Abstract
It has been proposed that the deficit in β-cell mass in type 1 diabetes (T1D) may be due, in part, to β-cell degranulation to chromogranin-positive hormone-negative (CPHN) cells. The frequency and distribution of pancreatic CPHN cells were investigated in 19 children with T1D compared with 14 nondiabetic (ND) children. We further evaluated these cells for replication and expression of endocrine lineage markers Nkx6.1 and Nkx2.2, and compared these frequencies with those previously reported in CPHN cells in adults with T1D. In contrast to adults’ cells, pancreatic CPHN cells were comparably abundant (percentage of endocrine cells ± standard error of the mean, 1.4 ± 0.2 vs 1.0 ± 0.2 in patients with T1D vs ND subjects, respectively; P = not significant) and comparably distributed in children with T1D vs ND donors. Replication of CPHN cells was detected but unchanged in children with T1D vs ND children, as was the percentage of CPHN cells expressing Nkx6.1 or NKx2.2. In children with T1D, the frequency of pancreatic CPHN cells was not increased, and this differed from adults with T1D.
Pancreatic nonhormone-expressing endocrine cells are not more frequent in children with type 1 diabetes compared with nondiabetic children.
Type 1 diabetes (T1D) results from insufficient β-cell mass due to autoimmune destruction. It afflicts approximately 1 million people in the United States. As defined by the US-based SEARCH for Diabetes in Youth study, approximately 22 of 100,000 children younger than 20 years develop T1D annually in the United States [1]; the prevalence in this age group is one per 526 children [2]. However, incidence in most countries appears to be rising [2, 3], particularly in the youngest children who also have more rapid onset of disease [4]. Although the concept that autoimmunity is causal in β-cell loss in T1D is well accepted [5, 6], intriguing concepts have arisen suggesting that loss of β cells in type 1 and 2 diabetes may be due, in part, to degranulation of β cells and/or transdifferentiation of β cells to other endocrine cell types [7–9]. However, an alternative explanation for the increased presence of hormone-negative endocrine cells in the pancreas in diabetes is that they are the result of ongoing attempted β-cell regeneration. Support for this possibility arises from the comparable pattern and distribution of such cells in late gestation and early infancy in humans [10].
There appears to be ongoing β-cell turnover in adults with longstanding T1D, although the source of these cells is unknown [11–13]. We previously examined pancreas tissue from adult brain-dead organ donors with T1D vs nondiabetic (ND) control subjects and found an increase in endocrine cells that did not express any known pancreatic hormone occurring in a distribution comparable to that in the developing human pancreas [14]. To determine whether the increased presence and scale of nonhormone-expressing endocrine cells in the pancreas differ in children with T1D, in whom the severity of the disease may be greater but in the setting of potentially greater plasticity in the pancreas, we investigated a cohort of children with T1D and age- and body mass index (BMI)-matched ND control subjects available through the outstanding repository of human pancreas assembled by the Network for Pancreatic Organ Donors with Diabetes (nPOD) consortium based at the University of Florida.
Using this resource, we investigated if is there an increase in the frequency of nonhormone-expressing endocrine cells in the pancreas in children with T1D, and we examined the distribution of these cells within the pancreas in both groups of children. In normal late gestation and early infancy, nonhormone-expressing endocrine cells are most abundant as scattered foci in the exocrine pancreas and are believed to represent newly forming and differentiating pancreatic endocrine cells.
1. Materials and Methods
A. Study Subjects
A-1. Design and case selection
All pancreata from the donors with T1D and the ND donors were procured from brain-dead organ donors by the Juvenile Diabetes Research Foundation nPOD, a program coordinated by the University of Florida in Gainesville, Florida [15]. All procedures were in accordance with federal guidelines for organ donation and the University of Florida Institutional Review Board.
Case characteristics.
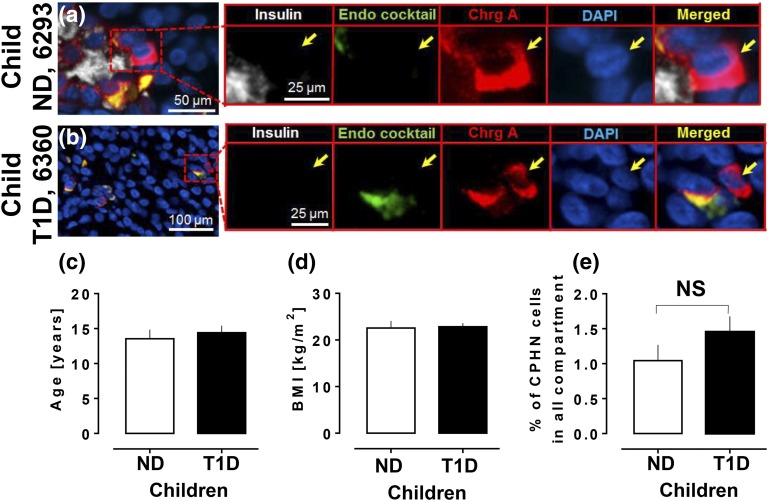
Pancreata were procured from 19 child donors (defined as <20 years of age) with T1D and 14 ND child donors matched for age [14.9 ± 0.9 years vs 13.5 ± 1.3 years, T1D and ND, respectively; P = not significant (ns); Fig. 1(c)], sex (T1D group: 12 boys, seven girls; ND group: nine boys, five girls), and BMI [22.8 ± 0.8 kg/m2 vs 22.6 ± 1.5 kg/m2, T1D vs ND, respectively; P = ns; Fig. 1(d); Tables 1 and 2].
Figure 1.
CPHN cells are present in comparable frequency in the pancreas in children with and without T1D. (a, b) Examples of CPHN cells in the pancreas of (a) a ND child donor and (b) a child donor with T1D. Scale bars: (a) 50 μm and (b) 100 μm. Insets in both panels show higher magnification of the indicated region in the low-power images (scale bars, 25 μm). Individual layers were stained for insulin (white), endocrine cocktail (glucagon, somatostatin, pancreatic polypeptide, and ghrelin: green), chromogranin A (red), and 4′,6-diamidino-2-phenylindole (blue) are shown along with the merged image. Yellow arrows indicate CPHN cells. All the children with T1D and the ND children analyzed in this study were matched for (c) age and (d) BMI. (e) There was no change in frequency of CPHN cells in all compartments of the pancreas sections in children with T1D compared with ND control subjects. ChrgA, chromogranin A; DAPI, 4′,6-diamidino-2-phenylindole; endo cocktail, endocrine cocktail.
Table 1.
Characteristics of Patients With T1D
| Type 1 Diabetes | Autoantibodies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case ID | Age, y | Sex | BMI, kg/m2 | GADA Result | IA 2A Result | MIAA Result | Zn T8A Result | Total Positive Autoantibody Count | Duration of Diabetes, y | Insulin Status | |
| 1 | 6062 | 10.7 | M | 21.9 | N/A | N/A | N/A | N/A | N/A | 6 | No insulin |
| 2 | 6063 | 4.4 | M | 23.8 | − | − | + | − | 1 | 3 | No insulin |
| 3 | 6064 | 19.6 | F | 22.6 | + | + | + | − | 3 | 9 | No insulin |
| 4 | 6089 | 14.3 | M | 26 | − | − | + | − | 1 | 8 | No insulin |
| 5 | 6083 | 15.2 | F | 18.4 | − | − | + | − | 1 | 11 | No insulin |
| 6 | 6087 | 17.5 | M | 21.9 | − | − | + | + | 2 | 4 | No insulin |
| 7 | 6145 | 18 | M | 23.1 | + | − | + | + | 3 | 11 | No insulin |
| 8 | 6148 | 17.1 | M | 23.9 | + | − | + | − | 2 | 7 | No insulin |
| 9 | 6261 | 16 | M | 20.7 | + | − | + | − | 2 | 14 | No insulin |
| 10 | 6360 | 4.8 | F | 26.1 | − | − | + | − | 1 | 2.5 | No insulin |
| 11 | 6046 | 18.8 | F | 25.2 | − | + | − | + | 2 | 8 | Abundant insulin+ islets |
| 12 | 6049 | 15 | F | 20.8 | + | − | + | − | 2 | 10 | Rare insulin + cells |
| 13 | 6084 | 14.2 | M | 26.3 | − | − | + | − | 1 | 4 | Some insulin+ cells in islets, patchy |
| 14 | 6195 | 19.2 | M | 23.7 | + | + | + | + | 4 | 5 | Rare insulin + cells |
| 15 | 6243 | 13 | M | 21.3 | − | − | + | − | 1 | 5 | Abundant insulin+ islets |
| 16 | 6265 | 11 | M | 12.9 | + | − | + | − | 2 | 8 | Rare insulin + cells |
| 17 | 6268 | 12 | F | 26.6 | − | − | + | − | 1 | 3 | Rare insulin + cells |
| 18 | 6306 | 19 | M | 24.5 | − | − | + | − | 1 | 5 | Abundant insulin+ islets |
| 19 | 6342 | 14 | F | 24.3 | − | + | + | − | 2 | 2 | Abundant insulin+ islets |
| Mean | 14.9 | 22.8 | |||||||||
| SEM | 0.9 | 0.8 | |||||||||
Abbreviations: +, positive; −, negative; F, female; GADA, glutamic acid decarboxylase; IA 2A, IA-2 antibody; M, male; MIAA, micro insulin autoantibody; N/A, not applicable; SEM, standard error of the mean; Zn T8A, zinc transporter antibody.
Table 2.
Characteristics of Patients Without T1D
| Control Subjects Without T1D | ||||
|---|---|---|---|---|
| Case ID | Age, y | Sex | BMI, kg/m2 | |
| 1 | 6047 | 7.8 | M | 23.9 |
| 2 | 6096 | 16 | F | 18.8 |
| 3 | 6098 | 17.8 | M | 22.8 |
| 4 | 6130 | 5 | M | 18.5 |
| 5 | 6336 | 14 | F | 28.9 |
| 6 | 6335 | 18 | M | 23.6 |
| 7 | 6153 | 15.2 | M | 20.5 |
| 8 | 6318 | 10 | F | 17.6 |
| 9 | 6293 | 9 | F | 18.6 |
| 10 | 6075 | 16 | M | 14.9 |
| 11 | 6279 | 19 | M | 34 |
| 12 | 6172 | 19.2 | F | 32.4 |
| 13 | 6230 | 16 | M | 18.9 |
| 14 | 6381 | 6.6 | M | 22.6 |
| Mean | 13.5 | 22.6 | ||
| SEM | 1.3 | 1.5 | ||
Pancreatic weights were available for 15 of the 19 donors with T1D and all 14 of the ND donors. Pancreas weight was lower in T1D donors than in ND donors, as previously reported [16] (33.1 ± 3.9 g vs 52.8 ± 5.4 g, T1D vs ND, respectively; P < 0.01).
B. Pancreas Acquisition and Processing
nPOD uses a standardized preparation procedure for pancreata recovered from cadaveric organ donors [17]. The pancreas is divided into three main regions (i.e., head, body, and tail), and by serial transverse sections throughout the medial to lateral axis, allowing for sampling of the entire pancreas organ while maintaining anatomic orientation. Because preparation is completed within 2 hours, tissue integrity is maintained. Tissues intended for paraffin blocks are trimmed to pieces no larger than 1.5 × 1.5 cm and fixed in 10% neutral buffered formalin for 24 ± 8 hours. Fixation is terminated by transfer to 70% ethanol, and samples are subsequently processed and embedded in paraffin. Mounted transverse sections from the paraffin-embedded tissue blocks were obtained from the body of the pancreas in most cases; where blocks of pancreas body were unavailable, sections from the head of the pancreas were used.
C. Immunostaining
C-1. For assessment of presence and frequency of CPHN cells
All staining was performed at the University of California, Los Angeles. Paraffin tissue sections from each subject were stained for chromogranin A, insulin, glucagon, somatostatin, pancreatic polypeptide, and ghrelin. Standard immunohistochemistry protocol was used for fluorescent immunodetection of various proteins in pancreatic sections [14].
Briefly, slides were incubated at 4°C overnight with a cocktail of primary antibodies prepared in antibody solution (3% bovine serum albumin in Tris-buffered saline with polysorbate 20) at the following dilutions: rabbit anti-chromogranin A [1:200; Resource Identification Initiative (RRID): AB_789299; catalog no. NB120-15160; Novus Biologicals, Littleton, CO]; mouse anti-glucagon (1:1000; catalog no. G2654-0.2ML; RRID: AB_259852; Sigma-Aldrich, St. Louis, MO); guinea pig anti-insulin (1:200; RRID: AB_306130; catalog no. 7842; Abcam, Cambridge, MA); rat anti-somatostatin (1:300; RRID: AB_2255365; catalog no. MAB354; EMD Millipore, Billerica, MA); goat anti-pancreatic polypeptide (1:3000; RRID: AB_2169058; catalog no. EB06805; Everest Biotech, Ramona, CA); and rat anti-ghrelin (1:50; RRID: AB_2637039; catalog no. MAB8200; R&D Systems, Minneapolis, MN). The primary antibodies were detected by a cocktail of appropriate secondary antibodies (Jackson ImmunoResearch, Westgrove, PA) conjugated to Cy3 (1:200, for chromogranin A, RRID: AB_2313568), fluorescein isothiocyanate [1:200 each, to detect glucagon (RRID: AB_2340795), somatostatin (RRID: AB_2340653), pancreatic polypeptide (RRID: AB_2340402), and ghrelin (RRID: AB_2340653)] or Cy5 [1:100, to detect insulin (RRID: AB_2340477)].
Slides were counterstained to mark the nuclei, using a mounting medium containing 4′,6-diamidino-2-phenylindole (Vectashield; Vector Laboratories, Burlingame, CA) and viewed using a Leica DM6000 microscope (Leica Microsystems, Deerfield, IL). Images were acquired using the ×20 objective (×200 magnification) using a Hamamatsu Orca-ER camera (catalog no. C4742-80-12AG; Indigo Scientific, Bridgewater, NJ) and Openlab software (Improvision, Lexington, MA).
C-2. Assessment of potential endocrine lineage and frequency of replication of CPHN cells
To investigate the potential endocrine cell lineage of CPHN cells in children and adults with T1D, we evaluated Ki67 as a endocrine replication marker (mouse anti-Ki67, 1:50; RRID: AB_2142367; catalog no. M7240; Agilent Technologies), Nkx2.2 as a panendocrine transcription factor (mouse anti-Nkx2.2, 1:50; RRID: AB_531794; catalog no. 74.5A5; DHSB), and Nkx6.1 as a β-cell transcription factor (mouse anti-Nkx6.1, 1:300; RRID: AB_532378; catalog no. F55A10; DSHB) using, in each case, a secondary antibody conjugated to Cy3 (RRID: AB_2340813). We combined this evaluation with a cocktail of all known pancreatic hormones, as described previously [11, 14]. Sections were evaluated from children [six T1D donors (case identification numbers [case IDs] 6063, 6089, 6265, 6261,6083, and 6084) and six age- and BMI-matched ND donors (case IDs 6047, 6130, 6336, 6075, 6293, and 6153)]. Slides were blocked and incubated sequentially with the same antibodies as described by Moin et al. [14]. Slides were counterstained to mark the nuclei using a mounting medium containing 4′,6-diamidino-2-phenylindole (Vectashield, Vector Laboratories, Burlingame, CA), and viewed and imaged using a Leica DM6000 microscope (Leica Microsystems, Deerfield, IL).
C-3. Comparison of CPHN cells in childhood and adults for replication (Ki67) and endocrine lineage (Nkx6.1 and Nkx2.2)
Once we established that, in contrast to adults with T1D [14], the frequency of CPHN cells in children with T1D did not differ from control children, we then compared the frequency of replication of CPHN (by Ki67) cells and the expression of the transcription factors Nkx6.1 and Nkx2.2 between adults and children. To do so, we included four adult donors with T1D (case IDs 6138, 6051, 6076, and 6050) and four without (case IDs 6015, 6021, 6029, and 6134) in which we previously reported the frequency of Ki67, Nkx6.1 and Nkx2.2 in CPHN cells [14]. We also analyzed three additional cases of adult T1D (case IDs 6036, 6045, and 6068) and adult ND (case IDs 6012, 6126, and 6104) for these same nuclear markers and compared the data with the selected subset of T1D and ND children (children with T1D, n = 6; ND children, n = 6; adults with T1D, n = 7; ND adults, n = 7).
D. Morphometric Analysis
One section of pancreas per subject was stained. Endocrine cells from 50 islets, as well as all endocrine cells present as single cells or in clusters in the exocrine pancreas contained within those islet-containing fields per subject were imaged at ×20 magnification. An islet was defined as a grouping of four or more endocrine cells. A cluster was defined as a grouping of three or fewer chromogranin-positive cells. Islets were selected by starting at the top left corner of the pancreatic tissue section and working across the tissue from left to right and back again in a serpentine fashion, imaging all islets in this systematic excursion across the tissue section. Analysis was performed in a blinded fashion by four of the authors (A.S.M.M., M.C., A.O., and J.C.), and all CPHN cells identified were confirmed by a second observer (A.E.B.). The endocrine cells contained within each islet were manually counted and recorded as follows: (1) the number of cells staining for chromogranin A; (2) the number of cells staining for the endocrine hormone cocktail; and (3) the number of cells staining for insulin. Thus, cells staining for chromogranin A but not the other known pancreatic hormones (i.e., insulin, glucagon, somatostatin, pancreatic polypeptide, or ghrelin) were noted.
At ×200 magnification, using the Leica DM6000 with Hamamatsu Orca-ER camera and a ×0.7 C-mount, each field of view was calculated to be 0.292 mm2. Within the fields imaged to obtain the 50 islets per subject, all single endocrine cells and clusters of endocrine cells (two or three adjacent endocrine cells) were counted and recorded as described in the previous paragraph.
E. Statistical Analysis
Statistical analysis was performed using the Student t test with GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA). Data in graphs and tables are presented as means ± standard error of the mean. Findings were assumed statistically significant at P < 0.05.
F. Study Approval
These studies in humans were reviewed and approved by the institutional review boards of the University of Florida and the University of California, Los Angeles. Informed consent was not required because the subjects were brain-dead organ donors.
2. Results
A. CPHN Cells Did Not Occur More Frequently in Children With T1D Than in ND Control Subjects
The mean number of endocrine cells counted within islets for the children with T1D was 1896 ± 160 cells per donor, and 2336 ± 240 cells per donor for the ND children. The mean number of endocrine cells counted in clusters for the children with T1D was 247 ± 22 cells per donor, and 97 ± 12 cells per donor for the ND children. The mean number of CPHN cells counted in islets for the children with T1D was 8.4 ± 2.0 cells per donor, and 15 ± 5.0 per donor for the children with ND.
CPHN cells were identified in pancreas tissue of ND children [Fig. 1(a)] and children with T1D [Fig. 1(b)]. The mean number of CPHN cells per individual identified in clusters for the T1D group was 18 ± 2.0 cells per donor, and 10.4 ± 2.3 cells per donor for the ND group. The percentage of CPHN cells counted in clusters was higher than that in islets both in T1D and ND groups (10.1% ± 1.3% vs 0.6% ± 0.1% of clustered CPHN cells vs islet CPHN cells in the T1D group, and 7.6% ± 0.7% vs 1.34% ± 0.1% of clustered CPHN cells vs islet CPHN cells in the ND group).
There was no increase in the frequency of CPHN cells in children with T1D compared with ND children (1.4% ± 0.2% vs 1.0% ± 0.2% of endocrine cells, T1D vs ND groups, respectively; P = ns), in either islets or scattered cells, or in(islets and clusters combined [Fig. 1(e)].
B. Replication of CPHN Cells in Children and Adults With T1D
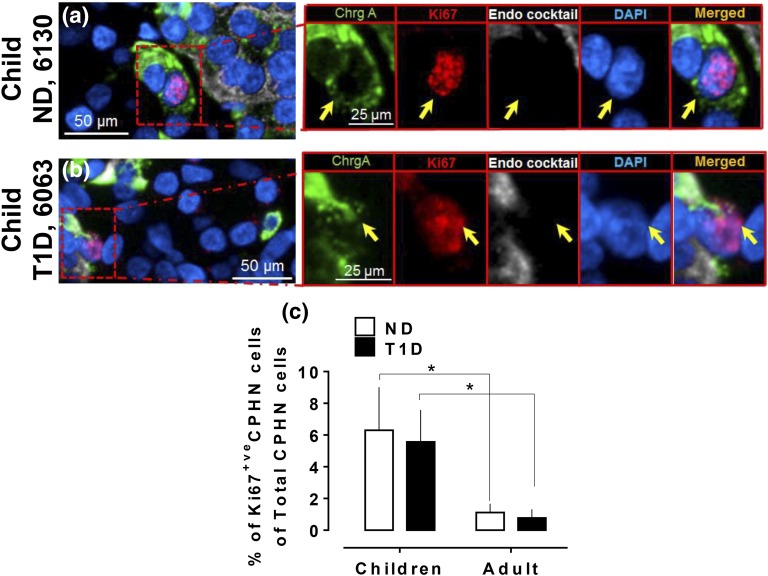
CPHN cells expressing Ki67 were identified in pancreas tissue of children with T1D and ND children (Fig. 2) at a comparable frequency (5.5% ± 2.0% vs 6.3% ± 2.7% of Ki67-positive CPHN cells in all compartments, T1D vs ND groups, respectively; P = ns). The frequency of replication of CPHN cells was higher in children with T1D and ND children compared with either adult group. Therefore, as has previously been reported for β cells [18], CPHN cells also have a greater capacity for replication in children than adults. The frequency of CPHN replication for T1D children and T1D adults was 5.5% ± 2.0% in children vs 0.7% ± 0.5% in adults of Ki67-positive CPHN cells relative to the total number of CPHN cells in all compartments (P < 0.05). The frequencies of CPHN replication for ND children and ND adults was 6.3% ± 2.7% and 1.1% ± 0.5%, respectively, of Ki67-positive CPHN cells relative to the total number of CPHN cells in all compartments [P < 0.05; Fig. 2(c)].
Figure 2.
The frequency of replication of CPHN cells in the pancreas is comparable in children with and without T1D and more frequent than in adults. Representative images of CPHN and Ki67 staining in the pancreas in (a) a child ND donor and (b) a child T1D donor. Individual layers stained for ChrgA (green), endocrine cocktail (insulin, glucagon, somatostatin, pancreatic polypeptide, and ghrelin: white), Ki67 (red), and DAPI (blue) are shown along with the merged image. Yellow arrows indicate CPHN cells. (c) Comparison of Ki67-positive CPHN cells between children and adults (in both T1D and ND) in all compartments of pancreas. Scale bars: 50 µm for lower-power images and 25 µm for insets.
C. CPHN Cells With a Potential β-Cell Lineage Were Not More Abundant in Children With T1D Compared With Control Subjects But Were Increased in Adults With T1D
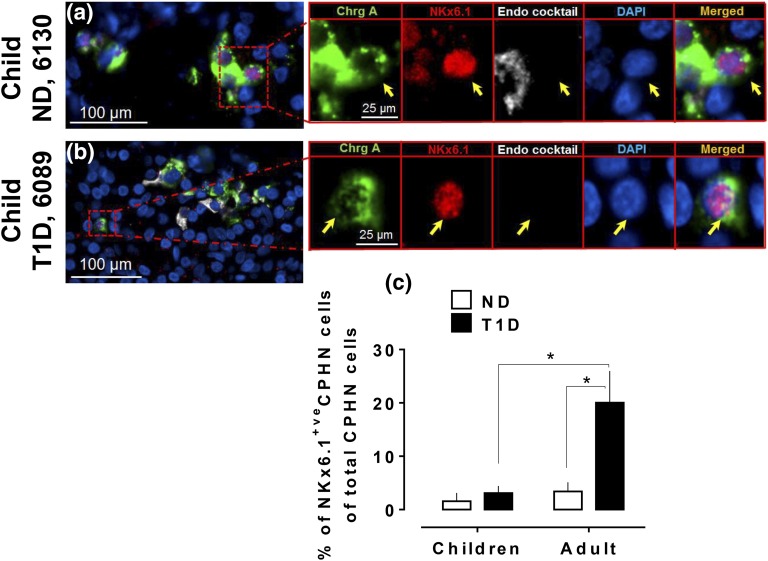
The percentage of pancreatic CPHN cells expressing NKx6.1 in children with T1D was comparable to ND control subjects (3.0% ± 1.3% vs 1.5% ± 1.5% of NKx6.1-positive CPHN cells in all compartments, T1D vs ND groups, respectively; P = ns; Fig. 3). This, again, differed from the findings in adults with T1D, in whom there was a sixfold increase in the percentage of CPHN cells that expressed NKx6.1 compared with ND organ donors [20.0% ± 6.0% vs 3.4% ± 1.7% of NKx6.1-positive CPHN cells of total CPHN cells, T1D vs ND adult groups, respectively; P < 0.05; Fig. 3(c)].
Figure 3.
The frequency of Nkx6.1-positive nuclei in CPHN cells in the pancreas is comparable in children with and without T1D and is lower than in adults with T1D. Representative images of CPHN and NKx6.1 staining in the pancreas of (a) a child ND donor and (b) a child T1D donor. Individual layers stained for ChrgA (green), endocrine cocktail (insulin, glucagon, somatostatin, pancreatic polypeptide and ghrelin: white), NKx6.1 (red), and DAPI (blue) are shown along with the merged image. Yellow arrows indicate CPHN cells. (c) Comparison of NKx6.1-positive CPHN cells between children and adults (those with and those who were ND) in all compartments of pancreas. Scale bars: 100 µm for lower-power images and 25 µm for insets.
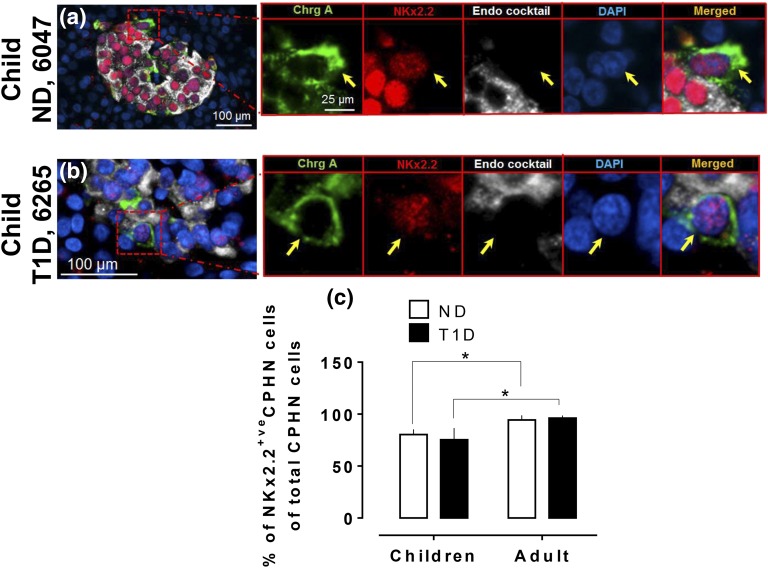
Nkx2.2 marks multipotent pancreatic progenitors, but, unlike mouse pancreas, it is detected in human pancreas progenitors after endocrine commitment [19]. There was no difference in the frequency of pancreatic CPHN cells expressing NKx2.2 in children with T1D vs ND control subjects (75.3% ± 11.2% vs 80.2% ± 5.0% of NKx2.2-positive CPHN, T1D vs ND children groups, respectively; P = ns; Fig. 4). However, the frequency of NKx2.2-positive CPHN cells was lower in T1D and ND children compared with T1D and ND adult donors [75.3% ± 11.2% vs 96.1% ± 2.5% in children with T1D vs adults with T1D, respectively; and 80.2% ± 5.0% vs 94.4% ± 4.3%, ND children vs ND adults, respectively, of NKx2.2-positive CPHN cells of total CPHN cells in all compartments; P < 0.05 in both comparisons; Fig. 4(c)].
Figure 4.
The frequency of Nkx2.2-positive nuclei in CPHN cells in the pancreas is comparable in children with and withoutT1D and lower than in adults both with and without T1D. Representative images of CPHN and NKx2.2 staining in the pancreas of (a) a child ND donor and (b) a child T1D donor. Individual layers stained for ChrgA (green), endocrine cocktail (insulin, glucagon, somatostatin, pancreatic polypeptide and ghrelin: white), NKx6.1 (red), and DAPI (blue) are shown along with the merged image. Yellow arrows indicate CPHN cells. (c) Comparison of NKx2.2-positive CPHN cells between children and adults (those with T1D and those who were ND) in all compartments of pancreas. Scale bars are 100 µm for lower-power images and 25 µm for insets.
3. Discussion
We found no increase in frequency in pancreatic endocrine cells expressing no known pancreatic hormone in children with T1D. This is in notable contrast to the marked increase in these CPHN cells in adults with T1D [14]. Several possibilities could account for this difference.
First, it is possible that, in adults, rather than CPHN cells being newly formed endocrine cells, they could be endocrine cells undergoing dedifferentiation secondary to metabolic or autoimmune stress. However, the location of the increased CPHN cells in adults with T1D, largely found as scattered cells or small foci in the exocrine pancreas rather than in established islets, weighs against dedifferentiation of mature endocrine cells. Indeed, the distribution of the more frequent CPHN cells in adults with T1D closely resembles that in the newly forming endocrine pancreas in late gestation and early infancy [10].
A second possibility is that the mechanism of neogenesis in production of new endocrine cells in the pancreas is more robust in adults than in children. Expansion of the β-cell mass in young children is mediated through replication of existing β cells, and so plausibly any effort at β-cell regeneration might seek that route. In contrast, in adult humans, β-cell replication is rare and perhaps the increased foci of CPHN cells in adults with T1D reveals attempted so-called neogenesis of endocrine cells from as-yet uncharacterized putative precursors [18].
A third possibility for the lack of increased CPHN cells in children with T1D compared with ND children is the increased replication of these cells in children compared with adults [18]. Replicating cells exposed to any DNA modifying stress are more vulnerable to apoptosis through failure of cell cycle checkpoints compared with cells in G0; therefore, it is plausible that any effort to enhance formation of new CPHN cells in childhood T1D is countered by enhanced apoptosis of these cells in the proximity of autoimmune-mediated stress [20].
Replication of most cell types is more frequent in childhood when compared with adults. Therefore, our finding that CPHN-cell replication was higher in children, regardless of diabetic status, when compared with adults is not surprising.
However, it is notable that although, in adults, the percentage of Nkx6.1-positive CPHN cells was raised in T1D, this was not the case in childhood T1D. Autoimmune-mediated destruction of β cells is more rapid in children than adults with T1D, implying more robust autoreactivity [6]. Given the epitope-spreading characteristic of autoimmunity and that Nkx6.1-positive CPHN cells are fated to a β-cell lineage, one might reasonably expect a more rapid clearance of Nkx6.1-expressing CPHN cells in children than in adults with T1D.
Another possibility is that inflammatory mediators could be preventing β-cell differentiation by inhibiting the transcription factors that preserve a mature β-cell phenotype [21]; as noted, the immune assault and local inflammation may be more intense in the children with T1D. Although children, regardless of diabetic status, had a lower percentage of Nkx2.2-positive CPHN cells than adults, the differences were much less marked than those seen with the percentage of Nkx6.1-positive CPHN cells, raising the possibility that, in children, Nkx2.2 cells are prevented from differentiating further into Nkx6.1-positive cells as a consequence of the more intense inflammatory milieu.
4. Conclusion
In summary, pancreatic CPHN cells were comparable in abundance in children with T1D and ND children. This finding differs from the increased frequency of these cells in adults with T1D, as does the lower frequency of CPHN cells expressing NK6.1 in children with T1D compared with control subjects. The importance of characterizing pancreatic changes in both children and adults with T1D is again emphasized because the pattern and progression of T1D differs markedly between these age groups and, therefore, effective strategies to intervene most likely also differ.
Acknowledgments
We thank Bonnie Lui from the Larry L. Hillblom Islet Research Center at the University of California, Los Angeles, for editorial assistance.
Acknowledgments
This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International. Organ procurement organizations partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/our-partners.php. This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant DK077967 and Larry Hillblom Foundation Grant 2014-D-001-NET to P.C.B.; and the nPOD through a pilot grant from the Helmsley Charitable Trust George S. Eisenbarth nPOD Award for Team Science (Grant 2015PG-T1D052, Subaward 664215) to A.E.B.
Author contributions: A.S.M.M., M.C., A.O., J.C., and A.E.B. performed the studies, the microscopy, and the morphologic analysis. A.S.M.M., S.D., P.C.B., and A.E.B. researched data; wrote, reviewed, and edited the manuscript; and contributed to the discussion.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Case ID
- case identification number
- ChrgA
- chromogranin A
- CPHN
- chromogranin-positive hormone-negative
- DAPI
- 4′,6-diamidino-2-phenylindole
- ND
- nondiabetic
- nPOD
- Network for Pancreatic Organ Donors with Diabetes
- ns
- not significant
- RRID
- Resource Identification Initiative
- T1D
- type 1 diabetes.
References and Notes
- 1.Lawrence JM, Imperatore G, Dabelea D, Mayer-Davis EJ, Linder B, Saydah S, Klingensmith GJ, Dolan L, Standiford DA, Pihoker C, Pettitt DJ, Talton JW, Thomas J, Bell RA, D’Agostino RB Jr; SEARCH for Diabetes in Youth Study Group . Trends in incidence of type 1 diabetes among non-Hispanic white youth in the U.S., 2002-2009. Diabetes. 2014;63(11):3938–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamman RF, Bell RA, Dabelea D, D’Agostino RB Jr, Dolan L, Imperatore G, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Pihoker C, Rodriguez BL, Saydah S; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vehik K, Dabelea D. The changing epidemiology of type 1 diabetes: why is it going through the roof? Diabetes Metab Res Rev. 2011;27(1):3–13. [DOI] [PubMed] [Google Scholar]
- 4.Leete P, Willcox A, Krogvold L, Dahl-Jørgensen K, Foulis AK, Richardson SJ, Morgan NG. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65(5):1362–1369. [DOI] [PubMed] [Google Scholar]
- 5.Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4(2):110–125. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M. Current concepts on the pathogenesis of type 1 diabetes--considerations for attempts to prevent and reverse the disease. Diabetes Care. 2015;38(6):979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Rodrigo Sandoval P, Masini M, Marselli L, Suleiman M, Ratner LE, Marchetti P, Accili D. Evidence of beta-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab. 2016;101(3):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150(6):1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57(11):2883–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler AE, Dhawan S, Hoang J, Cory M, Zeng K, Fritsch H, Meier JJ, Rizza RA, Butler PC. β-cell deficit in obese type 2 diabetes, a minor role of β-cell dedifferentiation and degranulation. J Clin Endocrinol Metab. 2016;101(2):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–2228. [DOI] [PubMed] [Google Scholar]
- 12.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–1720. [DOI] [PubMed] [Google Scholar]
- 13.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59(10):2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moin AS, Dhawan S, Shieh C, Butler PC, Cory M, Butler AE. Increased hormone-negative endocrine cells in the pancreas in type 1 diabetes. J Clin Endocrinol Metab. 2016;101(9):3487–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O’Neill A, Staeva T, Nierras C, Moraski J, Rowe P, Gianani R, Eisenbarth G, Crawford J, Schatz D, Pugliese A, Atkinson M. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28(7):608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308(22):2337–2339. [DOI] [PubMed] [Google Scholar]
- 17.Campbell-Thompson ML, Montgomery EL, Foss RM, Kolheffer KM, Phipps G, Schneider L, Atkinson MA. Collection protocol for human pancreas. J Vis Exp. 2012;23(63):e4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capito C, Simon MT, Aiello V, Clark A, Aigrain Y, Ravassard P, Scharfmann R. Mouse muscle as an ectopic permissive site for human pancreatic development. Diabetes. 2013;62(10):3479–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritzel RA, Butler PC. Replication increases beta-cell vulnerability to human islet amyloid polypeptide-induced apoptosis. Diabetes. 2003;52(7):1701–1708. [DOI] [PubMed] [Google Scholar]
- 21.Ortis F, Naamane N, Flamez D, Ladrière L, Moore F, Cunha DA, Colli ML, Thykjaer T, Thorsen K, Orntoft TF, Eizirik DL. Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes. 2010;59(2):358–374. [DOI] [PMC free article] [PubMed] [Google Scholar]