Abstract
Myometrium holds the structural integrity for the uterus and generates force for parturition with its primary component, the smooth muscle cells. The progesterone receptor mediates progesterone dependent signaling and connects to a network of pathways for regulation of contractility and inflammatory responses in myometrium. Dysfunctional progesterone signaling has been linked to pregnancy complications including preterm birth. In the present review, we summarize recent findings on modifiers and effectors of the progesterone receptor signaling. Discussions include novel conceptual discoveries and new development in legacy pathways such as the signal transducers NF-κB, ZEB, micro RNA and the unfolded protein response pathways. We also discuss the impact of progesterone receptor isoform composition and ligand accessibility in modification of the progesterone receptor genomic actions.
Keywords: Progesterone Receptor, Myometrium, Uterus, Progesterone Receptor isoforms, micro RNA
1. Introduction
The uterus goes through extensive structural remodeling and functional adaptation over the course of pregnancy. To accommodate fetal growth, myometrium expands its size through hyperplasia and hypertrophy of smooth muscle cells at distinct phases (Shynlova et al., 2010; Shynlova et al., 2006). Functionally, while myometrium remains quiescent throughout gestation, at term, inflammation responses stimulate coordinated contraction for laboring and parturition. At the postpartum stage, uterine involution occurs and its size returns to the pre-pregnancy stage ready for pregnancy again (Hsu et al., 2014). Dysregulated functional adaptation may result in premature laboring and subsequent preterm birth.
Preterm birth is defined as delivery before 37 weeks of pregnancy and is the leading cause of perinatal lethality. Premature infants resulted from preterm birth often suffer complications of retinopathy, jaundice, anemia, infections, respiratory distress, neurological disorders, cardiovascular defects and necrotizing enterocolitis. According to data from the Centers for Disease Control and Prevention, the United States preterm birth rate raises to 9.63% in 2015 after a 7-year decrease from 2007 to 2014 with higher occurrences in African American and American Indian or Alaska Native women. While many pharmacological agents for treatment of premature uterine contraction have beneficial effect in the first 48 hours, the steroid hormone progesterone (P4) serves as the most promising agent to maintain uterine quiescence and prevent preterm birth beyond the acute phase (Navathe and Berghella, 2016).
P4 is produced by ovaries, placenta and adrenal glands and its actions are primarily mediated by the progesterone receptor (PGR). PGR is expressed in the central nervous system, ovaries, breasts, and the female reproductive tracts, including the vagina, cervix, fallopian tubes and uterine endometrium and myometrium. PGR is important for female sexual behavior, ovulation, the establishment and maintenance of pregnancy and development of mammary gland (Bhurke et al., 2016; Diep et al., 2015). In uterine myometrium, P4 signaling maintains uterine quiescence by suppressing prostaglandin and oxytocin dependent inflammatory responses and contractile activities before term. At term, in a species dependent manner, either withdrawal of P4 by a decrease in hormone levels or alteration of PGR signaling relieves the suppression on inflammation and contraction, which allows the myometrium to adopt a contractile phenotype for laboring. Multiple mechanisms, including P4 metabolism, regulation of PGR gene expression, PGR post-translation modifications and PGR coregulators, that mediate or regulate uterine P4/PGR signaling have been identified and reviewed extensively (Bhurke et al., 2016; Grimm et al., 2016; Patel et al., 2015; Renthal et al., 2015; Rubel et al., 2016; Szwarc et al., 2014). Here we focus on discussing uterine P4/PGR signaling modifiers and effectors in myometrial physiology and diseases.
2. Ligand availability
Withdrawal of P4 signaling may occur at the level of ligand availability, which can be achieved by reduction of P4 synthesis and/or an increase of P4 degradation. Indeed, P4 degradation has been reported at the source (ovary) and target (myometrium) tissues. Prostaglandin signaling is required for parturition at term (Sugimoto et al., 1997). In ovaries, prostaglandin signaling attenuates progesterone synthesis by lowering the levels of steroidogenic acute regulatory protein (STAR) (Fiedler et al., 1999). Emerging evidence suggests that the prostaglandin-STAR pathway is modulated in part by fetal-maternal crosstalk via Surfactant Protein-A and Platelet-Activating Factor that are secreted by fetal lungs under the control of Steroid Receptor Coactivators 1 and 2 (SRC-1 and SRC-2) (Gao et al., 2015). It is proposed that, at maturation, signals from fetal lungs down-regulate ovarian P4 synthesis that leads to reduction of P4 signaling, and subsequent initiation of parturition (Mendelson et al., 2016). Moreover, prostaglandin also elevates transcription factor Nur77 (also known as nuclear receptor subfamily 4, group A, member 1, Nr4a1) levels to promote expression of 20α-hydroxysteroid dehydrogenase (20α-HSD, also known as aldo-keto reductase family 1, member C18, Akr1c18) for P4 degradation (Stocco et al., 2000). 20α-HSD is essential for P4 removal and timely induction of parturition, as evidenced by higher serum progesterone levels and prolonged gestation in 20α-HSD knockout mice (Piekorz et al., 2005). Ovarian 20α-HSD expression is also repressed by signal transducer and activator of transcription 5B (Stat5b) (Piekorz et al., 2005) and positively regulated by mastermind like domain containing 1 (Mamld1) (Miyado et al., 2015). Stat5b null mice exhibit elevated ovarian 20α-HSD expression, low serum P4 levels and early abortion of pregnancy (Piekorz et al., 2005; Udy et al., 1997). In contrast, ablation of Mamld1 leads to reduced 20α-HSD levels, higher serum P4 concentration and delayed parturition (Miyado et al., 2015). Interestingly, Mamld1 is required to suppress Stat5b expression in ovaries at 18.5 dpc while Mamld1 deficiency does not alter expression of prostaglandin F receptor and Nur77 (Miyado et al., 2015). These findings suggest that Mamld1 and other regulators jointly suppresses Stat5b expression to increase 20α-HSD levels in a prostaglandin-independent manner.
Increasing evidence indicates that regulation of ligand availability at the local microenvironment contributes, in part, to functional P4 withdrawal. In addition to ovaries, 20α-HSD is also expressed in uterine myometrium, adrenal glands, thymocytes and kidney (Akinola et al., 1997; Choi et al., 2008; Egert and Maass, 1973; Hirabayashi et al., 2001; Nadeem et al., 2016). In laboring human myometrium, increased 20α-HSD expression is associated with a reduction of nuclear P4 levels (Nadeem et al., 2016; Walsh et al., 1984), suggesting that myometrium 20α-HSD may play a role in regulating local P4 levels. During gestation, P4/PGR signaling promotes expression of the zinc finger E-box binding homeobox 1 (Zeb1, see more discussion in section 5) transcription factor in the myometrium (Renthal et al., 2010). ZEB1 then increases STAT5b protein levels by directly suppressing expression of miR-200a, a STAT5b upstream repressor. Decreased miR-200a also relieves the repression on zinc finger E-box binding homeobox 2 (ZEB2) whose increasing levels further suppress miR-200a expression. As a result, elevated STAT5b subsequently decreases levels of 20α-HSD to prevent P4 removal (Williams et al., 2012a). In the presence of ligand, PGR isoform B (PGR-B) moves into nuclei to represses expression of contractile genes reducing myometrium contraction (Nadeem et al., 2016). On the other hand, when P4 levels are lower, reduced P4/PGR signaling decreases ZEB1/2 and subsequently increases miR-200a expression, leading to reduced STAT5b levels, elevated 20α-HSD expression, and further reduction of ligand availability (Renthal et al., 2010; Williams et al., 2012a). Without ligand binding, PGR-B resides in cytosol while unliganded PGR isoform A (PGR-A) translocates to nuclei and promotes expression of contractile genes for subsequent increase of uterine contraction (Nadeem et al., 2016). Taken together, P4/PGR signaling feeds forward to maintain ligand availability by reducing the P4 metabolizing rate through activation of the ZEB1/2-miR-200-STAT5b pathway. At the onset of parturition, prostaglandin signaling decreases P4 levels by reducing synthesis and increasing degradation of P4, eventually leading to withdrawal of P4 signaling (Figure 1). Importantly, this mechanism provides a plausible explanation on the association between myometrial reduction of liganded PGR and preterm labor (Nadeem et al., 2016). Moreover, regulation of local ligand availability may also partially explain the P4 functional withdrawal in human when circulating P4 levels remain unchanged around parturition.
Figure 1.

Modulation of the progesterone availability.
3. Composition of PGR isoforms
The two PGR isoforms PGR-A and PGR-B are transcribed from the same genomic locus by two different promoters, resulting in a 114-kDa PGR-B and a 94-kDa PGR-A proteins (Kastner et al., 1990). Upon ligand stimulation, PGR-A and PGR-B have the capacity to modulate distinct sets of downstream target genes, suggesting that the P4 signaling is mediated by a combined action of both PGR isoforms (Khan et al., 2012; Tan et al., 2012). Therefore, it is logical to assume that changes in relative abundance of PGR-A and PGR-B may result in different translation of P4 signaling and subsequent physiological responses. This notion finds support from observations that alterations of PGR-A and PGR-B relative abundance have been associated with various physiological and pathological conditions, including breast cancer metastasis and uterine myometrium contractility (Chai et al., 2012; Khan et al., 2012; Merlino et al., 2007). In human myometrium, PGR-B is the more abundant isoform at the preterm stage and maintains its levels at both non-laboring and laboring term stages. In contrast, PGR-A levels rise over the course of pregnancy and eventually exceed PGR-B in the laboring myometrium (Chai et al., 2012; Merlino et al., 2007). Because the resulting increase of PGR-A to PGR-B ratio occurs concomitant with the P4 functional withdrawal and the transition from quiescent to laboring state, such correlation implicates that regulation of the PGR-A/PGR-B ratio may contribute to preparation and switch of myometrium into a contractile state.
PGR isoforms can be either activators or repressors through preferential interaction with different co-regulators (Giangrande et al., 2000; Jacobsen and Horwitz, 2012; Nadeem et al., 2016; Yore et al., 2010). With respect to the myometrium, PGR-A preferentially interacts with AP-1 subunits FOSL1 and FOSL2 in comparison to PGR-B, while PR-B exhibits a stronger interaction with JUN in nuclei of hTERT-HM human myometrial cells (Nadeem et al., 2016). The functional significance of such preferential interactions is exemplified by an observation that PGR-A works as an activator in the presence of FOSL2 and JUND while exhibits a repressive function on the GJA1 promoter in Syrian hamster myometrium cells (Nadeem et al., 2016). In contrast, PGR-B only suppresses GJA1 promoter activities in the presence of JUNB and JUND, but not in the context of FOSL2 and JUND (Nadeem et al., 2016). These findings implicate that, during gestation, the more abundant PGR-B interacts with JUN-JUN dimers to recruit p54/mSin3A/HDAC repressors to suppress expression of contractile genes. During transition to the laboring stage, elevated PGR-A and FOS levels reach a threshold to change the myometrial genomic profile in favor of contraction via the interaction between PGR-A and JUN-FOS heterodimers (Mitchell and Lye, 2002; Nadeem et al., 2016). Additionally, PGR-A can also attenuate PR-B transcription activities in hTERT-HM cells (Peters et al., 2016), possibly through a dominant-negative effect (Vegeto et al., 1993). Moreover, IL-1β stimulated phosphorylation of PR-A at serine 345 (S345) is required for the PR-A dependent inhibition of PR-B actions on the inflammatory genes (Amini et al., 2016). This mechanism may further tilt the functional impact of rising PGR-A/PGR-B ratio in favor of a PGR-A dependent genomic regulation.
Transcriptional regulation of the PGR-A gene serves as one of the mechanisms to mediate the rise of PGR-A levels. Studies on human tissues and mouse models reveal that KRUPPEL-LIKE FACTOR 9 (KLF9) promotes PGR-A expression in myometrium. Klf9 knockout mice have reduced myometrium PGR-A levels at term, delayed parturition and resistance to RU486-induced pregnancy termination (Zeng et al., 2008). Klf9 ablation lowers PGR-A protein levels, reduces expression of contractile genes, including Gja1 and Oxtr, and decreases the binding capacity of NF-κB proteins p65 and p50 to their target genes. In humans, comparing term patients, myometrium of patients with late-term pregnancy exhibits lower levels of KLF9, PGR-A and PGR-B, as well as a lower PGR-A/PGR-B ratio and an increased anti-inflammatory and decreased pro-inflammation profile of gene expression. (Pabona et al., 2015). Moreover, knocking down KLF9 attenuates RU486-dependent suppression of pro-inflammatory gene expression. Collectively, these findings indicate that KLF9 promotes PGR-A expression resulting in a molecular profile favorable for inflammation contraction in myometrium.
Emerging evidence indicates that PGR-A gene expression is subject to epigenetic regulation. In human myometrium, the PGR-A promoter exhibits higher levels of active histone mark such as histone H3 lysine 4 trimethylation (H3K4me3), acetylated histone 3 and acetylated histone 4, compared with the PGR-B promoter (Chai et al., 2012). This finding suggests that the PGR-A promoter is relatively permissible to transcriptional regulation by other transcription factors such as KLF9. This is also in line with the observation that PGR-A mRNA levels increase over the course of pregnancy while the PGR-B mRNA abundance has minimal change (Chai et al., 2012). On the PGR-A promoter, laboring human myometrium exhibits higher H3K4me3 levels and reduced occupancy of the H3K4 demethylase, Jumonji AT-rich interactive domain 1A (JARID1A, also known as KDM5A), compared with quiescent myometrium (Chai et al., 2014). Because JARID1A can repress PGR expression in MCF-7 cells (Stratmann and Haendler, 2011), it is proposed that the reduced JARID1A occupancy may result in higher levels of H3K4me3, increased enhancer activities and subsequent further induction of PGR-A transcription in laboring myometrium. Additionally, another inverse correlation between PGR-A and the Histone Deacetylase 1 (HDAC1) mRNA levels has also been reported in human myometrium at the transition from quiescent to laboring stage. Further experimentations show that HDAC1 occupies the PGR-A promoter and suppresses PGR-A expression in cultured primary myometrial cells (Ke et al., 2016). These observations suggest that the PGR-A expression is regulated by HDAC1 dependent histone modifications. In summary, in response to as yet unidentified signals, the epigenetic regulators JARID1A and HDAC1 may modulate PGR-A promoter activities through modifying the histone methylation and acetylation of myometrium during pregnancy.
PGR-A levels can also be regulated at the post-translational level as evidenced by the stabilization of that myometrial PGR-A protein in response to inflammatory stimuli (Peters et al., 2016). In human myometrial tissue explants and myometrial cell lines, IL-1β attenuates progesterone dependent reduction of PR-A protein levels. IL-1β does not alter PR-A mRNA abundance but blockage of proteasome activities can sustain PR-A protein levels in the presence of progesterone. Thus, IL-1β may regulate PR-A stability through post-translational modifications, possibly via the p38 mitogen-activated protein kinase dependent phosphorylation (Jung et al., 2002; Khan et al., 2011). In summary, multiple mechanisms regulate the increase of PGR-A protein levels, subsequently leading to change of P4 signaling profile via switching from PGR-B to PGR-A dominance to stimulate uterine contraction and parturition (Figure 2).
Figure 2.
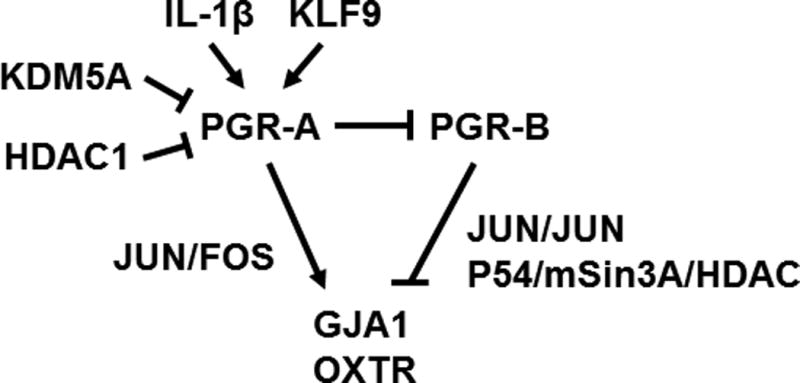
The progesterone receptor isoform regulatory network.
4. The PGR-NF-kB axis
P4 acts through PGR to suppress the inflammatory response in uterus (Lydon et al., 1995; Tibbetts et al., 1999). In human myometrial cells, P4 treatment attenuates interleukin-1 β (IL-1β) induced prostaglandin-endoperoxide synthase 2 (PTGS2, also known as COX-2) expression via blocking the occupancy of the protein complex NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) on the PTGS2 promoter (Hardy et al., 2006). P4 signaling also induces expression of kappa light polypeptide gene enhancer in B cells inhibitor, alpha (IκBα) to sequester and keep NF-κB inactive (Hardy et al., 2006). In addition, three other mechanisms have been proposed for regulation of NF-κB activities by P4/PGR signaling. 1) P4/PGR signaling may block IL-1β induced and proteasome dependent IκBα protein degradation (Renthal et al., 2015). 2) PGR may physically interact with NF-κB and inhibit NF-κB activities (Kalkhoven et al., 1996; Renthal et al., 2015). 3) PGR induces dual specificity phosphatase 1 (DUSP1) expression to reduce phosphorylation of mitogen-activated protein kinase 1 and 3 (ERK1/2) and subsequently decrease NF-κB p65 subunit nuclear localization, as evidenced in the T47D breast cancer cell line (Chen et al., 2011). In summary, P4/PGR signaling targets multiple points of the NF-κB pathway to suppress NF-κB dependent induction of pro-inflammatory gene expression (Figure 3).
Figure 3.

Regulation of NF-kB signaling by P4/PGR.
Mounting evidence indicates sirtuins as a regulator of the NF-kB pathway. Sirtuins, known as inflammation and metabolic regulators, are histone deacetylases capable of epigenetically modulating transcription. SIRT1 and SIRT2 suppress NF-κB activities by deacetylating p65 and subsequently decrease proinflammatory cytokine production (Rothgiesser et al., 2010; Yang et al., 2012). SIRT6 deacetylates histone H3 lysine 9 (H3K9) at promoters of NF-κB target genes, decreases RELA promoter occupancy and suppresses expression of NF-κB downstream targets (Kawahara et al., 2009). In uteri, laboring myometrium exhibits a lower level of SIRT3 compared with non-laboring myometrium at term (Lim et al., 2016), coincided with the withdrawal of P4 signaling. In cultured primary myometrial cells, SIRT3 levels are reduced by IL-1β and TNFα treatment, while knocking down SIRT3 expression augments NF-κB activities and increases expression of downstream cytokines and chemokines (Lim et al., 2016). These findings suggest a role of SIRT3 in maintaining uterine quiescence by suppressing the inflammatory response during gestation. Although it is unclear how SIRT3 interacts with NF-κB and whether PGR is involved, given the role of sirtuin family proteins in modulating activities of nuclear hormone receptors such as estrogen receptor and Liver X Receptor (Li et al., 2007; Moore and Faller, 2013), it would be interesting to examine potential interactions between PGR and SIRT3 in the myometrium.
5. The PGR-ZEB-microRNA regulatory circuit
ZEB1 and ZEB2 belong to the ZEB family transcription factors that are pivotal for embryonic development and tumor progression (Vandewalle et al., 2009; Zhang et al., 2015). Studies in mouse models reveal that Zeb1 mediates transforming growth factor β (TGFβ) dependent expression of smooth muscle genes and is required for differentiation of vascular smooth muscle (Nishimura et al., 2006), while Zeb2 is essential for normal vagina development (Miyoshi et al., 2006). In both human and mouse uteri, ZEB1 and ZEB2 are prominently expressed in quiescent myometrium and their levels are reduced at laboring (Renthal et al., 2010), concomitant with the temporal change of P4 signaling. The functional relationship between P4/PGR signaling and ZEB1/2 expression finds support from the requirement of P4 signaling in sustaining Zeb1 and Zeb2 expression with Zeb1 being a potential direct downstream target of PGR (Renthal et al., 2010). Results from mouse and cell models further suggest that ZEB1 occupies the promoters of gap junction protein, alpha 1 (Gja1) and oxytocin receptor (Oxtr) genes and directly represses transcription of these classic marker genes for uterine contraction (Renthal et al., 2010). These findings collectively indicate that ZEB1/2 mediates P4/PGR signaling in silencing the myometrium activities through regulation of contractile gene expression.
Results from a series of studies indicate that a regulatory network, consisting of ZEB1/2 and microRNAs (miR), modulates myometrial molecular profiles during the transition from quiescent to laboring. These interactions include reciprocal repression between ZEB1/2 and miR200 family, positive regulation of miR-199a/214 cluster expression by ZEB1 and repression of ZEB1 by miR-10b (Guo et al., 2015; Renthal et al., 2010; Tang et al., 2015; Williams et al., 2012a; Williams et al., 2012b). Expression of ZEB1/2 and miR-200b/429 correlates inversely in myometrium (Renthal et al., 2010). ZEB1 occupancy is observed on the genomic locus of miR-200b/429 cluster while such occupancy is stronger in quiescent than in laboring myometrium. Also, the 3’ untranslated regions of both ZEB1 and ZEB2 mRNA contain binding sites for miR-200b/429. Functionally, P4 elevates Zeb1/2 levels and suppresses miR-200b/429 expressions. On the other hand, miR-200b/429 overexpression reduces ZEB1 and ZEB2 expression in human myometrial cells. These findings together suggest that ZEB1/2 and miR-200b/429 exert a mutually repressive function. During gestation, P4/PGR signaling sustains a high level of ZEB1/2 that suppresses expression of miR-200 family to maintain their own abundance and ligand availability in myometrium (discussed in section 6). At the laboring stage, P4 withdrawal reduces ZEB1 expression and relives ZEB1/2’s repression effect on miR-200b/429, whose elevated expression further decrease ZEB1/2 levels.
P4 signaling also promotes microRNAs expression to reduce levels of contractile genes. In both human and mice, miR-199a-3p and miR-214 are more abundant in the quiescent myometrium than during labor (Williams et al., 2012b). P4 treatment and ZEB1 overexpression both can increase miR-199a-3p and miR-214 expressions, while reduced ZEB1 levels lead to lower abundance of miR-199a-3p and miR-214. In addition, ZEB1 occupancy is observed on the genomic locus of miR-199a/214 cluster and ZEB1 exhibits a much stronger occupancy pattern in quiescent myometrium. Moreover, miR-199a-3p and miR-214 both target the mRNA of PTGS2, while overexpression of either microRNA attenuate IL-1β induced increase of PTGS2 protein abundance and tumor necrosis factor α (TNFα) stimulated myometrial cell contraction. Taken together, these results reveal that ZEB1-miR-199a/214 mediated a P4/PGR dependent suppression of inflammatory gene expression, in addition to the aforementioned NF-κB pathway. Of note, estrogen treatment is able to reduce the abundance of myometrial miR-199a-3p and miR-214. Similarly, miR-181a, another anti-inflammatory microRNA that shows decreased expression near term, is also negatively regulated by estrogen (Gao et al., 2016). Given that ERα directly represses miR-181a transcription, it would be interesting to examine whether the miR-199a/214 cluster is regulated by estrogen with a similar mechanism.
Emerging data shows increased miR-10b expression in the human preterm myometrium compared with myometrium not in labor (Tang et al., 2015). Studies in endometrial epithelial cells reveal that miR-10b targets ZEB1 and PIK3CA mRNAs and reduces their protein abundance (Guo et al., 2015). Collectively, although more investigations are needed, it is tempting to speculate that the elevated miR-10b could be a contributing factor to tilt the balance between ZEB1 and miR-200 family toward the contractile profile, subsequently leading to early P4 withdrawal and preterm birth. In summary, P4/PGR signaling via the ZEB transcription factors controls multiple microRNAs with either pro- or anti-contractile/inflammatory roles to regulate the myometrial function (Figure 4).
Figure 4.

Interaction among P4/PGR, ZEB1/2 and microRNAs for regulation of contractile genes.
6. Endoplasmic reticulum stress and unfolded protein response
Protein production, including polypeptide synthesis, protein folding and some post-translational modifications, takes place in the organelle endoplasmic reticulum (ER). When accumulation of unfolded proteins stresses ER, the unfolded protein response (UPR) elicits pro-survival signaling to reduce translation rates, facilitate protein degradation, and induce ER chaperones rectifying the ER stress. Emerging evidence suggests smooth muscle UPR as a regulator of gestational length and P4 signaling as a UPR modulator (Kawakami et al., 2014; Kyathanahalli et al., 2015). Physiological UPR is present in uteri of pregnant mice and is thought to sustain enough active caspase 3 and 7 that keeps GJA1 protein at low level in order to maintain uterine quiescence (Kyathanahalli et al., 2015). At late gestation, decreased UPR results in a reduction of active caspase 3/7, which increases GJA1 protein levels by decreasing its degradation and leads to subsequent increased muscle contraction (Kyathanahalli et al., 2015). P4 treatment delays UPR reduction, maintains levels of active caspase 3/7, and sustains a lower level of GJA1. In contrast, RU486 suppresses UPR, decreases abundance of active caspase 3/7, and leads to elevated levels of GJA1(Kyathanahalli et al., 2015). Moreover, P4 also promotes uterine expression of Caspase 3, possibly through PGR binding motifs in the promoter region (Jeyasuria et al., 2009). Taken together, these findings indicate an additional layer of P4 regulation through post-translational modulation of GJA1 levels via UPR and Caspase 3/7 dependent mechanisms (Figure 5).
Figure 5.

Interplay between P4/PGR signaling and the unfolded protein response in control of contractile marker gene expression.
In uteri, P4 signaling interacts with another ER stress transducer, cAMP Response Element-Binding 3-like protein 1 (CREB3L1, also known as OASIS), that mediates crosstalk among subcellular organelles to enhance the protein processing capacity of endoplasmic reticulum (Kondo et al., 2011). CREB3L1 is a transcription factor with a transmembrane domain that allows its association with ER. Once activated by regulated intramembrane proteolysis, CREB3L1 translocates to the nucleus and exerts genomic actions (Mellor et al., 2013; Murakami et al., 2009). CREB3L1 also acts downstream of PGR to promote ERK signaling for stromal cell decidualization (Ahn et al., 2016). In human uteri, detection of CREB3L1 protein is reported in both epithelial and stromal compartments of endometrium with the strongest expression in the decidual cells at the late secretory stage when the P4 signaling is prominent (Ahn et al., 2016). Although it is not clear whether CREB3L1 is expressed in the myometrium, its mRNA and protein can be detected in various smooth muscle tissues including bladder, stomach and intestine. Based on the mouse model, endometrial CREB3L1 expression depends on PGR mediated P4 signaling through mechanisms yet to be identified (Ahn et al., 2016). Functionally, CREB3L1 is required for the decidualization process as evidenced by the failure of decidualization and reduced expression of decidualization marker genes in human endometrial stromal cells after knocking down the CREB3L1 in vitro (Ahn et al., 2016). Decidual cells exhibit distinct features compared with stromal cells they derived from, including larger cell size, enlarged and dilated rough ER, increased in size of the Golgi complexes and increased secretion of prolactin and IGFBP-1 (Lane et al., 1994). These changes are indicative of major structural and functional alterations of ER in response to P4 stimulation. In light of the roles of CREB3L1 in the decidualization response and in ER-nucleus interaction, it is tempting to speculate that the CREB3L1 may utilize similar mechanisms as those in ER stress response to mediate P4-induced ER and Golgi changes during decidualization.
Notably, excessive ER stress results in preterm delivery with the most impact at late gestation in the tunicamycin treated mouse model (Kawakami et al., 2014; Kyathanahalli et al., 2015). Moreover, ER stress is associated with fetal growth restriction and endometriosis (Ahn et al., 2016; Lian et al., 2011; Yung et al., 2008). These findings are significant because ER stress has been linked to numerus environmental insults (Kitamura, 2013) and pregnant women are exposed to various environmental stimulations over the course of pregnancy.
7. Concluding remarks and future perspectives
In myometrium, P4/PGR signaling is connected to a complex regulatory network through the NF-κB, ZEB-microRNAs and UPR pathways as well as direct transcriptional regulation, which together modulate the levels and activities of downstream effectors. On the other hand, the overall P4/PGR signaling dependent molecular profiles are modified by the combined activities of PGR isoforms, the concerted efforts of co-regulators and the ligand availability. P4/PGR signaling mediates and utilizes these interconnected pathways, as summarized in Figure 6, to determine the state of myometrium over the entire course of pregnancy. Although the proof of concept is performed on a few model genes, a better understanding on the genomic impact of PGR-A and PGR-B isoforms in myometrium at the global level and in a physiological context would benefit discovery of novel and distinct pathways specific for myometrium. In addition, modifiers that result in preterm birth and fetal growth restriction and are associated with the P4/PGR signaling network (e.g. microRNAs, ER stress and premature loss of liganded PGR in nuclei) are of particular interest in understanding mechanisms that elicit and mediate such associations and the extent of the individual modifier’s pathological impact.
Figure 6.
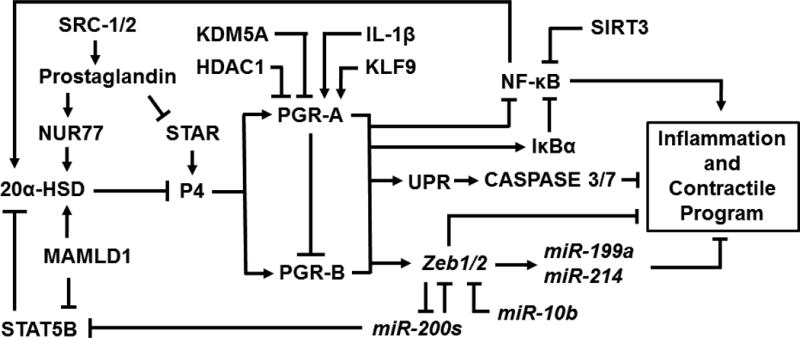
A genetic network of PGR signaling for regulation of uterine myometrium states in adult.
P4 supplement is currently in use for prevention of preterm birth and can prevent about one-third of recurrent preterm birth (Norwitz and Caughey, 2011). The fact that P4 fails to benefit many more patients highlights not only the complex etiology behind the preterm birth but also a few emerging questions in the myometrial biology. It is not clear whether all myometrial smooth muscle cells are the same and can respond equally to P4. Heterogeneity of smooth muscle cells are present in the arteries with respect to response to growth stimuli (Topouzis and Majesky, 1996). It is also believed that predisposed subpopulations of smooth muscle cells may contribute to pathological alterations in atherosclerosis and restenosis (Hao et al., 2003). Examining the molecular signature of individual myometrial smooth muscle cells with single cell transcriptome profiling may help to address these questions. Meanwhile, at the laboring stage, coordinated contraction of each individual smooth muscle cells is essential to generate effective force for parturition. Emerging evidence indicates the presence of a group of specialized cells named “interstitial cells of Cajal” or “telocytes” in uterine myometrium (Allix et al., 2008; Cretoiu et al., 2013). This type of cells serves as pacemaker cells in various several smooth muscle tissues generating electric waves to trigger coordinated muscle contraction. What factors these telocytes interact with and whether P4 signaling affects telocytes’ development and functions remain to be defined.
Acknowledgments
This work is supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institute of Health: Project Z1AES103311-01.
References
- Ahn JI, Yoo JY, Kim TH, Kim YI, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Ahn JY, Lim JM, Jeong JW. cAMP-Response Element-Binding 3-Like Protein 1 (CREB3L1) is Required for Decidualization and its Expression is Decreased in Women with Endometriosis. Curr Mol Med. 2016;16:276–287. doi: 10.2174/1566524016666160225153659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinola LA, Poutanen M, Vihko R, Vihko P. Expression of 17beta-hydroxysteroid dehydrogenase type 1 and type 2, P450 aromatase, and 20alpha-hydroxysteroid dehydrogenase enzymes in immature, mature, and pregnant rats. Endocrinology. 1997;138:2886–2892. doi: 10.1210/endo.138.7.5258. [DOI] [PubMed] [Google Scholar]
- Allix S, Reyes-Gomez E, Aubin-Houzelstein G, Noel D, Tiret L, Panthier JJ, Bernex F. Uterine contractions depend on KIT-positive interstitial cells in the mouse: genetic and pharmacological evidence. Biol Reprod. 2008;79:510–517. doi: 10.1095/biolreprod.107.066373. [DOI] [PubMed] [Google Scholar]
- Amini P, Michniuk D, Kuo K, Yi L, Skomorovska-Prokvolit Y, Peters GA, Tan H, Wang J, Malemud CJ, Mesiano S. Human Parturition Involves Phosphorylation of Progesterone Receptor-A at Serine-345 in Myometrial Cells. Endocrinology. 2016;157:4434–4445. doi: 10.1210/en.2016-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhurke AS, Bagchi IC, Bagchi MK. Progesterone-Regulated Endometrial Factors Controlling Implantation. Am J Reprod Immunol. 2016;75:237–245. doi: 10.1111/aji.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai SY, Smith R, Fitter JT, Mitchell C, Pan X, Ilicic M, Maiti K, Zakar T, Madsen G. Increased progesterone receptor A expression in labouring human myometrium is associated with decreased promoter occupancy by the histone demethylase JARID1A. Mol Hum Reprod. 2014;20:442–453. doi: 10.1093/molehr/gau005. [DOI] [PubMed] [Google Scholar]
- Chai SY, Smith R, Zakar T, Mitchell C, Madsen G. Term myometrium is characterized by increased activating epigenetic modifications at the progesterone receptor-A promoter. Mol Hum Reprod. 2012;18:401–409. doi: 10.1093/molehr/gas012. [DOI] [PubMed] [Google Scholar]
- Chen CC, Hardy DB, Mendelson CR. Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1) J Biol Chem. 2011;286:43091–43102. doi: 10.1074/jbc.M111.295865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Ishida M, Matsuwaki T, Yamanouchi K, Nishihara M. Involvement of 20alpha-hydroxysteroid dehydrogenase in the maintenance of pregnancy in mice. J Reprod Dev. 2008;54:408–412. doi: 10.1262/jrd.20045. [DOI] [PubMed] [Google Scholar]
- Cretoiu SM, Cretoiu D, Marin A, Radu BM, Popescu LM. Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction. 2013;145:357–370. doi: 10.1530/REP-12-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol. 2015;54:R31–53. doi: 10.1530/JME-14-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egert D, Maass H. On the fate of progesterone in the uterus of pregnant and non-pregnant rats. Acta Endocrinol Suppl (Copenh) 1973;173:47. doi: 10.1530/acta.0.072s047. [DOI] [PubMed] [Google Scholar]
- Fiedler EP, Plouffe L, Jr, Hales DB, Hales KH, Khan I. Prostaglandin F(2alpha) induces a rapid decline in progesterone production and steroidogenic acute regulatory protein expression in isolated rat corpus luteum without altering messenger ribonucleic acid expression. Biol Reprod. 1999;61:643–650. doi: 10.1095/biolreprod61.3.643. [DOI] [PubMed] [Google Scholar]
- Gao L, Rabbitt EH, Condon JC, Renthal NE, Johnston JM, Mitsche MA, Chambon P, Xu J, O'Malley BW, Mendelson CR. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest. 2015;125:2808–2824. doi: 10.1172/JCI78544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Wang G, Liu WN, Kinser H, Franco HL, Mendelson CR. Reciprocal Feedback Between miR-181a and E2/ERalpha in Myometrium Enhances Inflammation Leading to Labor. J Clin Endocrinol Metab. 2016;101:3646–3656. doi: 10.1210/jc.2016-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Molecular and cellular biology. 2000;20:3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm SL, Hartig SM, Edwards DP. Progesterone Receptor Signaling Mechanisms. J Mol Biol. 2016;428:3831–3849. doi: 10.1016/j.jmb.2016.06.020. [DOI] [PubMed] [Google Scholar]
- Guo Y, Lang X, Lu Z, Wang J, Li T, Liao Y, Jia C, Zhao W, Fang H. MiR-10b Directly Targets ZEB1 and PIK3CA to Curb Adenomyotic Epithelial Cell Invasiveness via Upregulation of E-Cadherin and Inhibition of Akt Phosphorylation. Cell Physiol Biochem. 2015;35:2169–2180. doi: 10.1159/000374022. [DOI] [PubMed] [Google Scholar]
- Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol. 2003;23:1510–1520. doi: 10.1161/01.ATV.0000090130.85752.ED. [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- Hirabayashi K, Suzuki M, Takahashi M, Nishihara M. Expression of ovarian 20alpha-hydroxysteroid dehydrogenase in rat thymus. Endocrine journal. 2001;48:557–563. doi: 10.1507/endocrj.48.557. [DOI] [PubMed] [Google Scholar]
- Hsu KF, Pan HA, Hsu YY, Wu CM, Chung WJ, Huang SC. Enhanced myometrial autophagy in postpartum uterine involution. Taiwan J Obstet Gynecol. 2014;53:293–302. doi: 10.1016/j.tjog.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol. 2012;357:18–29. doi: 10.1016/j.mce.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyasuria P, Wetzel J, Bradley M, Subedi K, Condon JC. Progesterone-regulated caspase 3 action in the mouse may play a role in uterine quiescence during pregnancy through fragmentation of uterine myocyte contractile proteins. Biol Reprod. 2009;80:928–934. doi: 10.1095/biolreprod.108.070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YD, Fan F, McConkey DJ, Jean ME, Liu W, Reinmuth N, Stoeltzing O, Ahmad SA, Parikh AA, Mukaida N, Ellis LM. Role of P38 MAPK, AP-1, and NF-kappaB in interleukin-1beta-induced IL-8 expression in human vascular smooth muscle cells. Cytokine. 2002;18:206–213. doi: 10.1006/cyto.2002.1034. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271:6217–6224. doi: 10.1074/jbc.271.11.6217. [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T, Yoshimi M, Kadota Y, Inoue M, Sato M, Suzuki S. Prolonged endoplasmic reticulum stress alters placental morphology and causes low birth weight. Toxicol Appl Pharmacol. 2014;275:134–144. doi: 10.1016/j.taap.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Ke W, Chen C, Luo H, Tang J, Zhang Y, Gao W, Yang X, Tian Z, Chang Q, Liang Z. Histone Deacetylase 1 Regulates the Expression of Progesterone Receptor A During Human Parturition by Occupying the Progesterone Receptor A Promoter. Reprod Sci. 2016;23:955–964. doi: 10.1177/1933719115625848. [DOI] [PubMed] [Google Scholar]
- Khan JA, Amazit L, Bellance C, Guiochon-Mantel A, Lombes M, Loosfelt H. p38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization. Mol Endocrinol. 2011;25:1710–1724. doi: 10.1210/me.2011-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JA, Bellance C, Guiochon-Mantel A, Lombes M, Loosfelt H. Differential regulation of breast cancer-associated genes by progesterone receptor isoforms PRA and PRB in a new bi-inducible breast cancer cell line. PLoS One. 2012;7:e45993. doi: 10.1371/journal.pone.0045993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M. The unfolded protein response triggered by environmental factors. Semin Immunopathol. 2013;35:259–275. doi: 10.1007/s00281-013-0371-y. [DOI] [PubMed] [Google Scholar]
- Kondo S, Saito A, Asada R, Kanemoto S, Imaizumi K. Physiological unfolded protein response regulated by OASIS family members, transmembrane bZIP transcription factors. IUBMB Life. 2011;63:233–239. doi: 10.1002/iub.433. [DOI] [PubMed] [Google Scholar]
- Kyathanahalli C, Organ K, Moreci RS, Anamthathmakula P, Hassan SS, Caritis SN, Jeyasuria P, Condon JC. Uterine endoplasmic reticulum stress-unfolded protein response regulation of gestational length is caspase-3 and-7-dependent. Proc Natl Acad Sci U S A. 2015;112:14090–14095. doi: 10.1073/pnas.1518309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane B, Oxberry W, Mazella J, Tseng L. Decidualization of human endometrial stromal cells in vitro: effects of progestin and relaxin on the ultrastructure and production of decidual secretory proteins. Hum Reprod. 1994;9:259–266. doi: 10.1093/oxfordjournals.humrep.a138492. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Lian IA, Loset M, Mundal SB, Fenstad MH, Johnson MP, Eide IP, Bjorge L, Freed KA, Moses EK, Austgulen R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta. 2011;32:823–829. doi: 10.1016/j.placenta.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Barker G, Menon R, Lappas M. A Novel Role for SIRT3 in Regulating Mediators Involved in the Terminal Pathways of Human Labor and Delivery. Biol Reprod. 2016;95:95. doi: 10.1095/biolreprod.116.142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes & development. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mellor P, Deibert L, Calvert B, Bonham K, Carlsen SA, Anderson DH. CREB3L1 is a metastasis suppressor that represses expression of genes regulating metastasis, invasion, and angiogenesis. Molecular and cellular biology. 2013;33:4985–4995. doi: 10.1128/MCB.00959-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson CR, Montalbano AP, Gao L. Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Lye SJ. Differential expression of activator protein-1 transcription factors in pregnant rat myometrium. Biol Reprod. 2002;67:240–246. doi: 10.1095/biolreprod67.1.240. [DOI] [PubMed] [Google Scholar]
- Miyado M, Miyado K, Katsumi M, Saito K, Nakamura A, Shihara D, Ogata T, Fukami M. Parturition failure in mice lacking Mamld1. Sci Rep. 2015;5:14705. doi: 10.1038/srep14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Maruhashi M, Van De Putte T, Kondoh H, Huylebroeck D, Higashi Y. Complementary expression pattern of Zfhx1 genes Sip1 and deltaEF1 in the mouse embryo and their genetic interaction revealed by compound mutants. Dev Dyn. 2006;235:1941–1952. doi: 10.1002/dvdy.20799. [DOI] [PubMed] [Google Scholar]
- Moore RL, Faller DV. SIRT1 represses estrogen-signaling, ligand-independent ERalpha-mediated transcription, and cell proliferation in estrogen-responsive breast cells. J Endocrinol. 2013;216:273–285. doi: 10.1530/JOE-12-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, Saitoh M, Nishimura R, Yoneda T, Kou I, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Wanaka A, Imaizumi K. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun. 2016;7:11565. doi: 10.1038/ncomms11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navathe R, Berghella V. Progesterone as a tocolytic agent for preterm labor: a systematic review. Curr Opin Obstet Gynecol. 2016;28:464–469. doi: 10.1097/GCO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- Nishimura G, Manabe I, Tsushima K, Fujiu K, Oishi Y, Imai Y, Maemura K, Miyagishi M, Higashi Y, Kondoh H, Nagai R. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Developmental cell. 2006;11:93–104. doi: 10.1016/j.devcel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Norwitz ER, Caughey AB. Progesterone supplementation and the prevention of preterm birth. Rev Obstet Gynecol. 2011;4:60–72. [PMC free article] [PubMed] [Google Scholar]
- Pabona JM, Zhang D, Ginsburg DS, Simmen FA, Simmen RC. Prolonged pregnancy in women is associated with attenuated myometrial expression of progesterone receptor co-regulator Kruppel-like Factor 9. J Clin Endocrinol Metab. 2015;100:166–174. doi: 10.1210/jc.2014-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21:155–173. doi: 10.1093/humupd/dmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters GA, Yi L, Skomorovska-Prokvolit Y, Patel B, Amini P, Tan H, Mesiano S. Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells. Endocrinology. 2016 doi: 10.1210/en.2016-1537. en20161537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20alpha-hydroxysteroid dehydrogenase. Mol Endocrinol. 2005;19:431–440. doi: 10.1210/me.2004-0302. [DOI] [PubMed] [Google Scholar]
- Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci U S A. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal NE, Williams KC, Montalbano AP, Chen CC, Gao L, Mendelson CR. Molecular Regulation of Parturition: A Myometrial Perspective. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci. 2010;123:4251–4258. doi: 10.1242/jcs.073783. [DOI] [PubMed] [Google Scholar]
- Rubel CA, Wu SP, Lin L, Wang T, Lanz RB, Li X, Kommagani R, Franco HL, Camper SA, Tong Q, Jeong JW, Lydon JP, DeMayo FJ. A Gata2-Dependent Transcription Network Regulates Uterine Progesterone Responsiveness and Endometrial Function. Cell Rep. 2016;17:1414–1425. doi: 10.1016/j.celrep.2016.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shynlova O, Kwong R, Lye SJ. Mechanical stretch regulates hypertrophic phenotype of the myometrium during pregnancy. Reproduction. 2010;139:247–253. doi: 10.1530/REP-09-0260. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, Lye SJ. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod. 2006;74:839–849. doi: 10.1095/biolreprod.105.048124. [DOI] [PubMed] [Google Scholar]
- Stocco CO, Zhong L, Sugimoto Y, Ichikawa A, Lau LF, Gibori G. Prostaglandin F2alpha-induced expression of 20alpha-hydroxysteroid dehydrogenase involves the transcription factor NUR77. J Biol Chem. 2000;275:37202–37211. doi: 10.1074/jbc.M006016200. [DOI] [PubMed] [Google Scholar]
- Stratmann A, Haendler B. The histone demethylase JARID1A regulates progesterone receptor expression. FEBS J. 2011;278:1458–1469. doi: 10.1111/j.1742-4658.2011.08058.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- Szwarc MM, Kommagani R, Lessey BA, Lydon JP. The p160/steroid receptor coactivator family: potent arbiters of uterine physiology and dysfunction. Biol Reprod. 2014;91:122. doi: 10.1095/biolreprod.114.125021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Ji H, Liu H, Gu W, Li X, Peng T. Identification and functional analysis of microRNA in myometrium tissue from spontaneous preterm labor. Int J Clin Exp Pathol. 2015;8:12811–12819. [PMC free article] [PubMed] [Google Scholar]
- Tibbetts TA, Conneely OM, O'Malley BW. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol Reprod. 1999;60:1158–1165. doi: 10.1095/biolreprod60.5.1158. [DOI] [PubMed] [Google Scholar]
- Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev Biol. 1996;178:430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–787. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Stanczyk FZ, Novy MJ. Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab. 1984;58:629–639. doi: 10.1210/jcem-58-4-629. [DOI] [PubMed] [Google Scholar]
- Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci U S A. 2012a;109:7529–7534. doi: 10.1073/pnas.1200650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Renthal NE, Gerard RD, Mendelson CR. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol. 2012b;26:1857–1867. doi: 10.1210/me.2012-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, Leung S, Zhong Z, Zhao H, Sweitzer S, Considine T, Riera T, Suri V, White B, Ellis JL, Vlasuk GP, Loh C. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS One. 2012;7:e46364. doi: 10.1371/journal.pone.0046364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yore MA, Im D, Webb LK, Zhao Y, Chadwick JG, Jr, Molenda-Figueira HA, Haidacher SJ, Denner L, Tetel MJ. Steroid receptor coactivator-2 expression in brain and physical associations with steroid receptors. Neuroscience. 2010;169:1017–1028. doi: 10.1016/j.neuroscience.2010.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. The American journal of pathology. 2008;173:451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Velarde MC, Simmen FA, Simmen RC. Delayed parturition and altered myometrial progesterone receptor isoform A expression in mice null for Kruppel-like factor 9. Biol Reprod. 2008;78:1029–1037. doi: 10.1095/biolreprod.107.065821. [DOI] [PubMed] [Google Scholar]
- Zhang P, Sun Y, Ma L. ZEB1: at the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]


