Abstract
Background
Ozone is a ubiquitous air pollutant with increasing concentrations in many populous regions. Toxicological studies show that ozone can cause oxidative stress and increase insulin resistance. These pathways may contribute to metabolic changes and diabetes formation. In this paper, we investigate the association between ozone and incident type 2 diabetes in a large cohort of African American women.
Methods
We used Cox proportional hazards models to calculate hazard ratios (HRs) for incident type 2 diabetes associated with exposure to ozone in a cohort of 45,231 African American women living in 56 metropolitan areas across the United States. Ozone levels were estimated using the U.S. EPA Models-3/Community Multiscale Air Quality (CMAQ) predictions fused with ground measurements at a resolution of 12 km for the years 2007–2008.
Results
The HR per interquartile range increment of 6.7 ppb of ozone was 1.18 (95% CI 1.04–1.34) for incident diabetes in adjusted models. This association was unaltered in models that controlled for fine particulate matter with diameter <2.5 μ (PM2.5). Associations were modified by nitrogen dioxide (NO2) levels, such that HRs for ozone levels were larger in areas of lower NO2.
Conclusions
Our results provide initial evidence of a positive association between in O3 and incident diabetes African American women. Given the ubiquity of ozone exposure and the importance of diabetes on quality of life and survival, these results may have important implications for the protection of public health.
Keywords: Ozone, Exposure, Air pollution, Diabetes, African American women
1.Introduction
Tropospheric ozone (O3) concentrations have increased by twofold since the 19th century, due largely to growing O3 precursor emissions associated with human activity (Parrish et al., 2012). O3 exhibits strong spatial and temporal heterogeneity (Cooper et al., 2014). In the United States nearly 130 million people live in areas that fail to comply with O3 standards set by the U.S. Environmental Protection Agency (City Rankings - American Lung Association|State of the Air, 2015). While other pollutants have shown marked improvement, ozone has not seen nearly the same decreases in many parts of the United States, particularly in more polluted areas such as Southern California (Gauderman et al., 2015). Higher and worsening concentrations have also been observed in densely populated areas of South and East Asia (Parrish et al., 2012). O3 is also an important greenhouse gas that contributes substantially to increased radiative forcing and resulting climate change (Intergovernmental Panel on Climate Change (IPCC), 2014). In the troposphere, ozone can elicit a wide range of adverse effects on human health, including: pulmonary dysfunction, hospitalization for respiratory causes, induction and exacerbation of asthma, and premature mortality from several causes, with specific risks observed for diabetic deaths (Berman et al., 2012; Mustafic et al., 2012; US EPA National Center for Environmental Assessment RTPNEMAG & Brown, 2013; Jerrett et al., 2009; Turner et al., 2016).
Growing epidemiological evidence implicates ambient air pollution as a contributor to the development of type 2 diabetes. While toxicological evidence suggests that PM2.5 exerts pro-diabetic effects, epidemiological data on the association of diabetes with PM2.5 exposure is inconsistent (Coogan et al., 2012; Chen et al., 2013; Puett et al., 2011). In addition, some studies have found markers of traffic-related air pollution such as NO2 to be associated with incident diabetes (Park et al., 2015). Recent meta-analyses reported increased relative risks of type 2 diabetes per 10 μg/m3 increase in exposure to PM2.5: 1.10 (95% CI: 1.02, 1.18) and to NO2: 1.08 (95% CI: 1.00, 1.17) (Eze et al., 2015). To date, no study has investigated whether ozone is associated with the onset of type 2 diabetes in humans.
Emerging evidence from animal experiments, however, suggests that O3 exposure may also have the capacity to induce metabolic insulin resistance. Vella et al. (2015) recently demonstrated that rats exposed to O3 for 16 h (as well as sub-acutely for 4 days at lower levels) developed elevations in fasting glucose levels and whole body insulin resistance (Vella et al., 2015). The insulin resistance was shown to be due to impaired insulin-signaling in muscle tissues as a consequence of oxidative stress-induced endoplasmic reticular stress pathways leading to c-Jun N-terminal kinase (JNK) activation. The investigators also provided evidence that these adverse responses to O3 inhalation were likely mediated by the formation of pro-oxidative molecules in the pulmonary alveolar fluid capable of translocating into the systemic circulation. Additional studies suggest that O3 could induce adverse systemic metabolic responses via activation of the sympathetic nervous system, by hypothalamic inflammation, or both (Bass et al., 2013). Hence, O3 may also work to induce diabetes mellitus through similar pathways as fine particulate matter with diameter <2.5 μm (PM2.5) (Rao et al., 2015). Specifically, both pollutants can cause oxidative stress in the lungs, which – if sustained over time – may lead to systemic pro-inflammatory and autonomic responses linked to numerous adverse health effects.
Based on the evidence from animal models and analogous findings on other common air pollutants, we hypothesized that ozone could contribute to the development of diabetes. We assessed this hypothesis in a large cohort of African American women.
2. Methods
2.1. Study population
In 1995, the Black Women’s Health Study (BWHS) began when 59,000 black women aged 21 through 69 were recruited largely though subscribers to Essence magazine, a publication targeted to black women (Rosenberg et al., 1995). A baseline questionnaire solicited information on demographics, medical conditions, reproductive history, and lifestyle factors. Follow up occurred biennially with Web-based and mailed health questionnaires. Follow-up of the baseline cohort has been completed for 88% of potential years of follow-up through 2011. The Institutional Review Board of Boston University School of Medicine approved the study protocol. Participants indicate consent by completing and returning the questionnaires.
Here we used data from the baseline questionnaire (1995) and eight subsequent follow-up cycles (1997–2011), provided by 45,231 women who lived in any of 56 U.S. metropolitan areas and who had complete body mass index (BMI) information at baseline. Those excluded because they did not live in the 56 metro areas (n = 11.914) did not differ statistically from the women included in terms of mean age, prevalence of diabetes or BMI. Follow up started at 30 years of age to exclude potential cases of type 1 diabetes, regardless of whether the age at enrollment was <30. For example, a woman who was 28 at enrollment in 1995 would not add to follow up time until 1997 when she turned 30. We excluded 2228 women with prevalent diabetes at baseline, which left a total of 43,003 women for analysis.
2.2. Diagnosis of diabetes
Incident cases of type 2 diabetes were ascertained by self-report of doctor-diagnosed diabetes at age 30 or older during follow-up. A validation study among 227 participants who met the ascertainment criteria confirmed type 2 diabetes in 96% of the women based on the data from their medical records or provided by their physicians (Krishnan et al., 2010).
2.3. Ascertainment of covariates
Self-reported data on alcohol consumption, smoking history, hours per week spent in vigorous activity, and weight and height (used to calculate BMI, weight in kg/height in m2) were obtained at baseline. All except height were updated with biennial follow-up questionnaires. Dietary data were obtained in 1995 and 2001 using a food frequency questionnaire modified from the 68-item short form Block-National Cancer Institute instrument (Block et al., 1990). We used factor analysis to identify two dietary patterns, one characterized by high intake of meat and fried food and the other by high intake of fruits and vegetables (Boggs et al., 2011). Educational attainment, household income, and parental history of diabetes were reported on various follow-up questionnaires.
We geocoded residential mailing addresses from 1995 to 2009 using TeleAtlas Road coverage as the reference layer. Geocoded addresses were then linked to U.S. Census data (block group level). Using factor analysis, we developed a neighborhood socioeconomic status (SES) score based on census variables indicating wealth, education, and income as described in detail elsewhere (Coogan et al., 2015).
2.4. Estimation of ozone
We estimated O3 concentrations from a Bayesian space-time fusion model known as the Downscaler, which was developed by the U.S. Environmental Protection Agency (Berrocal et al., 2012). The model estimates daily 8-hour maximum O3 concentrations for each census tract centroid in the contiguous United States. The model fuses data from the ground-based monitoring network with Community Model for Air Quality (CMAQ) model estimates with output on 12 * 12 km grids. We extracted daily estimates and averaged these for the years 2007–2008 to approximate the long-term average at all residential locations reported by BWHS participants over follow-up.
The Downscaler model underwent several validation steps (Berrocal et al., 2012). In brief, maps of the model output were produced for sub-regions of the United States and compared quantitatively and visually to monitoring locations, which showed the spatial patterns of predictions were largely consistent with monitored levels. The model performance was also assessed using the predictive mean absolute error (PMAE) of the space-time prediction, which showed the Downscaler outperformed either ordinary kriging models or CMAQ models alone. Correlations with hold-out cross-validation locations for daily predictions ranged from 0.61–0.86. These validation analyses suggested that the model predicted ambient ozone concentrations well.
2.5.Estimation of PM2.5
We used a hybrid modeling approach to estimate ambient PM2.5 for the years 1999–2008 at all participant residential addresses. Methods have been described in detail elsewhere (Beckerman et al., 2013). Briefly, we employed a two-stage modeling strategy that incorporated a land use regression (LUR) approach and a Bayesian Maximum Entropy (BME) approach. The model used data on traffic density and green space as fixed predictors. Validation of the final LUR-BME model in the cross-validation dataset showed strong agreement between observed and predicted PM2.5 levels with no evidence of bias; the cross-validation R2 ~ 0.79.
2.6. Estimation of NO2
We estimated annual NO2 levels for census block groups covering the years 2000–2010 with a land use regression model, (Novotny et al., 2011) and assigned them to all participant residential locations. The model used fixed-site ambient NO2 monitoring station data as the dependent variable and satellite-derived estimates of ground-level NO2 concentrations and ground-based datasets of land uses. The spatial LUR was derived from annual-average NO2 concentrations at 369 monitoring stations and from 81,670 satellite-derived ground-level NO2 estimates. The spatial model had good predictive power (R2 ~ 0.78). Temporal modeling incorporated 48,886 monthly-average monitoring station values to provide monthly averages by block. The R2 for the final spatiotemporal model was 0.80.
2.7. Estimation of temperature and heat
Given earlier findings on temperature modifications of ozone health effects (Jerrett et al., 2009), we assessed whether an interaction existed between ozone effects and temperature. Data were extracted from county-level estimates derived by the Centers for Disease Control (2014) North America Land Data Assimilation System (NLDAS) Daily Air Temperatures and Heat Index. We used the 10-year mean annual maximum temperature and heat indices (2000-2010) for each county and assigned this to study participants’ locations (Environments Outdoor Air - CDC Tracking Network, n.d.).
2.8. Statistical methods
We fit Cox proportional hazards models stratified by age in 1-year intervals, 2-year questionnaire cycle, and metro area (n = 56). We estimated hazard ratios (HR) and 95% confidence intervals (CI) to assess the association between air pollution and diabetes per interquartile range (IQR) of O3 (6.7 ppb). We calculated person-time from the start of follow-up in 1995 to the first occurrence of diabetes, loss to follow-up, death, or end of follow-up, whichever happened first. We assessed the proportional hazards assumptions by analyzing Schoenfeld residuals.
We began with a basic model including age, questionnaire cycle, and metro area in the strata statement. We then added covariates that individually changed the ozone coefficient by at least 10%: education (≤12,13–15, 16, ≥17); diet pattern as indicated by vegetable/fruit diet pattern score (quintiles) and meat/fried foods diet pattern score (quintiles); hours/week vigorous exercise (none, <5, ≥5); parental history of diabetes; body mass index (BMI = weight in kg/height2 in m as <25, 25–29,30–34, 35–39, ≥40); smoking status (never, past or current), and neighborhood SES (continuous based on factor analysis of census data). We subsequently included the co-pollutants, PM2.5 and NO2, as potential confounders. We tested for interactions between ozone and the variables included in the final model or the co-pollutants. Finally we conducted sensitivity analyses by excluding the three largest cities in the cohort (New York City, Los Angeles, and Chicago).
2.9. Assignment of the exposure surfaces
We assigned all air pollutant exposure to the residential addresses of the women. We then assigned the modeled exposure as the average of the pollutant concentration over the 2 years at the residential address lived at prior to diagnosis or the last follow up. We used this “proximate mean” approach to minimize the exposure classification that could result from using the baseline residential address for the entire follow up period. Due to the limited temporal resolution on some of the exposure models, we based the exposure assignment for the 2-year proximate mean on the overall mean of all years available for each pollutant (i.e., 2000–2010 for NO2, 2007–2008 for O3, and 1999–2008 for PM2.5).
After assignment, we conducted a series of descriptive analyses to ensure estimates were in likely ranges and distributions of the various exposures. We assessed the concentration-response function by plotting the hazard ratios against the ozone concentrations using a spline function with three degrees of freedom. All analyses were conducted using SAS v 9.3.
2.10. Role of the funding sources
The work presented in this paper was funded by the U.S. National Institute of Environmental Health Science and the U.S. Centers for Disease Control Environmental Public Health Tracking Program. None of the funding agencies played a direct role in the writing of this manuscript or the decision to submit it for publication.
3. Results
Table 1 shows the descriptive statistics of the analytic cohort by quintile of ozone exposure. As ozone levels increased, the proportion of smokers decreased and the proportion of never drinkers increased. There was little difference in mean BMI but the prevalence of obesity was slightly lower in the more highly polluted quintiles. In the lower quintiles of ozone, women were more likely to be at the lowest levels of income, education, and neighborhood SES.
Table 1.
Age-standardized baseline characteristics according to quintiles of O3 at 1995 address.
| Quintiles of O3 from lowest to highest levels based on the Downscaler model assigned to participants (ppb) | |||||
|---|---|---|---|---|---|
|
| |||||
| Q1 lowest | Q2 | Q3 | Q4 | Q5 highest | |
| Characteristics | 25.4–33.5 | 33.5–35.2 | 35.2–38.6 | 38.6–41.8 | 41.8–56.4 |
| Age, mean ± SD | 38.2 ± 10.8 | 39.4 ± 11.0 | 39.2 ± 10.6 | 38.4 ± 10.5 | 3830.3 ± 9.9 |
| BMI, mean ± SD | 27.8 ± 6.6 | 27.9 ± 6.7 | 27.9 ± 6.7 | 27.6 ± 6.4 | 27.2 ± 6.1 |
| BMI ≥ 30, % | 29 | 30 | 29 | 28 | 25 |
| Never drinker, % | 53 | 55 | 58 | 57 | 58 |
| Current/past smoker, % | 40 | 39 | 36 | 34 | 30 |
| Vigorous exercise ≥5 h/wk, % | 14 | 12 | 13 | 14 | 14 |
| Highest (healthiest) quintile of prudent diet score, % | 20 | 18 | 19 | 19 | 19 |
| Income ≤$25,000, % | 10 | 9 | 9 | 8 | 8 |
| Education ≤12 yrs, % | 19 | 18 | 18 | 18 | 16 |
| Lowest quintile area SES (%) | 27 | 22 | 21 | 17 | 11 |
| Parental history of diabetes, % | 25 | 25 | 26 | 26 | 26 |
Table 2 shows the association of ozone with incident diabetes in a basic model that included age, questionnaire cycle, and city, and in the fully adjusted model that included variables that met our confounding inclusion criteria. We observed no association between ozone and incident diabetes in the basic model. There was, however, an association between ozone and incident diabetes in the fully adjusted model (HR = 1.18, 95% CI 1.04, 1.34 over the IQR of ozone). Neighborhood SES was the variable most responsible for the increase in the adjusted HR (see Table 1S in the Online Appendix for the effect of individual variables on the ozone-diabetes association). The HR estimate increased to 1.20 (95% CI 1.05–1.37) with additional control for PM2.5. With further control for NO2, the estimate was reduced and included unity (HR = 1.13,95% CI 0.97–1.31).
Table 2.
Hazard ratios for incident diabetes per 6.7 ppb ozone with control for confounders and co-pollutants (4387 cases/453,221 person years).
| Model | HR (95% CI) |
|---|---|
| Basic modela | 1.00 (0.88, 1.13) |
| Basic + 10% criteriab | 1.18 (1.04, 1.34) |
| Basic + 10% criteria + PM2.5 | 1.20 (1.05, 1.37) |
| Basic + 10% criteria + NO2 | 1.13 (0.97, 1.31) |
| Basic + 10% criteria + PM2.5 + NO2 | 1.13 (0.97, 1.31) |
Basic model contains age, period, and city.
Covariates that met the 10% criteria include smoking status (never, past, current b15 cigarettes/day, current ≥15 cigarettes/day), years of education (≤12, 13–15, 16,≥17), hours/week vigorous exercise (none, <5, ≥5), vegetable/fruit diet pattern (quintiles), meat/fried food diet pattern (quintiles), parental history of diabetes (yes, no), BMI (weight in kg/height in m2 in m as <25, 25–29, 30–34, 35–39, ≥40) neighborhood SES (continuous based on factor analysis of census data).
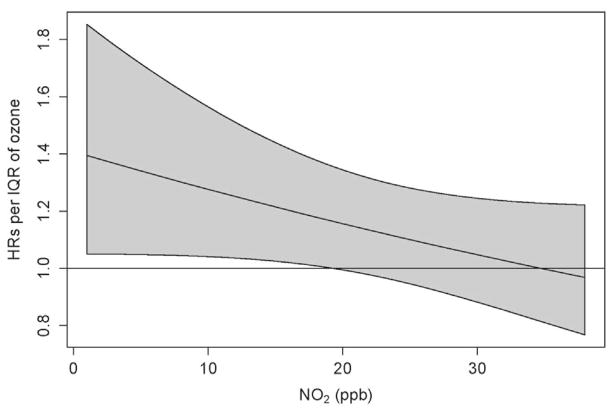
We assessed whether any of the more important predictors of or risk factors for diabetes modified the effect of ozone on diabetes onset (Table 3). There were no interactions that met the criteria for rejection of the null hypothesis. We found no evidence of effect modification by temperature or heat. For co-pollutants there was no interaction between PM2.5 and ozone (data not shown). We did, however, observe borderline evidence of an interaction between ozone and NO2 when the models were run with both pollution variables in continuous form (p = 0.09). Fig. 1 shows the effects of ozone by level of NO2, with generally decreasing effects as NO2 increased.
Table 3.
Hazard ratios for incident diabetes per 6.7 ppb O3 stratified by covariatesa.
| Cases/PYs | HRa (95% CI) | |
|---|---|---|
| Neighborhood SES | ||
| Quintile 1 lowest | 1081/85,569 | 1.07 (0.64, 1.78) |
| Quintile 2 | 934/86,213 | 0.80 (0.55, 1.15) |
| Quintile 3 | 879/88,921 | 1.29 (0.96, 1.74) |
| Quintile 4 | 833/95,834 | 1.20 (0.90, 1.60) |
| Quintile 5 highest | 660/96,683 | 1.69 (1.21, 2.36) |
| Interaction p-value | 0.27 | |
| BMI | ||
| <25 | 298/135,021 | 1.11 (0.64, 1.93) |
| 25–29 | 1184/154,702 | 1.08 (0.83, 1.40) |
| ≥30 | 2905/163,498 | 1.26 (1.08, 1.48) |
| Interaction p-value | 0.98 | |
| Age | ||
| <40 | 655/133,509 | 1.27 (0.93, 1.72) |
| 40–54 | 2200/224,159 | 1.21 (1.02, 1.44) |
| ≥55 | 1532/95,553 | 1.16 (0.93, 1.44) |
| Interaction p-value | 0.34 | |
| Education | ||
| ≤HS | 980/71,966 | 1.06 (0.77, 1.48) |
| Some college | 1476/142,585 | 1.13 (0.89, 1.43) |
| College graduate | 1926/238,024 | 1.19 (0.98, 1.44) |
| Interaction p-value | 0.65 | |
| Parental history of diabetes | ||
| Yes | 2072/135,222 | 1.14 (0.93, 1.40) |
| No | 2236/310,344 | 1.17 (0.99, 1.40) |
| Interaction p-value | 0.71 | |
| Presence of hypertension | ||
| No | 1932/310,669 | 1.10 (0.90, 1.33) |
| Yes | 2455/142,551 | 1.22 (1.02, 1.46) |
| Interaction p-value | 0.55 | |
| Vigorous exercise | ||
| <5 h/week | 4157/407,886 | 1.18 (1.03, 1.35) |
| ≥5 h/week | 187/41,596 | 1.37 (0.69, 2.70) |
| Interaction p-value | 0.66 | |
| Smoking | ||
| Never | 2344/281,409 | 1.13 (0.95, 1.36) |
| Past or current | 2037/170,892 | 1.18 (0.97, 1.45) |
| Interaction p-value | 0.51 | |
| Meat/fried food diet pattern score | ||
| Quintile 1 | 700/87,221 | 1.19 (0.82, 1.73) |
| Quintile 2 | 755/85,892 | 1.19 (0.85, 1.67) |
| Quintile 3 | 814/86,307 | 1.21 (0.89, 1.65) |
| Quintile 4 | 866/86,104 | 1.22 (0.89, 1.68) |
| Quintile 5 | 965/84,832 | 1.11 (0.80, 1.54) |
| Interaction p-value | 0.95 | |
| Vegetable/fruit diet pattern score | ||
| Quintile 1 | 810/79,953 | 1.39 (0.98, 1.96) |
| Quintile 2 | 835/83,068 | 0.94 (0.67, 1.33) |
| Quintile 3 | 798/86,354 | 1.26 (0.89, 1.79) |
| Quintile 4 | 843/89,243 | 1.21 (0.88, 1.66) |
| Quintile 5 | 814/91,738 | 1.22 (0.91, 1.64) |
| Interaction p-value | 0.87 | |
| Max air temperature | ||
| Tertile 1 | 1456/150,404 | 1.26 (0.91, 1.77) |
| Tertile 2 | 1390/144,718 | 1.22 (0.87, 1.72) |
| Tertile 3 | 1538/157,761 | 1.16 (0.99, 1.36) |
| Interaction p-value | 0.80 | |
| Max heat index | ||
| Tertile 1 | 1429/151,728 | 1.18 (1.01, 1.39) |
| Tertile 2 | 1164/125,095 | 0.95 (0.67, 1.36) |
| Tertile 3 | 1765/173,575 | 1.35 (0.96, 1.88) |
| Interaction p-value | 0.22 | |
We tested effect modification for all variables used as confounders. We also tested for modification with hypertension because there is evidence that hypertension increases the risk for diabetes formation. We hypothesized therefore that individuals with hypertension might be more susceptible to the effects of air pollution. There is also evidence that hypertension is associated with air pollution exposure, and it could be on the causal pathway from air pollution to diabetes. We therefore did not include this disease condition as a confounder, but only as a modifier. We also tested the heat stress variables because of prior evidence that the health effects of ozone may be modified by heat stress.
Fig. 1.
Interaction between Ozone and NO2.
There was no indication of the violation of proportional hazards assumption (p = 0.63 for ozone). Removal of New York City alone resulted in slightly higher hazard ratios, while removal of New York City and Los Angeles or New York City, Los Angeles and Chicago together more than doubled the HR to 1.48 (95% CI 1.18–1.85) (see Table 2S in the Online Appendix).
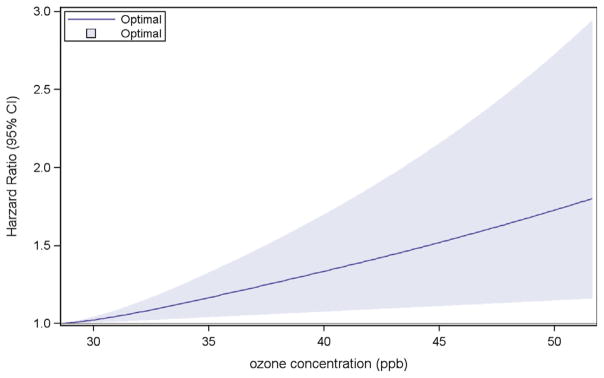
Fig. 2 presents the concentration-response model. The plot suggests a monotonic response function over the range of ozone exposures.
Fig. 2.
Concentration-Response Curve of Ozone and Incident Diabetes.
We also examined the within-metro correlations among the pollutants assessed with a focus on how O3 related to NO2 and PM2.5. The mean correlation between O3 and NO2 is −0.57, but the within-metro correlations range from −0.95 to a maximum of 0.21. Thus, the mean correlation is moderate, and there is substantial variation in the correlation between these two pollutants within the study cities. The within-metro correlations for O3 and PM2.5 average is − 0.29, with a wide range from a minimum of −0.8 to a maximum of 0.37. With these two pollutants, the average is correlation is moderately low, and there is also wide range of correlations among the 56 metros included in our study. Based on this empirical assessment, it appears unlikely that correlations of O3 with either PM2.5 or NO2 were likely to induce substantial collinearity in the statistical model that used within-metro contrasts as the primary exposure assessment.
4. Discussion
Based on analogous evidence of other ambient pollutants with oxidant potential and recent animal models indicating increased insulin resistance after exposure to ozone, we hypothesized that ambient ozone exposure could contribute to diabetes formation. We found initial support for an effect of ozone on incident diabetes in this large cohort of African American women. Our examination of the dose-response function suggested a monotonic relation between ozone and incident diabetes over the range of exposure. The results were changed little by the inclusion of PM2.5 as a co-pollutant. Our results, however, suggest that the association of ozone and diabetes incidence may depend partly on the levels of NO2 present.
Due to atmospheric chemistry processes, ozone is often inversely related to NO2 (Finlayson-Pitts & Pitts, 1997), and we found a mean within metro-correlation of −0.57. If both pollutants are risk factors for diabetes, and one is high in areas where the other is low, it is possible that we would see few effects of ozone where NO2 is high because higher rates of diabetes due to greater levels of NO2 would mask the effect of ozone. NOx was previously related to diabetes incidence among 4204BWHS participants who were residents of Los Angeles using a highly-resolved exposure prediction model: over 10 years of follow-up, the multivariable incidence rate ratio per 12.4 ppb NOx was 1.25 (95% CI 1.07– 1.46) (Coogan et al., 2012). Three other prospective studies have assessed NOx and diabetes incidence, with mixed results. In the Multi Ethnic Study of Atherosclerosis, the HR per 47.1 ppb NOx was 1.04 (95% CI 0.77, 1.40) (Park et al., 2015). In a German study, the relative risk for incident diabetes per 15 3g/m3 of NO2 was 1.42 (95% CI 1.16–1.73) (Krämer et al., 2010). In a Danish cohort, a 4% increase in diabetes incidence was observed when the stricter of 2 diabetes case definitions was used (HR = 1.04, 95% CI 1.00–1.08), with greater increases in non-smokers (HR = 1.12, 95% CI 1.05–1.20) and physically active people (HR = 1.10, 95% CI 1.03–1.16) (Andersen et al., 2012). Thus, the evidence of direct NO2 effects is only partially supported by existing studies, but the suggestive interaction shown here between NO2 and ozone merits further investigation in future studies. The finding here of an interaction, however, must be tempered by the limitation of temporal misalignment in the exposure models used to estimate ozone and NO2 concentration.
We found larger effects when the three biggest cities in the cohort were removed. This change in effect size probably occurred because of the lower incidence rates in the largest cities. Specifically, the rates for the three cities were: 8.15 in Los Angeles, 9.09 in Chicago, and 8.85 in New York; for the other 53 cities (excluding Los Angeles, Chicago, New York) the rate was 10.1. Pollution levels in the larger cities tended to be higher, and combined with the generally lower incidence rates, this could result in attenuation of the effect size when the three largest cities are included in the model.
This study has several strengths. First, we relied on a large, national cohort with well-validated ascertainment methods and substantial information on individual and neighborhood variables that could confound the relationship between ozone and diabetes. Second, we assigned exposures to three of the most common pollutants with well-validated models, all of which have potential to generate health effects. This allowed us to assess confounding and effect modification among the pollutants. Finally, we focused on African American women, who are at much higher risk of developing diabetes than the general population. While this allowed us to assess risks with some precision, focusing on this population also limits the generalizability of our findings.
Several weaknesses should also be noted. First, although the exposure models represent the best available for a national study, they offer different spatial resolutions, and for ozone the 12 km grid may be too coarse to capture micro-scale variation near roadways where ozone tends to be lower due to reactions with NO2 (Beckerman et al., 2008), or in areas without NO2 emissions, such as parks. This could introduce exposure:is classification that would tend to inflate confidence intervals or bias effects toward the null (Zeger et al., 2000). Second, we had only the residential addresses for the women, and it is likely that other exposures occurred at or around places of work or while the women were moving about outside their homes. This might be particularly important for ozone: other studies have found adverse respiratory effects from participation in outdoors sports in high ozone communities, suggesting that time outdoors exercising might be an important exposure window (McConnell et al., 2002). Finally the recruitment strategy may have resulted in selection bias. In BWHS, 97% of participants have at least a high-school education, as compared with 83% of the general population of African American women of the same ages (Department of Commerce. Education Attainment in the United States: March 1995. Washington, DC: Department of Commerce, Bureau of the Census, 1996). Thus the lowest level of education is underrepresented in BWHS. However, the annual incidence of diabetes in BWHS over follow-up was 9.5/1000 person-years, comparable to the incidence rate estimated for African Americans aged 20–79 in the National Health Interview Survey for 1997 (9.5/1000 person years) and 1999 (9.9/1000 per thousand person years) (Geis et al., 1980–2012).
Despite these caveats, our findings suggest initial support for the hypothesis that ozone contributes to diabetes incidence, and recent results from animal models support the biological plausibility of the association (Vella et al., 2015; Bass et al., 2013; Rao et al., 2015). Ozone continues to exceed standards in many parts of the United States and Europe (City Rankings - American Lung Association|State of the Air, 2015; US EPA National Center for Environmental Assessment RTPNEMAG & Brown, 2013; Summer 2014 Ozone Assessment —European Environment Agency, n.d.). For instance, recent studies from Southern California show that ozone has not appreciably improved over the past 10 years, while other pollutants such as PM2.5 and NO2 have declined precipitously (Gauderman et al., 2015). Ozone is also present at elevated levels in many parts of the world, particularly in heavily populated regions of Asia (Parrish et al., 2012). Our results, if confirmed in other U.S. populations and other regions of the world, may have important implications for diabetes prevention and for public health protection.
Supplementary Material
Acknowledgments
Sources of funding
This work was supported by the National Institutes of Environmental Health Science and the Centers for Disease Control.
This work was supported by the National Institutes of Environmental Health Science (Grant: ES019573) and the National Cancer Institute (UM1CA16497 and CA058420). Centers for Disease Control Environmental Public Health Tracking Program (Grant: 200-2010-37394). We thank Eunice Lee for assistance with submitting the paper and Bernardo Beckerman for helping with data preparation on the exposure assignments.
References
- Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, et al. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35(1):92–98. doi: 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass V, Gordon CJ, Jarema KA, MacPhail RC, Cascio WE, Phillips PM, et al. Ozone induces glucose intolerance and systemic metabolic effects in young and aged brown Norway rats. Toxicol Appl Pharmacol. 2013;273(3):551–560. doi: 10.1016/j.taap.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmos Environ. 2008;42(2):275–290. [Google Scholar]
- Beckerman BS, Jerrett M, Serre M, Martin RV, Lee S, van Donkelaar A, et al. A hybrid approach to estimating national scale spatiotemporal variability of PM2.5 in the contiguous United States. Environ Sci Technol. 2013;47:7233–7241. doi: 10.1021/es400039u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JD, Fann N, Hollingsworth JW, Pinkerton KE, Rom WN, Szema AM, et al. Health benefits from large-scale ozone reduction in the United States. Environ Health Perspect. 2012;120(10):1404–1410. doi: 10.1289/ehp.1104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal VJ, Gelfand AE, Holland DM. Space-time data fusion under error in computer model output: an application to modeling air quality. Biometrics. 2012;68(3):837–848. doi: 10.1111/j.1541-0420.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G, Hartman AM, Naughton DA. Reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- Boggs DA, Palmer JR, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Dietary patterns and 14-y weight gain in African American women. Am J Clin Nutr. 2011;94(6):86–94. doi: 10.3945/ajcn.111.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Burnett RT, KJCVPJ, GMSBRD, van DAJMMRV B, JR, CR Risk of Incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Can-ada. Environ Health Perspect. 2013;804(2):804–810. doi: 10.1289/ehp.1205958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- City Rankings - American Lung Association|State of the Air 2015 [Internet] [cited 2016 Feb 22]. Available from: http://www.stateoftheair.org/2015/city-rankings/
- Coogan PF, White LF, Jerrett M, Brook RD, JGS, Seto E, et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125(6):767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan PF, White LF, Yu J, Burnett RT, Seto E, Brook RD, et al. PM2.5 and diabetes and hypertension incidence in the black women’s health study. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper OR, Parrish DD, Ziemke J, Balashov NV, Cupeiro M, Galbally IE, et al. Global distribution and trends of tropospheric ozone: An observation-based review. Elem Sci Anthr. 2014;2:000029. [Google Scholar]
- Department of Commerce. Education Attainment in the United States: March 1995. Washington, DC: Department of Commerce, Bureau of the Census; 1996. [Google Scholar]
- Environments Outdoor Air - CDC Tracking Network, d. [cited 2016 Feb 22]. Available from. http://ephtracking.cdc.gov/showAirLanding.action.
- Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Kunzli N, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. 2015;123(5):381–389. doi: 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson-Pitts BJ, Pitts JN. Tropospheric air pollution: ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science. 1997;276(5315):1045–1052. doi: 10.1126/science.276.5315.1045. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905–913. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis LS, Wang J, Cheng YJ, Thompson RJ, Barker L, Li Y, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States. JAMA. 1980–2012;312:1218–1226. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, et al., editors. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2014. p. 1132. [Google Scholar]
- Jerrett M, Burnett RT, Pope CA, Ito K, Thurston G, Krewski D, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360(11):1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U, Herder C, Sugiri D, Strassburger K, Schikowski T, Ranft U, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118(9):1273–1279. doi: 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the black women’s health study. Am J Epidemiol. 2010;171(5):564–570. doi: 10.1093/aje/kwp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359(9304):386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Taffiet M, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307(7):713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- Novotny EV, Bechle MJ, Millet DB, Marshall JD. National satellite-based land-use regression: NO2 in the United States. Environ Sci Technol. 2011;45(10):4407–4414. doi: 10.1021/es103578x. [DOI] [PubMed] [Google Scholar]
- Park SK, Adar SD, O’Neill MS, Auchincloss AH, Szpiro A, Bertoni AG, et al. Long-term exposure to air pollution and type 2 diabetes mellitus in a multiethnic cohort. Am J Epidemiol. 2015;181(5):327–336. doi: 10.1093/aje/kwu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish DD, Law KS, Staehelin J, Derwent R, Cooper OR, Tanimoto H, et al. Long-term changes in lower tropospheric baseline ozone concentrations at northern mid-latitudes. Atmos Chem Phys. 2012;12(23):11485–11504. [Google Scholar]
- Puett RC, Hart JE, Schwartz J, FBH, Liese AD, Laden F. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect. 2011;119(3):384–389. doi: 10.1289/ehp.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, Montresor-Lopez J, Puett R, Rajagopalan S, Brook RD. Ambient air pollution: an emerging risk factor for diabetes mellitus. Curr Diab Rep. 2015;15(6):603. doi: 10.1007/s11892-015-0603-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Adams-Campbell L, Palmer JR. The black women’s health study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50(2):56–58. [PubMed] [Google Scholar]
- Summer 2014 Ozone Assessment –European Environment Agency. [Internet] [cited 2016 Feb 22]. Available from. http://www.eea.europa.eu/themes/air/ozone/air-pollution-by-ozone-across.
- Turner MC, Jerrett M, III, Pope AC, Krewski D, Gapstur SM, Diver WR, et al. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med. 2016;193(10):1134–1142. doi: 10.1164/rccm.201508-1633OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA National Center for Environmental Assessment RTPNEMAG, Brown, J. Final Report: Integrated Science Assessment of Ozone and Related Photochemical Oxidants. 2013G. (cited 2016 Feb 22) [Google Scholar]
- Vella RE, Pillon NJ, Zarrouki B, Croze ML, Koppe L, Guichardant M, et al. Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes. 2015;64(3):1011–1024. doi: 10.2337/db13-1181. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.