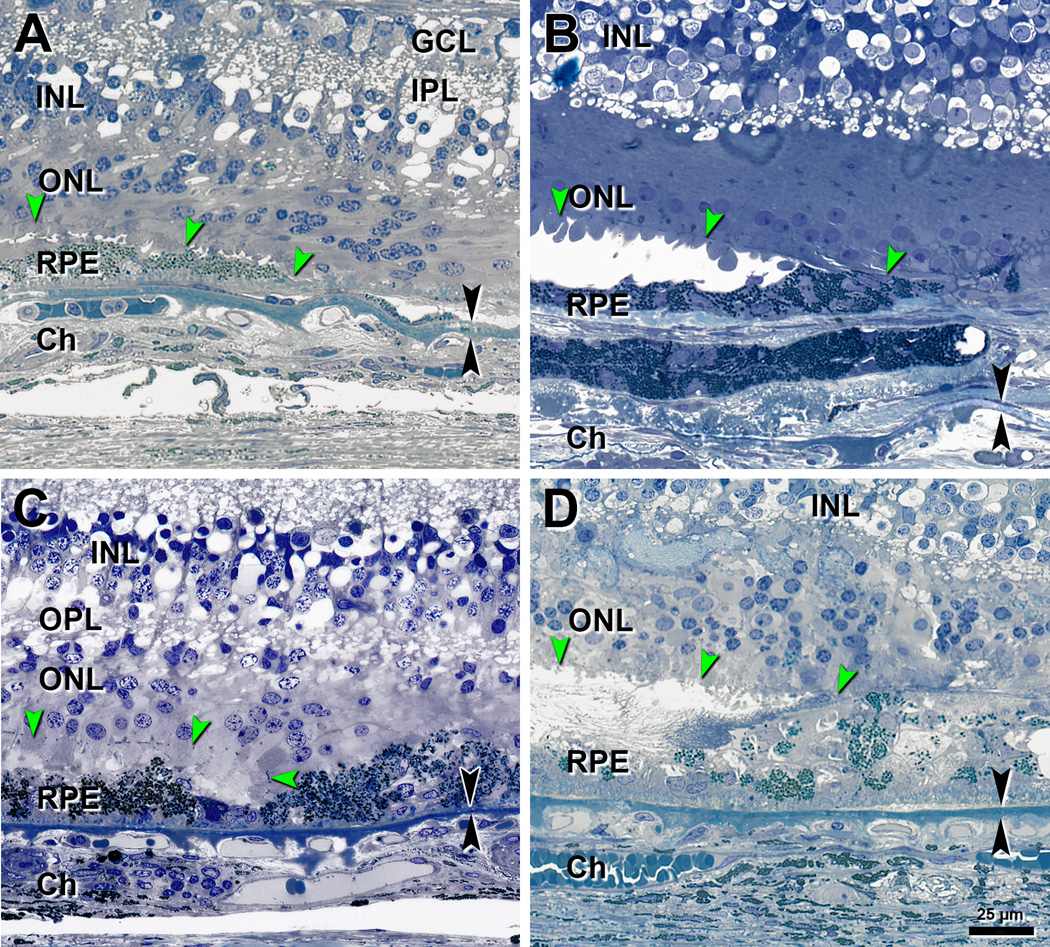
Figure 4. Descent of the external limiting membrane in 4 eyes with neovascular AMD.
Panoramic views of these eyes are shown in Figure 4. ELM, external limiting membrane (green arrowheads); GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium; Ch, choroid. Black arrowheads, Bruch’s membrane. Bar in D applies to all panels. A. Oblique ELM descent in an 87-year-old man. B. Oblique ELM descent in an 83-year-old woman. C. Curved ELM descent in an 80-year-old woman. D. Oblique ELM descent and subretinal fibrocellular scar in a 90-year-old-man.
Figure 5. RPE morphologies near the ELM descent in neovascular AMD eyes.
ELM descent (green arrowheads) and its projection on to Bruch’s membrane (red arrow) are shown. The ELM descent in all four eyes is shown at higher magnification in Figure 3. RPE and BLamD morphology and thickness were analyzed at −500 and −100 µm outside atrophy (yellow ticks to the left of the red arrows) and +500 and +100 µm inside atrophy (yellow ticks to the right of the red arrows). Submicrometer epoxy sections, toluidine blue stain. GCL = Ganglion cell layer, INL= Inner nuclear layer, ONL= Outer nuclear layer. A. A typical eye meeting criterion for inclusion. Outside atrophy: Very non-uniform and ‘sloughed’ RPE cells over a thin BLamD layer. Shed cone inner segments are found between the ONL and RPE40 (aqua arrowhead). Inside atrophy: a thicker layer of BLamD. The ONL stops at the ELM descent, which is oblique. 83-year-old woman. B. An atypical eye due to presence of melanotic RPE but still meeting criterion for inclusion. Outside atrophy: a continuous layer of cells containing dark polydispersed spherical granules and sub-RPE scar with melanotic cells (orange arrowhead). Inside atrophy: melanotic cells within fibrovascular scar. The ONL continues over the scar. The ELM descent is oblique. 86-year-old woman. C. Eye meeting criterion for inclusion. RPE layer is continuous, and photoreceptors are degenerate. The ELM descent is curved. An island of photoreceptors and several RPE cells survive over scar in the atrophic zone, at the right edge of the panel. 80-year-old woman. D. An eye not meeting criterion for inclusion due to sub-retinal fibrovascular scar with Entombed RPE and fibrin28 (pink arrowhead). Scar continues from the left edge to the ELM descent. Outer and inner segments of photoreceptors are degenerate. ONL continues over the scar. The ELM descent is oblique. 93-year-old woman. A–D. Complete histological sections are available at the Project MACULA website. A. http://projectmacula.cis.uab.edu/?p=1888. B. http://projectmacula.cis.uab.edu/?p=2999. C. http://projectmacula.cis.uab.edu/?p=1696. D. http://projectmacula.cis.uab.edu/?p=1704.
Figure 6 shows the distribution of RPE phenotypes relative to the ELM descent in 27 nvAMD eyes. At both assessment points on the non-atrophic side of the ELM descent, ‘very non-uniform’ cells predominate. ‘Sloughed’ and ‘bilaminar’ cells increase at −100 µm. ‘Shedding’, ‘vacuolated’ and ‘intraretinal’ cells are absent. On the atrophic side, ‘dissociated’ RPE is common. ‘Subducted’ cells are found on both sides of the ELM descent, with many fewer at −500 µm than at −100 µm. Only occasional cells are detected within the sub-RPE fibrovascular scar. ‘Melanotic’ cells are infrequent.
Figure 6. Distribution of RPE morphologies with respect to the ELM descent in neovascular AMD.
The ELM descent (red arrow) was the point of reference. The number of assessment locations is expressed at the right of each bar. Epithelial, non-epithelial, atrophic, and subducted morphologies28 are indicated by blue, green, orange, and brown bars respectively, with less affected at the top and more affected at the bottom. Percentages are referenced to the total number of RPE. RPE phenotypes do not obviously worsen in the junctional zone as the ELM descent is approached. Melanotic cells with spherical melanosomes (pink and yellow bars) are present in nvAMD eyes and not in GA eyes29.
Tables 1 and 2 show thicknesses of RPE layer and BLamD. RPE thickness across all morphologies did not differ significantly between −500 and −100 µm (11.8 ± 6.0 µm vs 12.8 ± 6.2 µm, p = 0.28). Similarly, overall BLamD thickness did not differ significantly between −500 and −100 µm (3.7 ± 3.7 µm vs 2.9 ± 3.2 µm, p = 0.08), with the exception of BLamD underlying ‘very non-uniform’ RPE being thinner at −100 µm (4.0 µm ± 3.9 µm vs 2.9 ± 3.4 µm, p = 0.04). To facilitate SDOCT metrics that are both histologically informed and feasible, combined thicknesses for the RPE + BLamD are shown in Table 3. Combining across all RPE morphologies, mean RPE + BLamD thickness at −500 µm and −100 µm was almost identical (15.7 ± 7.0 µm and 15.4 ± 7.7, respectively; p=0.91).
Table 1.
RPE thickness relative to the ELM descent in neovascular AMD eyes
| RPE Morphology |
−500 µm from ELM descent |
−100 µm from ELM descent |
||
|---|---|---|---|---|
| Thickness, mean ± SD, µm; N |
p value, − 500 vs −100 |
Thickness, mean ± SD, µm; N |
||
| Overall | 11.8 ± 6.0; 23 | 12.8 ± 6.2; 26 | 0.2847 | 12.3 ± 6.1; 27* |
| Non-uniform | 10.6 (n/a); 1 | --- | --- | 10.6 (n/a); 1 |
|
Very non- uniform |
10.8 ± 3.0; 20 | 11.0 ± 3.9; 20 | 0.8360 | 10.9 ± 3.5; 25 |
| Sloughed | 23.9 ± 8.9; 4 | 17.4 ± 4.5; 6 | 0.0600 | 19.8 ± 6.8; 8 |
| Shedding | --- | --- | --- | |
| Bilaminar | 17.7 ± 8.2; 2 | 22.3 ± 4.5; 6 | 0.3104 | 21.2 ± 5.4; 7 |
| Vacuolated | --- | --- | --- | |
| Intraretinal | --- | --- | --- | |
Notes: assessments were made in 27nv-AMD eyes lacking subretinal scar within ± 500 µm of the ELM descent and including eyes with sub-RPE scar
N indicates the number of donors in which measurements were made.
Thicknesses were not recorded for ‘dissociated’ RPE within the atrophic area.
P-value obtained via general estimating equations
SD, standard deviation
n/a, not applicable
For 22 eyes accessioned before 2006, overall mean RPE thickness was 12.3 ± 6.2 µm
Table 2.
Basal laminar deposit thickness relative to the ELM descent in neovascular AMD eyes
| RPE Morphology | −500 µm from ELM descent |
−100 µm from ELM descent |
−500 µm vs −100 µm |
+100 µm from ELM descent |
+500 µm from ELM descent |
+500 µm vs +100 µm |
Overall −500 µm and −100 µm |
Overall +100 µm and +500 µm |
|---|---|---|---|---|---|---|---|---|
| Thickness, mean ± SD, µm; N |
p value | Thickness, mean ± SD, µm; N | p value | Thickness, mean ± SD, µm; N |
||||
| Overall | 3.7 ± 3.7; 23 | 2.9 ± 3.2; 26 | 0.0844 | 3.3 ± 4.7; 27 | 4.2 ± 5.0; 26 | 0.0246 | 3.2 ± 3.5; 27* | 3.7 ± 4.8; 27 |
| Non-uniform | 2.2 (n/a); 1 | --- | --- | --- | --- | --- | 2.2 (n/a); 1 | --- |
| Very non-uniform | 4.0 ± 3.9; 20 | 2.9 ± 3.4; 20 | 0.0404 | --- | --- | --- | 3.5 ± 3.7; 25 | --- |
| Sloughed | 2.6 ± 2.6; 4 | 4.5 ± 3.7; 6 | 0.2204 | --- | --- | --- | 3.8 ±3.3; 8 | --- |
| Shedding | --- | --- | --- | --- | --- | --- | --- | --- |
| Bilaminar | 1.7 ± 2.4; 2 | 1.3 ± 1.7; 6 | 0.7621 | --- | --- | --- | 1.4 ± 1.7; 7 | --- |
| Vacuolated | --- | --- | --- | --- | --- | --- | --- | --- |
| Intraretinal | --- | --- | --- | --- | --- | --- | --- | --- |
| Dissociated | --- | --- | --- | 7.5 ± 6.5; 10 | 6.7 ± 7.1; 9 | 0.6461 | --- | 7.2 ± 6.6; 12 |
| Atrophy with BLamD | --- | --- | --- | 3.4 ± 2.0; 11 | 5.5 ± 3.7; 16 | 0.0407 | --- | 4.6 ± 3.2; 18 |
| Atrophy without BLamD | --- | --- | --- | 0; 10 | 0.6 ± 2.3; 12 | 0.2407 | --- | 0.3 ± 1.6; 15 |
Notes: assessments were made in 27nv-AMD eyes lacking subretinal scar within ± 500 µm of the ELM descent and including eyes with sub-RPE scar
N indicates the number of donors in which measurements were made.
P-value obtained via general estimating equations
SD, standard deviation
n/a, not applicable
For 22 eyes accessioned before 2006, overall mean BLamD thickness was 2.9 ± 3.3 µm
Table 3.
RPE + Basal laminar deposit thickness relative to the ELM descent in nvAMD
| RPE Morphology |
−500 µm from ELM descent |
−100 µm from ELM descent |
−500 µm vs −100 µm |
Overall |
|---|---|---|---|---|
| Thickness in µm, mean ± SD; N | p-value | Thickness in µm, mean ± SD; N |
||
| Overall | 15.7 ± 7.0; 23 | 15.4 ± 7.7; 26 | 0.9109 | 15.4 ± 7.3; 27* |
| Non-uniform | 12.8 (n/a); 1 | --- | --- | 12.8 (n/a); 1 |
|
Very non- uniform |
14.8 ± 5.3; 20 | 13.9 ± 5.8; 20 | 0.3788 | 14.3 ± 5.5; 25 |
| Sloughed | 26.6 ± 8.4; 4 | 21.8 ± 7.9; 6 | 0.1408 | 23.5 ± 8.0; 8 |
| Shedding | --- | --- | --- | --- |
| Bilaminar | 19.4 ± 10.6; 2 | 23.6 ± 3.6; 6 | 0.4423 | 22.6 ± 5.4; 7 |
| Vacuolated | --- | --- | --- | --- |
| Intraretinal | --- | --- | --- | --- |
Notes: Mean RPE + BLamD thickness by RPE grades in 27 nvAMD eyes from 27donors
N indicates the number of donors in which measurements were made.
P-value obtained via general estimating equations
SD, standard deviation
n/a, not applicable
For 22 eyes accessioned before 2006, overall mean RPE+BLamD thickness was 15.0 ± 7.4 µm
We compared our data in these 27 nvAMD eyes to 13 separately reported GA eyes analyzed by the same methods30 (Tables 4, 5). nvAMD eyes had proportionately fewer transitions than the GA eyes, which included multilobular GA.
Table 4.
Comparison of microstructure in neovascular AMD and geographic atrophy eyes
| # | Metric | Neovascular AMD (N=27) |
Geographic atrophy* (N=13) |
P |
|---|---|---|---|---|
| 1 | % of RPE phenotypes ≥ ’very non-uniform’ in non-atrophic area | |||
| −500 µm | 97.7% | 72.2% | 0.002 7 |
|
| −100 µm | 100.0% | 96.8% | n.s. | |
| 2 | RPE phenotypes in atrophic area |
‘dissociated’, ‘subducted’, ‘melanotic’ **, ‘entombed’ **, ‘entubulated’ † |
‘dissociated’, ‘subducted’, ‘entubulated’ † |
-- |
| 3 | BLamD thickness, non-atrophic area |
3.25 ± 3.46 µm | 7.99 ± 7.49 µm | 0.017 |
| 4 | BLamD thickness, atrophic area |
3.70 ± 4.81 µm | 6.63 ± 6.17 µm | 0.047 |
| 5 | Shape of ELM descent | |||
| Oblique | 47.9% | 31.9% | 0.046 | |
| Curved + reflected |
52.1% | 68.1% | ||
Table 5.
Comparison of RPE and BLamD thicknesses by location in neovascular AMD and geographic atrophy eyes
| # | Metric | −500 µm | −100 µm | P |
|---|---|---|---|---|
| 1 | RPE thickness | |||
| Neovascular AMD | 11.8 ± 6.0 | 12.8 ± 6.2 | n.s. | |
| Geographic atrophy | 12.1 ± 5.2 | 14.6 ± 6.9 | 0.039 | |
| 2 | BLamD thickness | |||
| Neovascular AMD | 3.7 ± 3.7 | 2.9 ± 3.2 | n.s. | |
| Geographic atrophy | 7.2 ± 6.1 | 8.4 ± 8.2 | n.s. | |
| 3 | RPE + BLamD thickness | |||
| Neovascular AMD | 15.7 ± 7.0 | 15.4 ± 7.7 | n.s. | |
| Geographic atrophy | 19.3 ± 8.2 | 23.1 ± 10.7 | 0.046 |
Notes: ±500, ±100, distances from ELM descent in µm, negative is non-atrophic, positive is atrophic;
Statistical evaluation: n.s., not significant at p< 0.05 level
Regarding distribution of RPE phenotypes, nvAMD eyes had mostly ‘very non-uniform’ cells at −500 and −100 µm with few “non-uniform” (age-normal) cells (Table 4, row 1), thus lacking noticeable worsening towards the ELM descent. In GA, the proportion of cells considered abnormal was significantly lower at −500 µm than at −100 µm, where many ‘shedding’, ‘sloughed’, and ‘intraretinal’ cells were found. Table 4, row 2 shows that the atrophic areas of nvAMD and GA eyes both exhibit ‘dissociated’ and ‘subducted’ cells29. However, fibrovascular scars in nvAMD are solely associated with RPE-derived phenotypes ‘entombed’ and ‘melanotic’. In this study ‘subducted’ are distributed on either side of the ELM descent in nvAMD eyes and are proportionately fewer at −500 µm than at −100 µm in nvAMD than GA.
Regarding layer thicknesses, in GA, RPE thickens significantly (20.6%) approaching the ELM descent but not in nvAMD (Table 5, row 1). BLamD thickness is 2-fold greater in GA relative to nvAMD both inside and outside the atrophic areas but does not vary significantly across the transition in either GA or nvAMD (Table 4, rows 3–4). Due to RPE thickening, the combination of RPE + BLamD together thickens by 19.6% near the ELM descent in GA but not in nvAMD (Table 5, row 3).
Regarding ELM shape, the percentage of descents that were oblique vs (curved + reflected) is significantly higher in nvAMD (47.9%) than in GA eyes (31.9%) (Table 4, row 5).
Five eyes accessioned after 2006 could have conceivably received intravitreal anti-VEGF therapy, and we found that the effect of excluding these eyes from the analysis was minimal. Thicknesses of RPE, BLamD, and RPE+BLamD in the remaining 22 eyes were almost identical to those found for the full dataset, and differences between nvAMD and GA eyes reached similar levels of statistical significance (notes to Tables 1–3). The proportion of oblique ELM shapes increased from 47.9% to 50.0%, which although a slightly larger effect, reduced the significance to borderline (p = 0.086).
Discussion
Our series is the largest to date providing high-resolution histology of intact nvAMD eyes. We used epoxy sections for a comprehensive and polychromatic visualization across the entire macula and focused on cardinal features of RPE ultrastructure and outer retinal neurodegeneration. We discretized continuous variation in RPE morphology with a system of cellular phenotypes and used unbiased systematic sampling41 to develop measures amenable to biometric analysis, which allowed us to compare eyes with nvAMD to eyes with GA. Previously, Sarks et al19 examined 18 eyes with disciform (fibrovascular) scars secondary to nvAMD and noted that the margin delimiting the atrophy surrounding the scar was not hyperpigmented, in contrast to eyes with GA, in which a hyperpigmented margin is common. Due to our excluding borders with sub-retinal fibrosis from analysis, our sample is not strictly comparable to this prior study19. Yet our data support the observation of absent hyperpigmentation by showing that the predominant RPE phenotype in the transition to atrophy is ‘very non-uniform’ and not ‘sloughed’ or ‘intraretinal’. Further, we found evidence for neither worsening of RPE morphology nor thickening of the combined RPE+BLamD layer, as the ELM descent is approached from the non-atrophic side. Our data extend the findings of Sarks et al19 by showing that ELM descents tend to be oblique rather than curved or reflected, as in GA. Histologically-guided metrics can potentially distinguish between GA and macular atrophy in SDOCT, with added value for interpreting FAF imaging.
Non-progressive dysmorphia in the transition to atrophy
We found little evidence for progressive RPE dysmorphia in the transition to atrophy that is well established for GA eyes27, 30, 31. We did note that ‘bilaminar’ increased near the ELM descent, perhaps because these are precursors of ‘entombed’ cells, which are specific to fibrovascular scars.29 In this and our recent GA study30 we considered fully pigmented, nucleated cells in the subretinal space and within the neurosensory to be RPE, because they contained numerous RPE-characteristic granules at a concentration similar to cells in the RPE layer.42 In our material, it is also possible to discern non-RPE cells such as lipid-filled monocytes.33, 34 In GA eyes we confirmed a dramatic and progressive deterioration in RPE health within 500 µm of the ELM descent27, 31. In the GA transition we found evidence for two main pathways of RPE fate, anterior migration and apoptosis.31, 32 The difference in RPE phenotype distribution between GA and nvAMD may be explained by the occurrence of acute, chronic or episodic exudation in nvAMD that is under different regulatory influences than those governing primary pigmentary degeneration, including the possibility of choriocapillary dysfunction early in the process.43, 44
The ELM Descent is a Marker for Atrophy
For the first time for nvAMD, we used as the border of atrophy the descent of the ELM towards Bruch’s membrane. Guided by an early description by Sarks39 we used this anatomical landmark in analyzing RPE phenotypes in GA30. The ELM comprises junctional complexes between the Müller cells and photoreceptor inner segments45–47 and together with junctional complexes among RPE, bounds the subretinal space48. The shape of the descent in atrophy is dictated by the outward and lateral extension of Müller cells53, 54. Published illustrations of immunoreactivity for glial fibrillary acidic protein show how Müller cells are reactive and the scaffolding of their apices perfectly outlines curved (Figure 2C of49; Figure 2H of50) and reflected (Figure 4C of49) ELM descents. Although we did not find similar published examples of oblique ELM descents, we believe that these fall along the same continuum. ELM descents of indeterminate shape were few in number and could be attributed to distortions due to fixation quality or tissue processing. We propose that curved and reflected ELM descents are related to the scrolling of Müller cells and cone photoreceptors seen in outer retinal tubulation.51–53 In this macula-specific gliosis, Müller cells protect surviving cones from the failing RPE-Bruch’s membrane complex by scrolling them into interconnecting tubes. We propose further that oblique ELM descents additionally reflect severe photoreceptor loss inherent in nvAMD (see below).
Interpretation of autofluorescence imaging
New information about RPE morphology in nvAMD can inform the interpretation of FAF imaging. Hyperautofluorescence can be explained by cell-autonomous and non-cell-autonomous mechanisms, some of which are assessable in the cross-sectional view of histology and SD-OCT. These include increased concentration of efficiently detected fluorophores, increased concentration of lipofuscin granules in individual cells, loss or re-positioning of melanosomes that block 488 nm autofluorescence, RPE hypertrophy resulting in taller individual cells, and superimposition of cells resulting from RPE migration31, 54. The latter two create a longer summation path length for exciting light through biologic fluorophores.30, 31 Reduced screening of RPE fluorophores due to reduced photoreceptor pigment can also lead to hyperautofluorescence55, 56. In contrast, hypoautofluorescence can be explained by loss of lipofuscin and melanolipofuscin granules singly or in aggregates, resulting in increasingly variable and overall reduction of FAF intensity.32 Hypoautofluorescence is also due to blocking by non-fluorescent material located in more anterior retinal layers.
Several FAF patterns have been described in nvAMD. First, a contiguous lawn of FAF indicates that RPE/photoreceptor complex is physically intact and metabolically active as driven by photoreceptor outer segment renewal and retinoid cycling.20 Second, a contiguous and well-demarcated area of greatly reduced FAF signal signifies photoreceptor death, with or without RPE loss1, 20, 21, 57 and in any case, predicts poor visual acuity at baseline and follow-up. These minimally AF areas may form a multilobular pattern typical of primary GA58 (Figure 7A), as the underlying degeneration progresses. Third, areas of absent FAF are preceded by and surrounded by areas of an abnormally granular FAF21, 57, seen as pigmentary disturbances in color imaging that are also associated with poor vision.4 Fourth, several published FAF images3, 9, 19, 22, 23, 57, 59 include a band of relatively uniform hyperautofluorescence with smoothly contoured edges surrounding atrophic areas (Figure 7B). This pattern recalls the inferior-angling gravitational tracks that typify central serous retinopathy60 and gives the impression of fluid having washed over the RPE, like a floodplain of exudation.
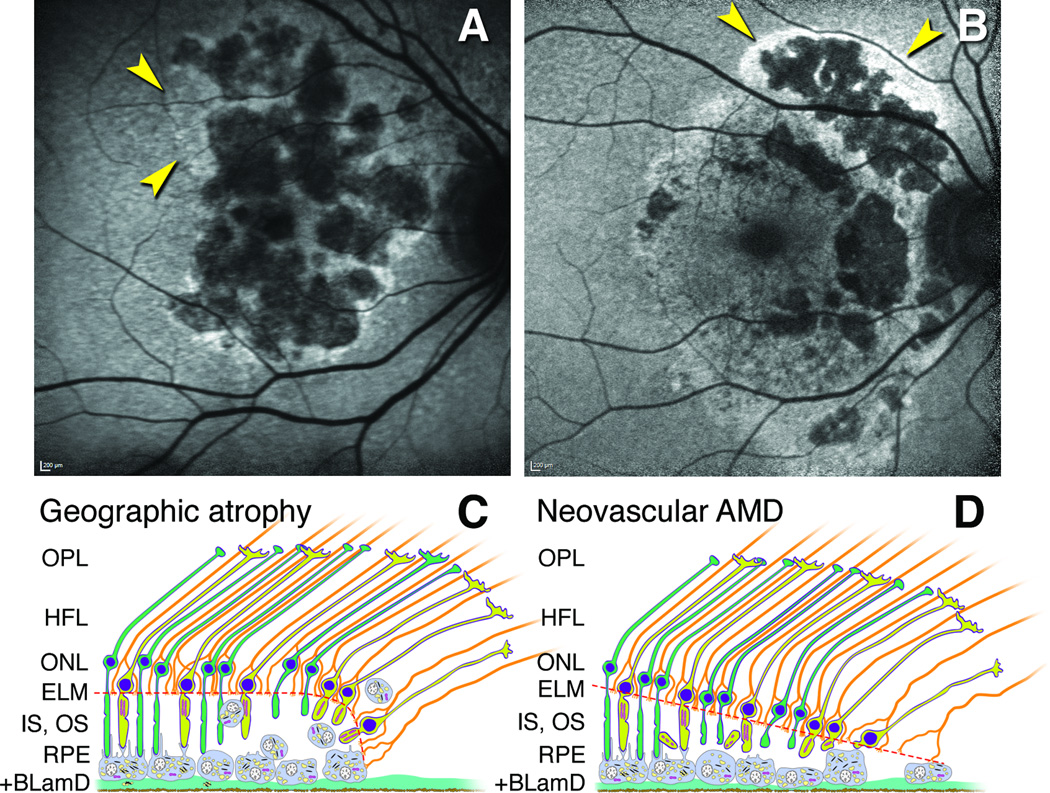
Figure 7. Proposed mechanisms for hyperautofluorescence in primary geographic atrophy and macular atrophy secondary to neovascularization in age-related macular degeneration.
A. Multilobular GA with variegated hyperautofluorescence in the margins (arrows). B. Neovascular AMD with multilobular atrophy and also hyperautofluorescence in a floodplain configuration (arrows), attributed to the history of exudation in this eye (image courtesy of N. Phasukkijwatana, MD, and D. Sarraf, MD). C,D. Schematics show outer retinal cells at the descent of the ELM (orange dashed line) towards Bruch’s membrane in GA (C) and nvAMD (D). The ELM is comprised of junctional complexes of Müller cells (orange) and photoreceptors (yellow, cones; green, rods). For simplicity, Bruch’s membrane and neovascular membranes are not shown. C. The RPE layer exhibits a progressive dysmorphia towards the ELM descent.27, 30, 31, 54 Hyperautofluorescence is attributable to stacked and rounded RPE cells that increase path length of exciting light through biologic fluorophores.31 D. In nvAMD, the RPE is non-uniform in morphology, without worsening toward the border. Photoreceptor loss is severe due to inner segment shedding40 believed secondary to episodic exudation. Reduced screening by photopigment in these shortened cells also contributes to hyperautofluorescence. In most cases, as in panel B, RPE dysmorphia and reduced photopigment screening may occur simultaneously.
Of relevance is our recent observation of a severe form of photoreceptor degeneration that occurs in 44% of nvAMD eyes and not in GA.40 Of our 27 study eyes, many with attached retinas, 7 exhibited cone inner segments containing mitochondria shed into the subretinal space where they interspersed with ‘sloughed’ RPE.40 Inner segment shedding is consistent with other neurodegenerations also involving release of cellular material61 and may be evoked by direct contact of photoreceptors with extravasated fluid. By markedly reducing the photopigment available for screening, this distinctive degeneration is a plausible contributor to a hyperautofluorescent exudative floodplain (Figure 7B, D). In contrast, hyperautofluorescence at the margin of primary GA (Figure 7A,C) is attributable to a progressive RPE dysmorphia (rounding and stacking) towards the ELM descent that increases path length for light. In nvAMD, shorter and/or fewer photoreceptors over an extended area outside the atrophic area will result in a straight, obliquely oriented ELM descent (Figure 7D), compared to curved ELM descents in primary GA (Figure 7C). In most nvAMD eyes, these processes probably occur simultaneous with an underlying primary GA. If this is true, then our observed differences in atrophy borders between GA and nvAMD eyes might have been larger, had we been able to separate primary GA from atrophy secondary to exudation. Only in eyes with simultaneous longitudinal OCT and FAF imaging1, 22 can these questions be definitively resolved.
Why RPE atrophy continues and possibly accelerates in the course of chronic VEGF suppression is not fully understood but several hypotheses exist. First, neovascularization may help sustain the diseased macula, in part by recapitulating choriocapillaris subjacent to RPE, and thus pharmaceutically suppressing neovascularization is deleterious.4 Further, the growth rate of atrophy is dependent on the type of neovascularization, with type 1 probably most resistant8, 10, 13. A re-direction of blood flow from the choriocapillaris serving RPE surrounding fibrovascular tissue to the scar itself has been suggested.19 Second, VEGF has neuroprotective functions that are blunted by therapy, possibly exacerbating vision loss.4 Third, atrophy is proposed to arise non-specifically in response to the exudative process itself, with episodic leakage over time, mechanical damage secondary to vascular outgrowth and contraction (dramatically demonstrated via OCT-angiography62), and concomitant inflammation and ischemia.3 Newly recognized forms of severe photoreceptor degeneration,40 the non-progressive nature of RPE change at the ELM descent herein described, and FAF signatures consistent with a floodplain3, 9, 19, 23, 57, 59 (Figure 7) support this third model, as do recent analysis of fellow eyes to those treated with anti-VEGF agents.24
Strengths, limitations, conclusions
Strengths of this study include a large number of nvAMD eyes analyzed at high-resolution, a cellular phenotyping system to discretize RPE degeneration, and unbiased sampling methods to include both the atrophic areas and surrounding less-affected tissue. Limitations were the small number of RPE flat mounts for en face viewing, lack of flat-mounts and histology from the same donor tissues, absence of Bruch’s membrane thicknesses to include with the RPE+BLamD thicknesses, and lack of clinical information about treatment history and subretinal and sub-RPE fibrovascular scar components.
Nevertheless, our study represents the first quantitative description of RPE morphology in intact nvAMD eyes. We formulated new hypotheses testable in longitudinal imaging of patients receiving anti-VEGF therapy and in animal models with genetic predisposition to neovascularization.63, 64 Importantly our data support three anatomical markers of potential utility in assessing via SDOCT and FAF whether RPE death in nvAMD is due to an underlying degeneration or secondary to exudation, with or without VEGF suppression. Relative to eyes with GA, eyes with nvAMD lack the progressive dysmorphia and thickening of the RPE layer toward the ELM descent, have thinner combined RPE+BLamD, and tend to have obliquely oriented, straight ELM descents. Analysis of large clinical imaging datasets will help determine whether these histological features are found with sufficient reliability to merit use as biomarkers. Histology of clinically imaged eyes will be essential for determining RPE changes relate to specific neovascularization patterns, and for testing hypotheses of how exudation impacts outer retinal cells.
Acknowledgments
CAC is supported by NEI EY06109, Macula Foundation, Inc., 2014 von Sallmann Prize and institutional support from the EyeSight Foundation of Alabama and Research to Prevent Blindness Inc.
ECZ is supported by the University of Milan.
TA is supported by DFG (German Research Foundation) AC265/1-1, AC265/2-1.
KBF is supported by the Macula Foundation, Inc.
Acquisition of donor eyes was supported by International Retinal Research Foundation, National Eye Institute P30 EY003039, and the Arnold and Mabel Beckman Initiative for Macular Research. Creation of Project MACULA was additionally supported from the Edward N. and Della L. Thome Memorial Foundation.
We thank the Alabama Eye Bank for timely retrieval of donor eyes; donor families for their generosity; Giovanni Staurenghi MD for facilitating the the participation of author ECZ; and David Fisher for graphical design of Figure 7.
Footnotes
Disclosures
KBF: Consultant for: Heidelberg Engineering, Optos, Optovue, Genentech, & Bayer HealthCare
CAC: Consultant for Genentech, Merck, Janssen Cell Therapy, Regeneron, Ora.
References
- 1.Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. PMID 23642856. [DOI] [PubMed] [Google Scholar]
- 2.Peden MC, Suner IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122:803–808. doi: 10.1016/j.ophtha.2014.11.018. PMID 25596618. [DOI] [PubMed] [Google Scholar]
- 3.Bhisitkul RB, Mendes TS, Rofagha S, et al. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA and HORIZON studies (SEVEN-UP Study) Am J Ophthalmol. 2015 doi: 10.1016/j.ajo.2015.01.032. PMID 25640411. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118:523–530. doi: 10.1016/j.ophtha.2010.07.011. PMID 20920825. [DOI] [PubMed] [Google Scholar]
- 5.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. PMID 22555112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–1267. doi: 10.1016/S0140-6736(13)61501-9. PMID 23870813. [DOI] [PubMed] [Google Scholar]
- 7.Young M, Chui L, Fallah N, et al. Exacerbation of choroidal and retinal pigment epithelial atrophy after anti-vascular endothelial growth factor treatment in neovascular age-related macular degeneration. Retina. 2014;34:1308–15. doi: 10.1097/IAE.0000000000000081. PMID 24451923. [DOI] [PubMed] [Google Scholar]
- 8.Grunwald JE, Pistilli M, Ying GS, et al. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015;122:809–816. doi: 10.1016/j.ophtha.2014.11.007. PMID 25542520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lois N, McBain V, Abdelkader E, et al. Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy. Retina. 2012;33:13–22. doi: 10.1097/IAE.0b013e3182657fff. PMID 22846802. [DOI] [PubMed] [Google Scholar]
- 10.Dhrami-Gavazi E, Balaratnasingam C, Freund KB. Type 1 neovascularization may confer resistance to geographic atrophy amongst eyes treated for neovascular age-related macular degeneration. Int J Retin Vitr. 2015;1:1–12. doi: 10.1186/s40942-015-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Mrejen S, Jung JJ, et al. Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Retina. 2015;35:176–186. doi: 10.1097/IAE.0000000000000374. PMID 25387047. [DOI] [PubMed] [Google Scholar]
- 12.Chae B, Jung JJ, Mrejen S, et al. Baseline predictors for good versus poor visual outcomes in the treatment of neovascular age-related macular degeneration with intravitreal anti-VEGF therapy. Invest Ophthalmol Vis Sci. 2015;56:5040–5047. doi: 10.1167/iovs.15-16494. PMID 26237196. [DOI] [PubMed] [Google Scholar]
- 13.Dansingani KK, Freund KB. Optical coherence tomography angiography reveals mature, tangled vascular networks in eyes with neovascular age-related macular degeneration showing resistance to geographic atrophy. Ophthalmic Surg Lasers Imaging Retina. 2015;46:907–912. doi: 10.3928/23258160-20151008-02. PMID 26469229. [DOI] [PubMed] [Google Scholar]
- 14.Ford KM, Saint-Geniez M, Walshe T, et al. Expression and role of VEGF in the adult retinal pigment epithelium. Investigative Ophthalmology & Visual Science. 2011;52:9478–9487. doi: 10.1167/iovs.11-8353. PMID 22058334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saint-Geniez M, Kurihara T, Sekiyama E, et al. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. PMID 19841260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurihara T, Westenskow PD, Bravo S, et al. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012;122:4213–4217. doi: 10.1172/JCI65157. PMID 23093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vujosevic S, Vaclavik V, Bird AC, et al. Combined grading for choroidal neovascularisation: colour, fluorescein angiography and autofluorescence images. Graefes Arch Clin Exp Ophthalmol. 2007;245:1453–1460. doi: 10.1007/s00417-007-0574-9. PMID 17429674. [DOI] [PubMed] [Google Scholar]
- 18.Dandekar SS, Jenkins SA, Peto T, et al. Autofluorescence imaging of choroidal neovascularization due to age-related macular degeneration. Arch Ophthalmol. 2005;123:1507–1513. doi: 10.1001/archopht.123.11.1507. PMID 16286612. [DOI] [PubMed] [Google Scholar]
- 19.Sarks J, Tang K, Killingsworth M, et al. Development of atrophy of the retinal pigment epithelium around disciform scars. Br J Ophthalmol. 2006;90:442–446. doi: 10.1136/bjo.2005.083022. PMID 16547324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaclavik V, Vujosevic S, Dandekar SS, et al. Autofluorescence imaging in age-related macular degeneration complicated by choroidal neovascularization: a prospective study. Ophthalmology. 2008;115:342–346. doi: 10.1016/j.ophtha.2007.04.023. PMID 17599415. [DOI] [PubMed] [Google Scholar]
- 21.Kumar N, Mrejen S, Fung AT, et al. Retinal pigment epithelial cell loss assessed by fundus autofluorescence imaging in neovascular age-related macular degeneration. Ophthalmology. 2012;120:334–341. doi: 10.1016/j.ophtha.2012.07.076. PMID 23137630. [DOI] [PubMed] [Google Scholar]
- 22.Schütze C, Wedl M, Baumann B, et al. Progression of retinal pigment epithelial atrophy in antiangiogenic therapy of neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159:1100–1114. e1. doi: 10.1016/j.ajo.2015.02.020. PMID 25769245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuroda Y, Yamashiro K, Tsujikawa A, et al. Retinal pigment epithelial atrophy in neovascular age-related macular degeneration after ranibizumab treatment. Am J Ophthalmol. 2016;161:94–103. e1. doi: 10.1016/j.ajo.2015.09.032. PMID 26432927. [DOI] [PubMed] [Google Scholar]
- 24.Bhisitkul RB, Desai SJ, Boyer DS, et al. Fellow eye comparisons for 7-year outcomes in ranibizumab-treated AMD subjects from ANCHOR, MARINA, and HORIZON (SEVEN-UP Study) Ophthalmology. 2016 doi: 10.1016/j.ophtha.2016.01.033. PMID 26996339. [DOI] [PubMed] [Google Scholar]
- 25.Curcio CA, Balaratnasingam C, Messinger JD, et al. Correlation of type 1 neovascularization associated with acquired vitelliform lesion in the setting of age related macular degeneration. Am J Ophthalmol. 2015 doi: 10.1016/j.ajo.2015.08.001. PMID 26255578. [DOI] [PubMed] [Google Scholar]
- 26.Grossniklaus HE, Hutchinson AK, Capone A, Jr, et al. Clinicopathologic features of surgically excised choroidal neovascular membranes. Ophthalmology. 1994;94:1099–1111. doi: 10.1016/s0161-6420(13)31216-0. PMID 7516516. [DOI] [PubMed] [Google Scholar]
- 27.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. PMID 2476333. [DOI] [PubMed] [Google Scholar]
- 28.Zanzottera EC, Messinger JD, Ach T, et al. The Project MACULA retinal pigment epithelium grading system for histology and optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015 doi: 10.1167/iovs.15-16431. PMID 25813989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanzottera EC, Messinger JD, Ach T, et al. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015;56:3269–3278. doi: 10.1167/iovs.15-16432. PMID 26024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanzottera EC, Ach T, Huisingh C, et al. Visualizing retinal pigment epithelium phenotypes in the transition to geographic atrophy in age-related macular degeneration. [7/8/16 accepted];Retina. 2016 doi: 10.1097/IAE.0000000000001276. PMID n.a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudolf M, Vogt SD, Curcio CA, et al. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology. 2013;120:821–828. doi: 10.1016/j.ophtha.2012.10.007. PMID 23357621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ach T, Tolstik E, Messinger JD, et al. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:3242–3252. doi: 10.1167/iovs.14-16274. PMID 25758814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curcio CA, Medeiros NE, Millican CL. The Alabama age-related macular degeneration grading system for donor eyes. Invest. Ophthalmol. Vis. Sci. 1998;39:1085–1096. PMID 9620067. [PubMed] [Google Scholar]
- 34.Pang CE, Messinger JD, Zanzottera EC, et al. The Onion Sign in neovascular age-related macular degeneration represents cholesterol crystals. Ophthalmology. 2015;122:2316–2326. doi: 10.1016/j.ophtha.2015.07.008. PMID 26298717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ach T, Huisingh C, McGwin G, Jr, et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55:4832–4841. doi: 10.1167/iovs.14-14802. PMID 25034602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curcio CA, Messinger JD, Sloan KR, et al. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–276. doi: 10.1097/IAE.0b013e31827e25e0. PMID 23266879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ooto S, Vongkulsiri S, Sato T, et al. Outer retinal corrugations in age-related macular degeneration. JAMA Ophthalmol. 2014;132:806–813. doi: 10.1001/jamaophthalmol.2014.1871. PMID 24801396. [DOI] [PubMed] [Google Scholar]
- 38.Green WR, Enger C. Age-related macular degeneration histopathologic studies: the 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. PMID 7692366. [DOI] [PubMed] [Google Scholar]
- 39.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br. J. Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. PMID 952802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litts KM, Messinger JD, Freund KB, et al. Inner segment remodeling and mitochondrial translocation in cone photoreceptors in age-related macular degeneration with outer retinal tubulation. Invest Ophthalmol Vis Sci. 2015;56:2243–2253. doi: 10.1167/iovs.14-15838. PMID 25758815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microscopy. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen KC, Jung JJ, Curcio CA, et al. Intraretinal hyperreflective foci in acquired vitelliform lesions of the macula: clinical and histologic study. Am J Ophthalmol. 2016;164:89–98. doi: 10.1016/j.ajo.2016.02.002. PMID 26868959. [DOI] [PubMed] [Google Scholar]
- 43.McLeod DS, Grebe R, Bhutto I, et al. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–4991. doi: 10.1167/iovs.09-3639. PMID 19357355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biesemeier A, Taubitz T, Julien S, et al. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35:2562–2573. doi: 10.1016/j.neurobiolaging.2014.05.003. PMID 24925811. [DOI] [PubMed] [Google Scholar]
- 45.Uga S, Smelser GK. Comparative study of the fine structure of retinal Muller cells in various vertebrates. Invest Ophthalmol. 1973;12:434–448. PMID 4541022. [PubMed] [Google Scholar]
- 46.Williams DS, Arikawa K, Paallysaho T. Cytoskeletal components of the adherens junctions between the photoreceptors and the supportive Muller cells. J Comp Neurol. 1990;295:155–164. doi: 10.1002/cne.902950113. PMID 2341633. [DOI] [PubMed] [Google Scholar]
- 47.Omri S, Omri B, Savoldelli M, et al. The outer limiting membrane (OLM) revisited: clinical implications. Clin Ophthalmol. 2010;4:183–195. doi: 10.2147/opth.s5901. PMID 20463783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bunt-Milam AH, Saari JC, Klock IB, Garwin GS. Zonula adherentes pore size in the external limiting membrane of the rabbit retina. Invest. Ophthalmol. Vis. Sci. 1985;26:1377–1380. PMID 4044165. [PubMed] [Google Scholar]
- 49.Wu KH, Madigan MC, Billson FA, Penfold PL. Differential expression of GFAP in early v late AMD: a quantitative analysis. Br J Ophthalmol. 2003;87:1159–1166. doi: 10.1136/bjo.87.9.1159. PMID 12928288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guidry C, Medeiros NE, Curcio CA. Phenotypic variation of retinal pigment epithelium in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:267–273. PMID 11773041. [PubMed] [Google Scholar]
- 51.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 1996;37:1236–1249. PMID 8641827. [PubMed] [Google Scholar]
- 52.Litts KM, Messinger JD, Dellatorre K, et al. Clinicopathological correlation of outer retinal tubulation in age-related macular degeneration. JAMA Ophthalmol. 2015;133:609–612. doi: 10.1001/jamaophthalmol.2015.126. PMID 25742505. [DOI] [PubMed] [Google Scholar]
- 53.Schaal KB, Freund KB, Litts KM, et al. Outer retinal tubulation in advanced age-related macular degeneration: optical coherence tomographic findings correspond to histology. Retina. 2015;35:1339–1350. doi: 10.1097/IAE.0000000000000471. PMID 25635579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bird AC, Phillips RL, Hageman GS. Geographic atrophy: a histopathological assessment. JAMA Ophthalmol. 2014;132:338–345. doi: 10.1001/jamaophthalmol.2013.5799. PMID 24626824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theelen T, Berendschot TT, Boon CJ, et al. Analysis of visual pigment by fundus autofluorescence. Exp Eye Res. 2008;86:296–304. doi: 10.1016/j.exer.2007.10.022. PMID 18096158. [DOI] [PubMed] [Google Scholar]
- 56.Joseph A, Rahimy E, Freund KB, et al. Fundus autofluorescence and photoreceptor bleaching in multiple evanescent white dot syndrome. Ophthalmic Surg Lasers Imaging Retina. 2013;44:588–592. doi: 10.3928/23258160-20131105-08. PMID 24221465. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki M, Gomi F, Sawa M, et al. Changes in fundus autofluorescence in polypoidal choroidal vasculopathy during 3 years of follow-up. Graefes Arch Clin Exp Ophthalmol. 2013;251:2331–2337. doi: 10.1007/s00417-013-2336-1. PMID 23604513. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz-Valckenberg S, Sahel JA, Danis R, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression study) Ophthalmology. 2016;123:361–368. doi: 10.1016/j.ophtha.2015.09.036. PMID 26545317. [DOI] [PubMed] [Google Scholar]
- 59.Ozkok A, Sigford DK, Tezel TH. Patterns of fundus autofluorescence defects in neovascular age-related macular degeneration subtypes. Retina. 2016;36:2191–2196. doi: 10.1097/IAE.0000000000001034. PMID 27078800. [DOI] [PubMed] [Google Scholar]
- 60.Spaide RF, Klancnik JM., Jr Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology. 2005;112:825–833. doi: 10.1016/j.ophtha.2005.01.003. PMID 15878062. [DOI] [PubMed] [Google Scholar]
- 61.Davis CH, Marsh-Armstrong N. Discovery and implications of transcellular mitophagy. Autophagy. 2014;10:2383–2384. doi: 10.4161/15548627.2014.981920. PMID 25484086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang D, Jia Y, Rispoli M, et al. Optical coherence tomography angiography of time course of choroidal neovascularization in response to anti-angiogenic treatment. Retina. 2015;35:2260–2264. doi: 10.1097/IAE.0000000000000846. PMID 26469535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu W, Jiang A, Liang J, et al. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2008;49:407–415. doi: 10.1167/iovs.07-0870. PMID 18172119. [DOI] [PubMed] [Google Scholar]
- 64.Kumar S, Berriochoa Z, Ambati BK, Fu Y. Angiographic features of transgenic mice with increased expression of human serine protease HTRA1 in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55:3842–3850. doi: 10.1167/iovs.13-13111. PMID 24854852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olsen TW, Feng X. The Minnesota Grading System of eye bank eyes for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:4484–4490. doi: 10.1167/iovs.04-0342. PMID 15557458. [DOI] [PubMed] [Google Scholar]
- 66.Curcio CA, Saunders PL, Younger PW, Malek G. Peripapillary chorioretinal atrophy: Bruch's membrane changes and photoreceptor loss. Ophthalmology. 2000;107:334–343. doi: 10.1016/s0161-6420(99)00037-8. PMID 10690836. [DOI] [PubMed] [Google Scholar]