Abstract
Compared to conventional core-shell structures, core-shell free nanoparticles with multiple functionalities offer several advantages such as minimal synthetic complexity and low production cost. In this paper, we present the synthesis and characterization of Nd3+ doped Na(Gd0.5Lu0.5)F4 as a core-shell free nanoparticle system with three functionalities. Nanocrystals with 20 nm diameter, high crystallinity and a narrow particle size distributions were synthesized by the solvothermal method and characterized by various analytical techniques to understand their phase and morphology. Fluorescence characteristics under near infrared (NIR) excitation at 808 nm as well as X-ray excitation were studied to explore their potential in NIR optical and X-ray imaging. At 1.0 mol% Nd concentration, we observed a quantum yield of 25% at 1064 nm emission with 13 W/cm2 excitation power density which is sufficiently enough for imaging applications. Under 130 kVp (5 mA) power of X-ray excitation, Nd3+ doped Na(Gd0.5Lu0.5)F4 shows the characteristic emission bands of Gd3+ and Nd3+ with the strongest emission peak at 1064 nm due to Nd3+. Furthermore, magnetization measurements show that the nanocrystals are paramagnetic in nature with a calculated magnetic moment per particle of ~570 μB at 2T. These preliminary results support the suitability of the present nanophosphor as a multimodal contrast agent with three imaging features viz. optical, magnetic and X-ray.
Keywords: NIR downconversion, Nanoparticle, Multimodal, Magnetic properties, X-ray absorption
1. Introduction
Rare earth doped materials find several potential applications in several areas such as lasers, optical temperature sensors, white light-emitting diodes, and dye-sensitized solar cells [1–6]. With the introduction of nanotechnology there are so much interest in the development and applications of rare earth based multifunctional nanomaterials. One of the most important areas is biomedical imaging, where nanomaterials with multiple imaging features have been used as contrast agents [7–9]. Several imaging modalities, such as X-ray/computed tomography (CT), magnetic resonance imaging (MRI), and optical imaging, currently use contrast agents to improve cell and tissue images. The variation of each imaging modality offers a unique advantage in spatial resolution, imaging depth, sensitivity, and ease of use.
Several multimodal inorganic contrast agents with core-shell structures have been reported in the past. For example, core-shell quantum dots (QD) have been proposed as a suitable multimodal agent due to their unique optical properties such as multiplexing capabilities, photo-stability, and high fluorescence yield [10–14]. Coupling the QDs with the magnetic metal ion core can lead to a dual model contrast agent. However, there are several significant shortcomings with these imaging agents. QDs show several undesirable properties such as luminescence blinking, high toxicity, and requiring precise size tuning [15,16]. Core-shell upconverting nanoparticles have been shown to be a viable multimodal imaging agent since they are excited in the near infrared (NIR) region and yield emission in the visible region with comparatively better signal-to-noise ratio, low photodamage, and better penetration depth [17]. With the inclusion of Gd3+ and Fe3+ ions in the overall core structure, multimodal imaging features can be achieved [18–20]. An un-doped outer shell has even been shown to enhance the multimodal features of the core material [19]. But, one of the major issues with core-shell structured nanoparticle is the synthesis complexity and the associated cost of production.
In order to circumvent these difficulties, a core-shell free structure was proposed where multiple functionalities can be easily achieved by doping metals with the desired properties into the same lattice. This can be easily accomplished in wide band gap materials such as oxides, halides, chalcogenides, etc. For example, trivalent rare earth metals are considered to be suitable dopants for narrow band tunable emission with a very wide range of excitation and emission in the UV–VIS–IR range [22]. Unlike QDs and organic dyes, these phosphors are free from photobleaching and do not need size control for color tunability. In addition, they have a long decay time and excellent IR absorbing features that enables imaging in the upconversion and downconversion mode. By replacing a fraction of the metal cations in the lattice with another cation having magnetic properties, a luminomagnetic nanostructures can be easily accomplished without the need of a core-shell structure. To date, several researchers have developed luminomagnetic nanocrystals (NCs) as potential multimodal imaging agents with core-shell free nanostructures [23–27].
This paper discusses the synthesis and characterization of core-shell free Na(Gd0.5Lu0.5)F4:Nd3+ (NGL) as a potential NIR sensitive luminomagnetic nanophosphor for multimodal imaging application with three functionalities viz. NIR emission, X-ray fluorescence, and magnetic properties. NGL belongs to the NaYF4 structure and is a wide band gap halide with an optical band gap of near 8 eV [28]. The multimodal properties of these NCs is achieved by utilizing the strong NIR fluorescence properties of the rare earth Nd3+, magnetic properties of Gd3+, and the high X-ray absorption properties of Gd3+ and Lu3+ in the NGL host. To the best of our knowledge, this is the first study reporting the synthesis and characterization of fully monodispersed water-soluble core-shell free nanophosphors with three imaging features.
2. Materials and methods
2.1. Synthesis
The nanocrystals were synthesized via solvothermal (ST) method using 99.9% purity precursor chemicals (GFS Chemicals, US). To make Nd3+ doped Na(Gd0.5Lu0.5)F4 (NGL), stoichiometric amounts of L(NO3)3 (L = Gd,Lu,Nd) and 1 g of the surfactant polyvinylpyrrolidone (PVP) were dissolved in 30 ml of ethylene glycol (Reagent, GFS Chemicals, US). The solution was then mixed with 0.21 g of sodium fluoride and 0.5 g ammonium fluoride in 20 ml of ethylene glycol. The resulting solution was heated to 200°C for 24 h in an 80 ml stainless steel autoclave. After the reaction was completed, the nanoparticles were precipitated by adding excess ethanol and separated by centrifuging at 10,000 rpm (Z323, Labnet, U.S.) and subsequently washed 6 times with ethanol. Finally, the particles were freeze dried (25EL Freezmobile, VirTis, US) for 15 h to obtain the powder.
2.2. Phase and morphology
The X-ray powder diffraction (XRD) measurements were done with an automated diffractometer (Ultima IV, Rigaku, Japan) with Cu Kα (λ = 1.5 A) using Ni-filtered Cu Kα radiation at 44 keV and 30 mA. The data was collected in the 20°–60° range using a step size of 0.05° and a count time of 0.15 s. The particle size and morphology of the NCs were characterized by using a high resolution transmission electron microscope (HRTEM, JEOL ARM 200F) operated at 200 keV along with selected area diffraction (SAED) was used to determine the crystallinity of the samples. Electron dispersive x-ray (EDX) spectrum was obtained using a probe size of 0.13 nm and probe current 86 pA. Digital micrograph software GATAN was used for the image analyze.
2.3. Spectroscopic characterization
Optical absorption spectrum of the powder was obtained using a spectrophotometer in the scattering mode (Perkin Elmer Lambda 19, US.). Fluorescence characterization of the synthesized particles were done by exciting the sample with a near infrared (NIR) 808 nm power tunable 14-Pin butterfly module laser diode (Axcel Photonics, B2-808-3000-150) that was controlled by a laser diode driver (Throlabs LM74S2 Driver, Thorlabs Laser Diode Control LDC2000-2A) and the emission from the sample was collected by the spectrofluorimeter (Quanta Master 51, Photon Technology International Inc., US) with an InGAS detector (Teledyne Judson Technologies, 062-8451, US). Photoluminescence decay curves were measured on a Quanta Master 40 system (Photon Technology International Inc., US) using the single shot transient digitizer technique with a nitrogen laser pumped dye laser (PTI part GL-3300 + GL-302) as excitation source. The nitrogen laser has an 800 ps pulse width that pumps a high resolution dye chamber to give 525 ± 0.04 nm light. The collected decay curve was analyzed using built in software provided by PTI. For the quantum yield (QY) measurements, a barium sulfate coated integrating sphere (203 mm in diameter) (Oriel, Model 70451) was used and is mounted on the side of the spectrofluorimeter sample chamber, opposite to the excitation source. Powder sample was held in a specially designed sample holder with a quartz window to hold the powder in place and mounted at the sample port of the integrating sphere. The diffuse fluorescence spectra from the sample and the laser profile were recorded with the spectrofluorimeter by exciting the sample with an 808 nm power tunable fiber coupled Fabry Perot laser diode (Axcel Photonics, B2-808-3000-150). Fluorescence output from the sphere was collected via a liquid light guide (LLG) with a specially designed baffle that prevents the direct entry of the exciting laser beam into the detector. The LLG collects the light from the sphere and feeds to the InGAS detector through the emission monochromator. More details regarding the QY can be seen in our previous publications [29,30].
2.4. Magnetic characterization
Magnetic properties of the samples were measured using a Superconducting Quantum Interference Device (SQUID) in the Magnet and Low Temperature Facility, at Northwestern University. The hysteresis loop of magnetization at room temperature was recorded with the magnetic field sweeping from −5 to 5 T. The sample weighed was determined to be 67 mg.
2.5. X-ray excited emission
To characterize the X-ray induced luminescence or X-ray-excited scintillation (XES), the samples were irradiated in a Faxitron X-ray Cabinet System (Model RX-650). The X-ray induced luminescence was collected in a coaxial transmission irradiation configuration with the sample centered under the X-ray source. Samples were made by pressing 10 mg of the synthesized powder materials using a 7 mm diameter mold in a hand-pellet press with consistent pressure. Pellet samples were then mounted on a piece of angled teflon using double sided tape and placed ~2 mm in front of an optical fiber in the Faxitron system. The emitted light was collected with an optical fiber attached to the Edinburgh Instruments FLS920 spectrometer. All samples were excited over an X-ray range of 20–130 kVp (5 mA).
3. Results and discussions
The crystalline phase of NGL was studied using the obtained XRD pattern shown in Fig. 1. The peaks were indexed with the α-NaGdF4 (PDF 027-0697) and β-NaGdF4 (PDF 027-0699) phases. This mixed phase was seen in all dopant concentrations and the compositions of these two phases are estimated to ~88% α-NaGdF4 phase (cubic) and ~12% β-NaGdF4 phase (hexagonal). The mixed phase in the system is most likely due to the precursor ratio of Na-Gd/Lu in the host crystal where the phase and size can be effected [31]. We determined that the NGL cubic phase is within the Fm-3m (225) space group with the cell parameters a = b = c = 5.5180 Å.
Fig. 1.

X-ray diffraction of NGL with the XRD cubic reference data α-NaGdF4 (PDF 027-0697) and β-NaGdF4 (PDF 027-0699). The ratio between the two phases was calculated to be ~88% and ~12% respectively.
A Transmission Electron Microscope (TEM) image of the 1mol% Nd3+ doped NGL is shown in Fig. 2(a), which reveal a quasispherical shape with an average size of 21.78 ± 2.24 nm (Fig. 2(c)). The SAED pattern and lattice fringes in the HRTEM image of NGL are shown in Fig. 2(b,d) which illustrates the high crystallinity of the nanoparticles. A line scan histogram of Fig. 2(d) is shown in the inset of Fig. 2(d) where the lattice spacing of NGL is about 0.318 nm which corresponds to the (111) plane of α-NaGdF4. The EDX was performed on NGL (Fig. 2(e)) where the mapping verified that the elements Gd, Lu, Na, and F were present in the nanoparticle whereas 1 mol% of Nd is below the EDX threshold to be detected. Using the EDX data, the percentage of each element was estimated to be ~24% of F, ~21% of Na, ~28% of Gd, ~27% of Lu.
Fig. 2.
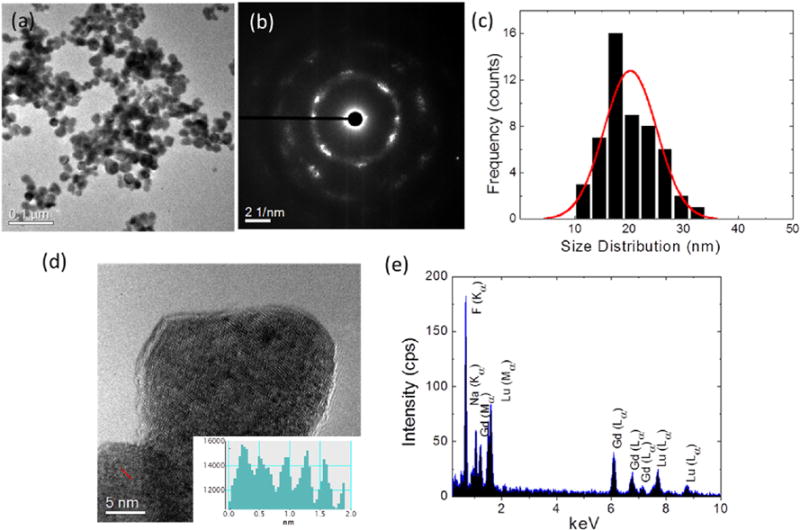
(a) TEM image of the Na(Gd0.5Lu0.5)F4:Nd3+, (b) the SAED pattern, (c) Particle size distribution (d) HRTEM of NGL with inset shows a line profile with a lattice spacing of 0.318 nm which corresponds to the (111) plane of α-NaGdF4, (e) EDX line scan mapping of the nanoparticle indicating the presence of elements F, Na, Gd, Lu.
The optical absorption spectrum of 2 mol% of Nd doped NGL is shown in Fig. 3(a) with spectral band notations for all observed transitions. The spectrum was obtained by converting the reflectance spectrum to absorbance by applying the Kubelka-Munk model [32]. The absorption spectrum shows all characteristics bands of Nd3+ with 574 and 794 nm as the two strongest bands (specifically the 2G7/2 and the 4F5/2 + 2H9/2). To explore the NIR fluorescence spectral properties as a function of Nd3+ concentration, the samples were excited with an 808 nm diode laser under identical experimental conditions with a laser power density of 12.74 W cm−2 and the emission spectra was collected in the 850–1900 nm range (Fig. 3(b)). The emission spectra shows four emission bands centered at 880, 1064, 1330 and 1800 nm which originate from the 4F3/2 to 4I9/2, 4I11/2, 4I13/2 and 4I15/2 respectively. As usual, the band at 1064 nm shows the greatest intensity followed by the band at 880 nm. The fluorescence branching ratios of the emission bands are respectively 37.91% for the 880 nm band, 44.80% for the 1064 nm band, 12.05% for the 1330 nm band, and 5.24% for the 1800 nm band. Furthermore, all emission bands show the characteristic Stark splitting indicating high crystallinity of the sample. Concentration dependent emission data shows that the emission is strongest in 1 mol% of Nd doped NGL.
Fig. 3.

(a) Absorption spectra of Na(Gd0.5Lu0.5)F4:Nd3+, (b) Emission spectra of Na(Gd0.5Lu0.5)F4:Nd3+, as a function of Nd concentration (0.1, 0.5, 1.0, 2.0 mol%) (c) Quantum yield of different emission bands as a function of Nd3+ concentration at a power density of 12.74 W/cm2. (d) Decay curves of 1064 nm emission for various Nd concentrations.
To determine the quantum yield (QY) of the NGL, an integrating sphere was calibrated with a material of known efficiency, and identical measurements were done following the standard reported procedure [29,30]. The QY was measured for different Nd concentrations and the results are plotted in Fig. 3(c). The QY of NGL with the Nd dopant concentration of 0.1, 0.5, 1.0, 2.0 mol% were measured to be 5.40, 5.86, 25.36, and 16.54% respectively with a power density of 12.74 W cm−2 under 808 nm excitation. Each individual emission band quantum yield was also measured and is shown in Fig. 3(c). The 1064 nm emission line showed the highest overall quantum yield, followed by the 880 nm band. Comparatively, the QY of our NGL particles is higher than the value of 22% obtained by Chen et al. in 3 mol% Nd doped NaGdF4 [21].
The fluorescence decay curves of the 1064 nm emission as a function of Nd3+ concentration is shown in Fig. 3(d). All decay curves are found to be double exponential in nature and the decay time was obtained by the expression:
where A1 and A2 are the fitting parameters and τ1 and τ2 are the fluorescence decay times. The average decay time was obtained using the expression [33].
Using the above expression the average decay time obtained for 0.1, 0.5, 1.0 and 2.0 mol% Nd3+ concentrations are respectively 247, 571, 511, and 330 μs. At 0,1 mol% Nd concentration the decay signal was not so strong to get a reliable decay time. However, from 0.5 mol% Nd concentration onward decay signal was strong enough to get reliable measurements. As usual, at higher dopant concentration the decay time shows a decreasing tendency due to the energy transfer between the Nd ions [33]. Longer decay times are desirable in time-gated fluorescence bioimaging where longer lifetimes can be more discernable against the noise from scattered light and autofluorescence [34].
The room temperature magnetization curve of NGL is shown in Fig. 4. By fitting the magnetization curve with the Langevin function, the magnetic moment per particle for NGL was found to be ~570 μB [35]. Considering the fact that these NCs contain 50% of Lu3+ and Gd3+, this value is comparable to that reported for similar NCs with 100% of either component, such as NaGdF4 [20,36].
Fig. 4.
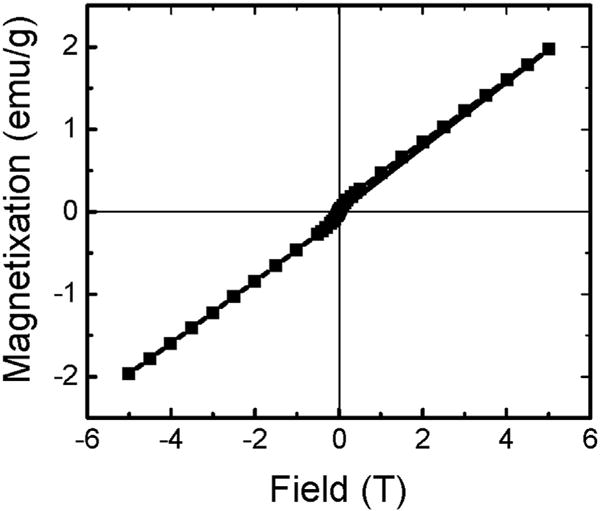
Magnetization curve of Na(Gd0.5Lu0.5)F4:Nd3+ at room temperature.
Fig. 5(a and b) shows the X-ray excited luminescence spectrum of NGL with 1.0 mol% Nd3+ concentration. The spectrum shows a total of 10 emission bands corresponding to various transitions in Gd3+ and Nd3+ as indicated. All of the emission bands are identified using the trivalent energy states of Gd3+ and Nd3+ and the emission mechanism is interpreted in the energy level diagram shown in Fig. 5(c). Gd3+ shows a single emission line at 311 nm corresponding to the transition 6P7/2→8S7/2 while the remaining emission bands originate from various excitation states of Nd3+. The emission intensity transitions under X-ray excitation is noticeably weaker compared to the NIR excitation at 808 nm which can be attributed to the high energy excitation of the NCs where the non-radiative losses are higher at various excited levels due to the thermalization of the 4f shell electrons with the conduction band levels. Furthermore, low X-ray excitation power density also contributes to the observed weaker emission whereas both Gd3+ and Lu3+ have high X-ray attenuation coefficients and can transfer the absorbed X-ray energy to lower lying energy levels of Nd3+ to enable the X-ray excited fluorescence in NGL.
Fig. 5.
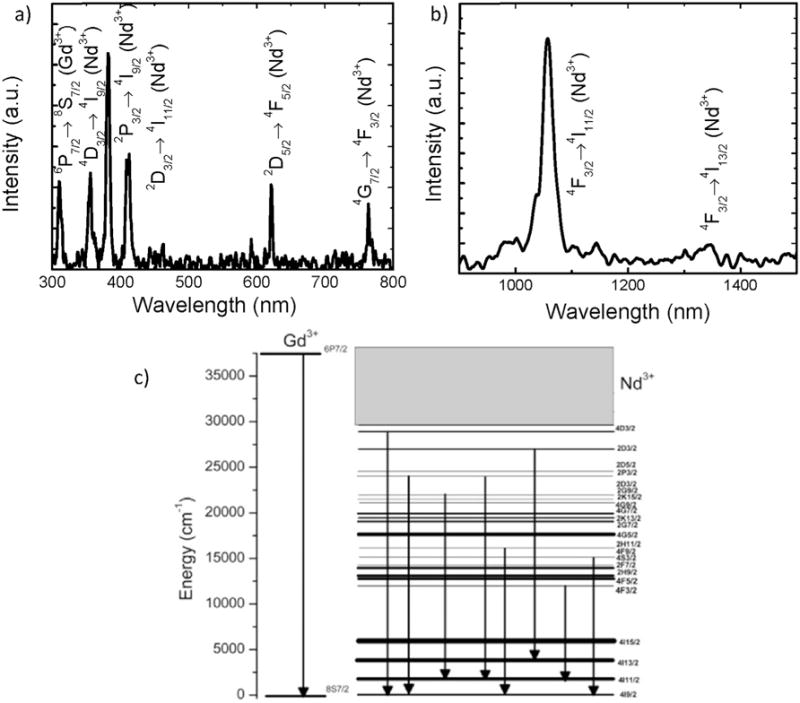
X-ray Emission spectrum of Na(Gd0.5Lu0.5)F4:Nd3+, in the a)visible and, b) NIR region at 130 kvP X-ray excitation. c) Energy level diagram interpretation of the X-ray excited luminescence spectrum of Na(Gd0.5Lu0.5)F4:Nd3+.
4. Conclusions
We have synthesized Nd3+ doped Na(Gd0.5Lu0.5)F4 nanophosphor in 20 nm size range. The crystal lattice was properly designed to incorporate multiple functionalities such as strong NIR emission, X-ray excited emission, and magnetic properties where the magnetic moment per particle for NGL was found to be ~570 μB. The NC shows very strong NIR emission at 1064 nm with a quantum yield of ~25.36% under an 808 nm excitation at a power density of 12.74 W/cm2, which is sufficiently large enough for NIR imaging applications. Under X-ray excitation, the NCs show emission from Gd3+ and Nd3+ indicating the suitability of the present nanomaterial as an X-ray contrast agent.
In addition, the magnetic properties of the material enable them to be useful in magnetic resonance imaging. Since the three functionalities are incorporated into a single nanoparticle without the need for core-shell structure, synthesis complexities are avoided and the efficiency of each of these functionalities can be controlled for by selecting the proper dopants as well as concentrations. It is anticipated that such a multifunctional material could provide several opportunities for future medical imaging applications. In conclusion, we successfully made a nanoparticle system that possess three functionalities and our preliminary observations show the potential application of these material as tri-modal imaging agents and further proof of concept experiments are underway to show their multimodal imaging applications.
Acknowledgments
The authors would like to acknowledge the financial support from the National Science Foundation Partnerships for Research and Education in Materials [NSF-PREM grant NO-DMR-0934218]. LCM would like to acknowledge the partial funding from the National Institutes of Health [NIGMS RISE GM060655], the National Center for Research Resources [G12RR013646-12], and the National Institute on Minority Health and Health Disparities [G12MD007591] from the National Institutes of Health. BWL and MJT gratefully acknowledge support from the Department of Homeland Security, Domestic Nuclear Detection Office - Academic Research Initiative [NSF-ECCS-11-40037]. Finally, we would like to thank Dr. Brian Yust, Francisco Pedraza, Diego Alducin, Dr. Arturo Ponce-Pedraza, Dr. Teja Guda, and Sergio A. Montelongo for their fruitful discussions and assistance in these studies.
References
- 1.Kaminskii AA. Laser Crystals: Their Physics and Properties. Springer Verlag; New York: 2013. [Google Scholar]
- 2.Peng Du, Laihui Luo, Qingying Yue, Weiping Li. The simultaneous realization of high- and low-temperature thermometry in Er3+/Yb3+-codoped Y2O3 nanoparticles. Mater Lett. 2015;143:209–211. [Google Scholar]
- 3.Ruiqing Li, Wenwen Zi, Linlin Li, Lu Liu, Junjun Zhang, Lianchun Zou, Shucai Gan. Monodisperse and hollow structured Y2O3:Ln3+ (Ln=Eu, Dy, Er, Tm) nanospheres: a facile synthesis and multicolor-tunable luminescence properties. J Alloys Compd. 2014;617:498–504. [Google Scholar]
- 4.Du Peng, Lim Joo Ho, Kim Sang Hun, Yu Jae Su. Facile synthesis of Gd2O3: Ho3+/Yb3+ nanoparticles: an efficient upconverting material for enhanced photovoltaic performance of dye-sensitized solar cells. Opt Mater Express. 2016;6:1896–1904. [Google Scholar]
- 5.Chander N, Khan AF, Komarala VK, Chawla S, Dutta V. Enhancement of dye sensitized solar cell efficiency via incorporation of upconverting phosphor nanoparticles as spectral converters. Prog Photovolt Res Appl. 2016;24:692–703. [Google Scholar]
- 6.Hongmei Lai, Yuanzhe Wang, Guoping Du, Weizhi Han. Dual functional YVO4: Eu3+,Bi3+@SiO2 submicron-sized core-shell particles for dye-sensitized solar cells: light scattering and downconversion. Ceram Int. 2014;40(4):6103–6108. [Google Scholar]
- 7.Fei He, Lili Feng, Piaoping Yang, Bin Liu, Shili Gai, Guixin Yang, Yunlu Dai, Jun Lin. Enhanced up/down-conversion luminescence and heat: simultaneously achieving in one single core-shell structure for multimodal imaging guided therapy. Biomaterials. 2016;105:77–88. doi: 10.1016/j.biomaterials.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Xuesong Zhai, Pengpeng Lei, Peng Zhang, Zhuo Wang, Shuyan Song, Xia Xu, Xiuling Liu, Jing Feng, Hongjie Zhang. Growth of lanthanide-doped LiGdF4 nanoparticles induced by LiLuF4 core as tri-modal imaging bioprobes. Biomaterials. 2015;65:115–123. doi: 10.1016/j.biomaterials.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Xiaoran Deng, Yunlu Dai, Jianhua Liu, Ying Zhou, Ping’an Ma, Ziyong Cheng, Yinyin Chen, Kerong Deng, Xuejiao Li, Zhiyao Hou, Chunxia Li, Jun Lin. Multifunctional hollow CaF2:Yb3+/Er3+/Mn2+-poly(2-Aminoethyl methacrylate) microspheres for Pt(IV) pro-drug delivery and tri-modal imaging. Biomaterials. 2015;50:154–163. doi: 10.1016/j.biomaterials.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Sitbon G, Bouccara S, Tasso M, Francois A, Bezdetnaya L, Marchal F, Beaumont M, Pons T. Multimodal Mn-doped I-III-VI quantum dots for near infrared fluorescence and magnetic resonance imaging: from synthesis to in vivo application. Nanoscale. 2014;6:9264–9272. doi: 10.1039/c4nr02239d. [DOI] [PubMed] [Google Scholar]
- 11.Hsu JC, Huang CC, Ou KL, Lu N, Mai FD, Chen JK, Chang JY. Silica nanohybrids integrated with CuInS2/ZnS quantum dots and magnetite nanocrystals: multifunctional agents for dual-modality imaging and drug delivery. J Mater Chem. 2011;21:19257. [Google Scholar]
- 12.Cheng CY, Ou KL, Huang WT, Chen JK, Chang JY, Yang CH. Gadolinium-based CuInS2/ZnS nanoprobe for dual-modality magnetic resonance/optical imaging. ACS Appl Mater interfaces. 2013;5:4389–4400. doi: 10.1021/am401428n. [DOI] [PubMed] [Google Scholar]
- 13.Guo W, Yang W, Wang Y, Sun X, Liu Z, Zhang B, Chang J, Chen X. Color tunable Gd-Zn-Cu-In-S/ZnS quantum dots for dual modality magnetic resonance and fluorescence imaging. Nano Res. 2014;7:1581–1591. doi: 10.1007/s12274-014-0518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Deng R, Ji X, Pan D. Alloyed Mn-Cu-In-S nanocrystals: a new type of diluted magnetic semiconductor quantum dots. Nanotechnology. 2012;23:255706. doi: 10.1088/0957-4484/23/25/255706. [DOI] [PubMed] [Google Scholar]
- 15.Patterson GH, Piston DW. Photobleaching in two-photon excitation microscopy. Biophys J. 2000;78:2159–2162. doi: 10.1016/S0006-3495(00)76762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia A, Gao Y, Zhou J, Li C, Yang T, Wu D, Wu L, Li F. Core-shell NaYF4: Yb3+,Tm3+@FexOy nanocrystals for dual-modality T2-enhanced magnetic resonance and NIR-to-NIR upconversion luminescent imaging of small-animal lymphatic node. Biomaterials. 2011;32:7200–7208. doi: 10.1016/j.biomaterials.2011.05.094. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Liu Y, Tu D. Multimodal biosensing based on lanthanide-doped nano-bioprobes, in. Lanthanide-doped Luminescent Nanomaterials, Springer Berlin Heidelberg. 2014:165–187. [Google Scholar]
- 19.Cheng L, Yang K, Li Y, Zeng X, Shao M, Lee ST, Liu Z. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials. 2012;33:2215–2222. doi: 10.1016/j.biomaterials.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zhao Z, Zhang X, Cordes DB, Weeks B, Qiu B, Madanan K, Sardar D, Chaudhuri J. Magnetic and optical properties of NaGdF4:Nd3+, Yb3+, Tm3+ nanocrystals with upconversion/downconversion luminescence from visible to the near-infrared second window. Nano Res. 2014;8:636–648. [Google Scholar]
- 21.Chen G, Ohulchanskyy TY, Liu S, Law WC, Wu F, Swihart MT, Agren H, Prasad PN. Core/shell NaGdF4:Nd3+/NaGdF4 nanocrystals with efficient near-infrared to near-infrared downconversion photoluminescence for bioimaging applications. ACS Nano. 2012;6:2969–2977. doi: 10.1021/nn2042362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun LD, Wang YF, Yan CH. Paradigms and challenges for bioapplication of rare earth upconversion luminescent nanoparticles: small size and tunable emission/excitation spectra. Acc Chem Res. 2014;47:1001–1009. doi: 10.1021/ar400218t. [DOI] [PubMed] [Google Scholar]
- 23.Zeng S, Wang H, Lu W, Yi Z, Rao L, Liu H, Hao J. Dual-modal upconversion fluorescent/X-ray imaging using ligand-free hexagonal phase NaLuF4:Gd/Yb/Er nanorods for blood vessel visualization. Biomaterials. 2014;35:2934–2941. doi: 10.1016/j.biomaterials.2013.11.082. [DOI] [PubMed] [Google Scholar]
- 24.Zeng S, Xiao J, Yang Q, Hao J. Bi-functional NaLuF: Gd3+/Yb3+/Tm3+ nanocrystals: structure controlled synthesis, near-infrared upconversion emission and tunable magnetic properties. J Mater Chem. 2012;22:9870–9874. [Google Scholar]
- 25.Mimun LC, Ajithkumar G, Pokhrel M, Yust BG, Elliott ZG, Pedraza F, Dhanale A, Tang L, Lin AL, Dravid VP, Sardar DK. Bimodal imaging using neodymium doped gadolinium fluoride nanocrystals with near-infrared to near-infrared downconversion luminescence and magnetic resonance properties. J Mater Chem B Mater Biol Med. 2013;1:5702–5710. doi: 10.1039/C3TB20905A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pokhrel M, Mimun LC, Yust B, Kumar GA, Dhanale A, Tang L, Sardar DK. Stokes emission in GdF3:Nd3+ nanoparticles for bioimaging probes. Nanoscale. 2014;6:1667–1674. doi: 10.1039/c3nr03317a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju Q, Chen X, Ai F, Peng D, Lin X, Kong W, Shi P, Zhu G, Wang F. An upconversion nanoprobe operating in the first biological window. J Mater Chem B. 2015;3:3548–3555. doi: 10.1039/c5tb00025d. [DOI] [PubMed] [Google Scholar]
- 28.Chong K, Hirai T, Kawai T, Hashimoto S, Ohno N. Optical properties of Bi3 ions doped in NaYF4. J Lumin. 2007;122–123:149–151. [Google Scholar]
- 29.Kumar GA, Pokhrel M, Sardar DK. Absolute quantum yield measurements in Yb/Ho doped M2O2S (M= Y, Gd, La) upconversion phosphor. Mater Lett. 2013;98:63–66. [Google Scholar]
- 30.Pokhrel M, Kumar GA, Sardar DK. High upconversion quantum yield at low pump threshold in Er3+/Yb3+ doped La2O2S phosphor. Mater Lett. 2013;99:86–89. [Google Scholar]
- 31.Dühnen S, Rinkel T, Haase M. Size control of nearly monodisperse β-NaGdF4 particles prepared from small α-NaGdF4 nanocrystals. Chem Mater. 2015;27:4033–4039. [Google Scholar]
- 32.Molenaar R, Bosch JJ Ten, Zijp JR. Determination of Kubelka-Munk scattering and absorption coefficients by diffuse illumination. Appl Opt. 1999;38:2068–2077. doi: 10.1364/ao.38.002068. [DOI] [PubMed] [Google Scholar]
- 33.Tan Mei Chee, Kumar GA, Riman RE, Brik MG, Brown E, Hommerich U. Synthesis and optical properties of infrared-emitting YF3:Nd nanoparticles. J Appl Phys. 2009;106:063118-1–063118-12. [Google Scholar]
- 34.Jin Dayong, Piper James A. Time-gated luminescence microscopy allowing direct visual inspection of lanthanide-stained microorganisms in background-free condition. Anal Chem. 2011;83:2294–2300. doi: 10.1021/ac103207r. [DOI] [PubMed] [Google Scholar]
- 35.Mendoza D, Morales F, Walter J. Magnetization studies in quasi two-dimensional palladium nanoparticles encapsulated in a graphite host. J Phys Condens Matter. 1999;11:317–322. [Google Scholar]
- 36.Zhao Q, Shao B, Lu W, Lv W, Jiao M, Zhao L, You H. beta-NaGdF4 nanotubes: one-pot synthesis and luminescence properties. Dalton Trans. 2015;44:3745–3752. doi: 10.1039/c4dt03619k. [DOI] [PubMed] [Google Scholar]


