Abstract
Certain cognitive deficits in schizophrenia appear to emerge from altered postnatal development of the dorsolateral prefrontal cortex (DLPFC). Dendritic spines on DLPFC layer 3 pyramidal cells are essential for certain cognitive functions, change in density over development, and are reduced in number in schizophrenia. Altered expression of molecular regulators of actin filament assembly and stability, which are essential for spine formation and maintenance, is thought to contribute to the pathogenesis of spine deficits in the disease. However, the normal developmental expression patterns of these molecular regulators of dendritic spines, which might provide insight into the timing of spine deficits in schizophrenia, are unknown. Therefore, we quantified the expression from birth to adulthood of key transcripts regulating dendritic spine density in monkey DLPFC. Layer 3 pyramidal cells, and tissue samples containing layers 3 or 6, were captured by laser microdissection and selected transcripts were quantified using PCR. In layer 3 pyramidal cells, the expression levels of most of the transcripts studied changed early, and not late, in postnatal development. These developmental shifts in expression were generally not detected in tissue homogenates of layers 3 or 6, suggesting that the changes may be enriched in layer 3 pyramidal cells. The timing of these shifts in expression suggests that early, rather than later, postnatal development may be a vulnerable period for layer 3 pyramidal neurons. Disruption of the normal developmental trajectories of these transcripts may contribute to layer 3 pyramidal neuron spine deficits in individuals who are later diagnosed with schizophrenia.
Keywords: CDC42, dendritic spines, development, layer 3, pyramidal cells, schizophrenia
Introduction
The disease process of schizophrenia appears to involve altered development of the dorsolateral prefrontal cortex (DLPFC) (Hoftman et al., 2017; Lewis and Levitt, 2002; Selemon and Zecevic, 2015). The DLPFC mediates certain cognitive functions, such as working memory, which improve substantially throughout postnatal development (Luna et al., 2010) and are impaired in individuals with schizophrenia (Kahn and Keefe, 2013). Thus, disturbances in the normal developmental trajectories of elements of DLPFC circuitry that are critical for working memory could give rise to its impairment in schizophrenia.
Pyramidal cells in DLPFC layer 3 play a critical role in working memory (Goldman-Rakic, 1995), and in schizophrenia, these neurons exhibit a lower density of dendritic spines (Garey et al., 1998; Glantz and Lewis, 2000; Konopaske et al., 2014). In contrast, pyramidal cells in layers 5 and 6 appear to have a normal complement of spines (Kolluri et al., 2005). The apparent specificity of the spine deficit to layer 3 pyramidal neurons is thought to be developmental in nature given the laminar differences in the developmental patterns of spine density, and of the excitatory synapses they receive, in both human (Huttenlocher, 1979; Petanjek et al., 2011) and non-human primate DLPFC (Anderson et al., 1995; Bianchi et al., 2013; Bourgeois et al., 1994). For example, the densities of dendritic spines and excitatory synapses on layer 3 pyramidal cells increase rapidly during the neonatal period, reaching a plateau in early childhood that persists until late childhood, followed by a protracted period of pruning over adolescence until stable adult levels are achieved (Anderson et al., 1995; Bourgeois et al., 1994; Petanjek et al., 2011). In contrast, DLPFC layers 5 and 6 exhibit a more modest or absent postnatal pruning of both excitatory synapses (Bourgeois et al., 1994) and dendritic spines (Petanjek et al., 2011).
Dendritic spine formation and maintenance is influenced by a number of gene products that regulate actin filament assembly and stability (Koleske, 2013). Indeed, the spine deficits on layer 3 pyramidal cells in schizophrenia appear to reflect altered expression of some of these gene products (Datta et al., 2015a; Hill et al., 2006; Ide and Lewis, 2010) (Fig. 1). For example, cell division cycle 42 (CDC42) is crucial for spinogenesis (Threadgill et al., 1997), whereas p21 activated serine/threonine kinase 1 (PAK1) regulates the stability of existing spines (Hayashi et al., 2004); expression of both of these transcripts is downregulated in layer 3 pyramidal cells in schizophrenia (Datta et al., 2015a). However, the relationship of the expression of these transcripts with changes in spine density over postnatal development is not clear. Moreover, prior work suggests that schizophrenia is associated with a blunting of normal developmental processes in the DLPFC (Fish et al., 2013; Hoftman et al., 2015; Hyde et al., 2011); yet it is not known if the spine deficits, and the altered expression of spine-related transcripts, reflect a blunting of spinogenesis early in development or an exaggerated pruning of spines during adolescence (McGlashan and Hoffman, 2000). Thus, understanding the normal developmental trajectories in the expression of these transcripts could inform the timing of their alterations, and when spine deficits arise, during the pathogenesis of schizophrenia.
Figure 1.
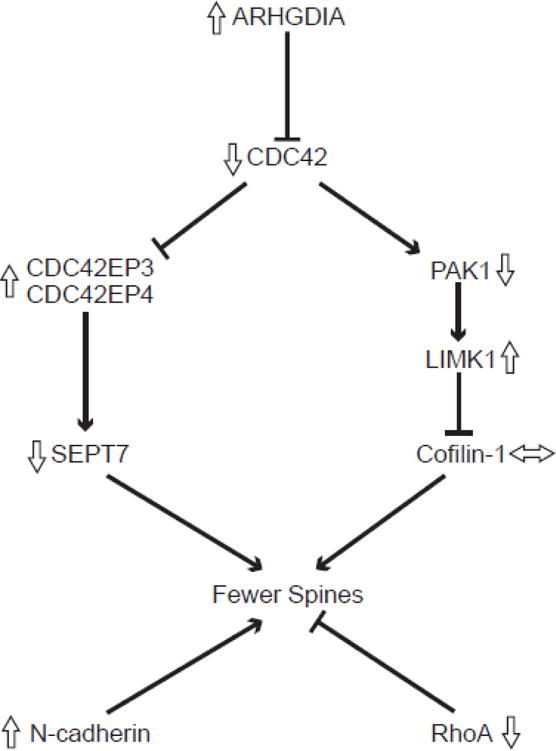
Signaling pathways for selected transcripts that regulate dendritic spine density. Lines with arrowheads indicate activation and those with blunted ends indicate inhibition. Open arrows indicate the direction of altered expression of each transcript in layer 3 pyramidal cells in schizophrenia (Arion et al., 2015; Datta et al., 2015a; Ide and Lewis, 2010). The protein products of these transcripts contribute to spine stability through regulation of the assembly and stability of actin filaments (Koleske, 2013). Rho GTPases are dendritically translated, are active in the spine, and have well elucidated roles in the morphogenesis and maintenance of dendritic spines, including molecular signaling cascades such as the cell division cycle 42 (CDC42) pathway (Tada and Sheng, 2006). In this pathway, CDC42 is negatively regulated by a Rho GDP dissociation inhibitor alpha (ARHGDIA) (Gorvel et al., 1998) and acts through the CDC42-p21-activated serine/threonine kinase (PAK)-LIM domain kinase (LIMK) pathway to promote spine stability by phosphorylating and deactivating the neuronal cofilin, cofilin-1, an actin severing protein (Bernstein and Bamburg, 2010). In a separate pathway, negative regulation by CDC42 on CDC42 effector proteins 3 and 4 (CDC42EP3 and CDC42EP4) promotes spine outgrowth by opening the barrier at the base of dendritic spine necks formed by septin molecules such as Septin-7 (SEPT7) and allowing influx of molecules important in spine growth into the spine head (Joberty et al., 2001). RhoA is another GTPase which has a negative influence on dendritic spine maintenance, contributing to spine loss (Tashiro et al., 2000). In addition to the role that GTPases play in dendritic spine maintenance, other molecules contribute to the stability of spines. For example, neuronal cadherin (N-cadherin) has been shown to contribute significantly to nascent synapse stabilization and subsequent spine formation (Takeichi, 2007).
To address these questions, we investigated the developmental trajectories of a subset of spine-related transcripts, which show altered expressed in schizophrenia (Fig. 1), selectively in layer 3 pyramidal cells from the DLPFC of macaque monkeys. Because these transcripts may be involved in other cytoskeletal processes that are not unique to dendritic spines in pyramidal cells, we studied the same transcripts in tissue homogenates of layer 3 and layer 6, which differ in the developmental trajectories of dendritic spines (Petanjek et al., 2011) and axospinous synapses (Bourgeois et al., 1994).
Methods
Animals and Tissue Preparation
Rhesus (Macaca mulatta) monkeys (n=20) ranging in age from 3 days postnatal to 12 years old, ages which include the period between early neonatal to mid-life in this species, were used (Table 1). All monkeys were female except for one 3-day old male. The animals were divided into 5 age groups (n=4 animals per group) based on previously identified inflection points in the developmental trajectory of spine density on layer 3 pyramidal cells in monkey DLPFC (Anderson et al., 1995): neonatal (3–8 days old), a period when the density of dendritic spines is rapidly increasing; infant (3 months), when spine density reaches a plateau; prepubertal (15–18 months), the end of the plateau period immediately prior to the onset of spine pruning during adolescence; postpubertal (43–48 months), following the end of spine pruning; and adult (8–12 years) when the density of dendritic spines is at stable, mature levels. The developmental trajectories of dendritic spines on layer 3 pyramidal cells are very similar in monkey (Anderson et al., 1995) and human (Petanjek et al., 2011) DLPFC. The inflection points in these trajectories were used to define age groups which were named based on the corresponding period in human development.
Table 1.
Rhesus monkeys used in study
| Age Group | Subject | Age (months) |
Sex | Perfusion Status | Biopsy | Weight (kg) |
Storage Time (months) |
|---|---|---|---|---|---|---|---|
| Neonatal | RH315 | 0.10 | M | − | − | 0.66 | 59 |
| RH311 | 0.23 | F | − | − | 0.66 | 64 | |
| RH285 | 0.27 | F | − | − | 0.62 | 108 | |
| RH331 | 0.27 | F | − | − | 0.55 | 14 | |
|
| |||||||
| Infant | RH310 | 3 | F | − | − | 1.14 | 65 |
| RH324 | 3 | F | − | − | 1.07 | 39 | |
| RH241 | 3 | F | + | + | 1.02 | 148 | |
| RH245 | 3 | F | + | + | 1.20 | 140 | |
|
| |||||||
| Prepubertal | RH264 | 15 | F | + | + | 2.50 | 126 |
| RH265 | 15 | F | + | + | 2.40 | 126 | |
| RH317 | 16 | F | − | − | 2.70 | 59 | |
| RH287 | 18 | F | − | − | 2.40 | 99 | |
|
| |||||||
| Postpubertal | RH239 | 43 | F | + | + | 5.50 | 148 |
| RH289 | 45 | F | − | − | 5.70 | 99 | |
| RH258 | 47 | F | + | + | 6.30 | 128 | |
| RH288 | 47 | F | − | − | 5.00 | 99 | |
|
| |||||||
| Adult | RH326 | 97 | F | − | − | 14.00 | 33 |
| RH259 | 104 | F | + | + | 6.4 | 127 | |
| RH260 | 138 | F | − | − | 9.5 | 127 | |
| RH354 | 155 | F | − | − | 7.7 | 5 | |
Monkeys younger than 6 months were housed with their mothers, juveniles 6–24 months were housed in groups, and those older than 24 months were housed either in pairs or in single cages in the same social setting. Seven animals were perfused transcardially with ice-cold artificial cerebrospinal fluid under deep anesthesia with ketamine and pentobarbital, as previously described (Gonzalez-Burgos et al., 2008). The remaining 13 monkeys were deeply anesthetized with ketamine and pentobarbital without transcardial perfusion. In all animals, the brain was removed intact and the right hemisphere was blocked, flash-frozen in isopentane, and stored at −80 °C. Housing and experimental procedures were conducted in accordance with the guidelines of the US Department of Agriculture and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
Laser Microdissection Procedure
Cryostat sections (12 μm thick) containing DLPFC areas 9 and 46 (Fig. 2A and B) were cut, thaw-mounted onto glass polyethylene naphthalate membrane slides, stored at −80 °C, and stained for Nissl substance using thionin on the day of microdissection as previously described (Datta et al., 2015b). Cortical layers were identified based on differences in the size and packing density of Nissl-stained cells (Fig. 2D). Individual layer 3 pyramidal cells, identified by the characteristic triangular morphology of the soma and a prominent apical dendrite projecting towards the pial surface (Fig. 2E), were microdissected using a Leica microdissection system with a 40× objective, as previously described (Datta et al., 2015b). Pyramidal cells were sampled throughout layer 3 of the DLPFC from the medial border of area 9 (i.e. the fundus of the cingulate sulcus) through the lateral border of area 46 (i.e. the ventral bank of the principal sulcus) (Fig 2B). For each monkey, 150 individual pyramidal cells from layer 3 were collected from each of 3 adjacent cryostat sections and the samples from each monkey were pooled (n=450 cells per monkey). To assess the cell type-specificity of our findings, samples of DLPFC layers 3 or 6 were each captured by laser microdissection (using a 5× objective) as a set of strips (total cross-sectional area ~7×106 μm2 per layer) (Fig. 2C). All samples were lysed in RLT Plus Buffer (QIAGEN) with β-mercaptoethanol, frozen and then RNA was extracted and purified using the RNeasy Plus Micro Kit (QIAGEN). Layer 6 was chosen for comparison to layer 3 due to the marked differences in the developmental trajectories of the dendritic spines on pyramidal neurons in these layers. In layer 3 of monkey DLPFC, the densities of both excitatory synapses and spines similarly increase during early development, plateau, and then decline during adolescence (Anderson et al., 1995; Bourgeois et al., 1994). In contrast, the density of excitatory synapses in layer 6 increases to a lesser degree during the early neonatal period, and then remains at a plateau into adulthood (Bourgeois et al., 1994).
Figure 2.
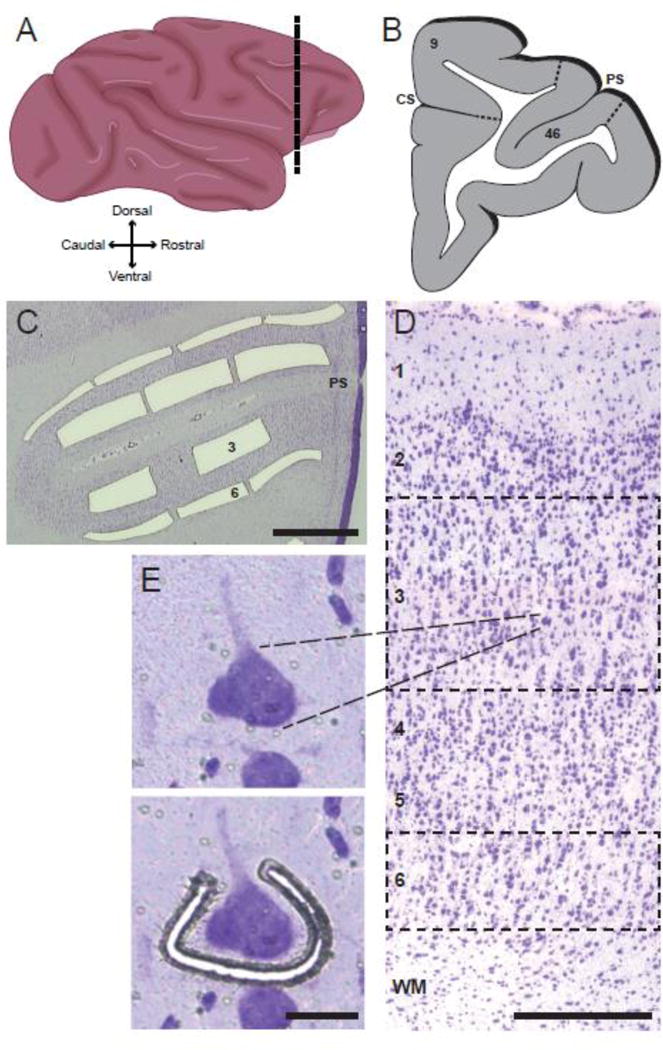
Laser microdissection approach for obtaining laminar and cellular samples for qPCR analyses. (A) Schematic drawing of a lateral view of the Rhesus monkey brain. Dashed line indicates the approximate location of the DLPFC regions studied. (B) Schematic drawing of a coronal section at the level of the dashed line in panel A indicating the locations of DLPFC areas 9 and 46 (PS = principal sulcus, CS = cingulate sulcus). (C) Nissl-stained section of the DLPFC at the level of the principal sulcus (PS) illustrating the laser microdissection of layers 3 and 6. Scale bar = 1.0 mm. (D) Lower power photomicrograph of a Nissl-stained section showing the location of each cortical layer; dashed lines illustrate the locations of samples dissected for laminar analysis of layers 3 and 6. Scale bar = 200 μm. (E) Higher magnification photomicrograph image of a layer 3 pyramidal cell before (top panel) and during (bottom panel) the process of laser microdissection. Scale bar = 25 μm.
Prior to the collection of cells for this study, the cell type-specific capture of pyramidal cells was assessed by comparing the expression of a pyramidal cell-specific marker, vesicular glutamate transporter 1 (vGlut1), with markers of oligodendrocytes (myelin basic protein, MBP) and GABAergic interneurons (vesicular GABA transporter, vGAT) in samples of layer 3 pyramidal cells and layer 3 homogenates by quantitative polymerase chain reaction (qPCR). The level of vGlut1 mRNA relative to vGAT mRNA was ~168-fold higher in layer 3 pyramidal cells compared to ~34-fold higher in layer 3 homogenates. Similarly, the level of vGlut1 mRNA to MBP mRNA was ~10-fold higher in layer 3 pyramidal cells compared to ~2-fold higher in layer 3 homogenates (data not shown). These results demonstrate that our dissection method resulted in an enriched collection of pyramidal cells with minimal contamination from other cell types.
Quantitative Polymerase Chain Reaction
Total RNA was converted to complementary DNA using the qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA). Forward and reverse primers were designed to target the mRNA transcripts of interest whose cognate proteins are known to be localized to dendritic spines and have known roles in regulating dendritic spine density. These transcripts have been previously reported to show upregulated (CDC42EP3, CDC42EP4, LIMK1, N-cadherin, ARHGDIA), downregulated (CDC42, PAK1, SEPT7, RhoA), or unchanged (cofilin-1) expression in DLPFC layer 3 pyramidal cells in schizophrenia (Arion et al., 2015; Datta et al., 2015a) (Fig. 1). Although some of the transcripts of interest have multiple isoforms, the amount of cDNA available from the cell collection was sufficient only for the study of representative isoforms. Consequently, we chose LIMK1 because of its well-studied role in dendritic spine stability, and because deletion of LIMK2 is insufficient to cause spine deficits (Ohashi, 2015). Similarly, we focused on PAK1 because it is localized to dendritic spines and deletion of PAK1 disrupts dendritic spine stability (Hayashi et al., 2007). All primers showed 90–100% efficiency, and each amplified product resulted in a single and specific amplicon (Supplementary Table 1). Two housekeeping genes (β-actin and cyclophilin-A), selected based on their stable expression across development in this species and in this cell type (Datta et al., 2015b; Hoftman et al., 2015), were used to normalize the target gene expression levels.
Levels of each transcript were assessed by qPCR using Power SYBR Green fluorescence and the ViiA™7 Real Time PCR System. Cycle threshold (CT) values were assessed for the normalizers and each gene of interest in quadruplicate, and the delta CT (dCT) for each target transcript was calculated by subtracting the mean CT of the two normalizer genes from the CT of the gene of interest. Because the dCT represents the log2-transformed expression ratio of each target transcript to the mean of the normalizer genes, the relative expression levels of the target transcripts are reported as the more intuitive expression ratio, or the 2−dCT.
Statistical Analysis
Differences between age groups were analyzed using a one-way ANOVA. An analysis was performed including storage time as a covariate. For each transcript studied, the effect of storage time was not significant (all F4,15<3.1, p>0.1) for samples of layer 3 pyramidal cells, layer 3 tissue homogenates, or layer 6 tissue homogenates. Consistent with these findings, tissue storage time did not significantly affect levels of these transcripts in a prior study of postmortem human brain tissue (Datta et al., 2015a). In the design of the experiment, we attempted to control for perfusion status by including 2 perfused and 2 unperfused animals in the infant, prepubertal, and postpubertal age groups. Tukey’s post-hoc test was used for comparisons between age groups, with significance set to α=0.05.
Results
Developmental Trajectories of Transcripts Downregulated in Schizophrenia
In monkey DLPFC, CDC42 expression (Fig. 3A) showed a significant downregulation with age in layer 3 pyramidal cells (F4,15=13.07, p<0.0001), with the greatest decline between the neonatal to prepubertal age groups (27.8% decrease, p=0.002). In contrast, no age-related differences in CDC42 mRNA levels were detected in layer 3 (F4,15=1.79, p=0.184) or 6 (F4,15=1.24, p=0.335) tissue homogenates.
Figure 3.
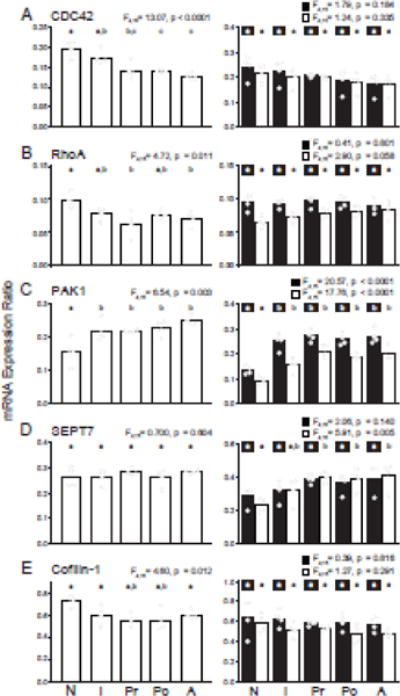
Developmental trajectories of the expression of transcripts which are downregulated [(A) CDC42, (B) RhoA, (C) PAK1, (D) SEPT7] or unchanged [(E) cofilin-1] in the DLPFC in schizophrenia. Bar graphs demonstrate the mean differences between age groups and individual animal data within each age group are plotted (N: neonatal, I: infant, Pr: prepubertal, Po: postpubertal, A: adult). Within each graph, bars not sharing the same letter are significantly different from each other. Panels in the left column show data from layer 3 pyramidal cells and panels in the right column show data from layer 3 (solid bars) and 6 (open bars) tissue homogenates for a given transcript.
Expression of RhoA (Fig. 3B) was also downregulated across development in layer 3 pyramidal cells (F4,15=4.72, p=0.011), with the greatest decline between the neonatal and prepubertal age groups (36.7% decrease, p=0.007). In contrast, RhoA mRNA levels did not change with development in layer 3 (F4,15=0.41, p=0.801) or 6 (F4,15=2.90, p=0.058) tissue homogenates.
In contrast to CDC42 and RhoA, PAK1 (Fig. 3C) levels increased with age (F4,15=6.54, p=0.003), and post hoc analysis showed an increase in expression only between the neonatal and infant age groups (37.8% increase, p=0.041). Levels of PAK1 mRNA also showed an upregulation of expression with age in both layers 3 (F4,15=20.57, p<0.0001) and 6 (F4,15=17.76, p<0.0001) tissue homogenates, but again only between the neonatal and infant age groups.
Expression of SEPT7 (Fig. 3D) did not differ with age in either layer 3 pyramidal cells (F4,15=0.700, p=0.604) or layer 3 tissue homogenates (F4,14=2.06, p=0.140), but did increase with age in layer 6 tissue homogenates (F4,15=5.91, p=0.005). The largest increase in layer 6 tissue homogenates occurred between the neonatal and prepubertal age groups (74.5% increase, p=0.011).
Developmental Trajectories of a Transcript Unchanged in Schizophrenia
Cofilin-1 (Fig. 3E) decreased with age in layer 3 pyramidal cells (F4,15=4.60, p=0.012), with the greatest decline between the neonatal and prepubertal age groups (24.4% decrease, p=0.015). No developmental changes were seen in layer 3 (F4,15 = 0.39, p=0.816) or 6 (F4,15=1.27, p=0.291) tissue homogenates.
Developmental Trajectories of Transcripts Upregulated in Schizophrenia
In monkey DLPFC, CDC42EP3 (Fig. 4A) was downregulated with age in layer 3 pyramidal cells (F4,15=7.02, p=0.002), with the greatest difference occurring between the neonatal and infant age groups (46.0% decrease, p = 0.004). In contrast, CDC42EP3 levels did not differ across groups in layer 3 (F4,15=1.97, p=0.152) or 6 (F4,15=2.78, p=0.065) tissue homogenates.
Figure 4.
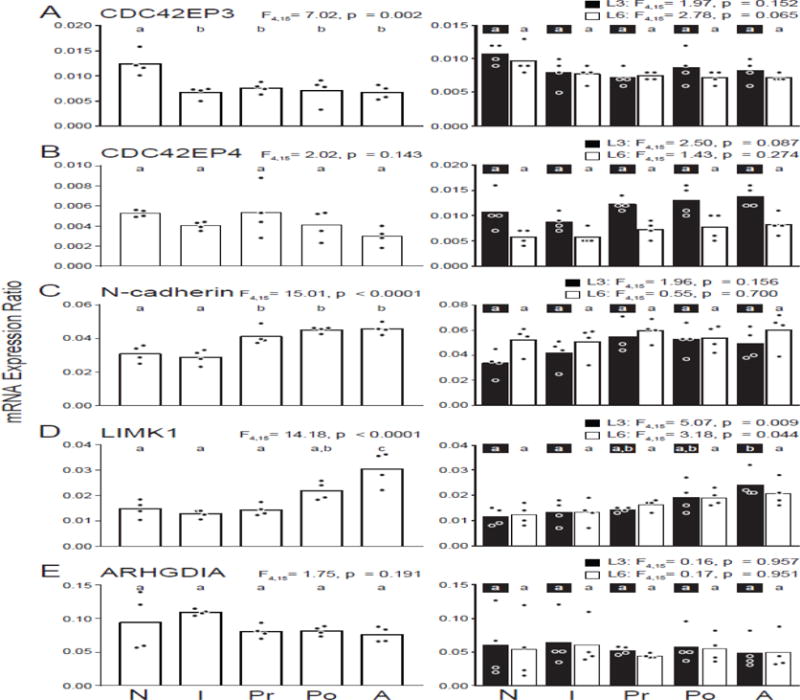
Developmental trajectories of the expression of transcripts that are upregulated [(A) CDC42EP3, (B) CDC42EP4, (C) N-cadherin, (D) LIMK1, (E) ARHGDIA] in the DLPFC in schizophrenia. Bar graphs demonstrate the mean differences between age groups and individual animal data within each age group are plotted (N: neonatal, I: infant, Pr: prepubertal, Po: postpubertal, A: adult). Within each graph, bars not sharing the same letter are significantly different from each other. Panels in the left column show data from layer 3 pyramidal cells and panels in the right column show data from layer 3 (solid bars) and 6 (open bars) tissue homogenates for a given transcript.
CDC42EP4 (Fig. 4B) showed a similar downward trajectory across development in layer 3 pyramidal cells, although these changes did not reach significance (F4,15=2.02, p=0.143). No significant differences were observed in either layer 3 (F4,15=2.50, p=0.087) or 6 (F4,15=1.43, p=0.274) tissue homogenates.
In contrast, N-cadherin (Fig. 4C) showed an upregulation with age in layer 3 pyramidal cells (F4,15=15.01, p<0.0001), and post hoc analysis revealed changes only between the infant and prepubertal age groups (40.8% increase, p=0.005). In contrast, tissue homogenates of layer 3 (F4,14=1.96, p=0.156) or layer 6 (F4,15=0.55, p=0.700) did not differ with age.
LIMK1 (Fig. 4D) was the only transcript to show a marked upregulation later in development (F4,15=14.18, p<0.0001), with the greatest change between the postpubertal and adult age groups (39.6% increase, p=0.049). Both layer 3 (F4,15=5.07, p=0.009) and layer 6 (F4,15=3.18, p=0.044) tissue homogenates showed a similar upregulation in LIMK1 expression only in the older age groups.
Finally, ARHGDIA (Fig. 4E) did not change with age in layer 3 pyramidal cells (F4,15=1.75, p=0.191), layer 3 tissue homogenates (F4,15=0.16, p=0.957) or layer 6 tissue homogenates (F4,15=0.17, p=0.951).
Specificity of Developmental Expression Changes to Layer 3 Pyramidal Cells
In layer 3 pyramidal cells, levels of 6 transcripts significantly differed between neonatal and adult animals (Table 2). However, only 2 of these transcripts (PAK1 and LIMK1) showed a similar significant difference between these age groups in layer 3 tissue homogenates, and only PAK1 and SEPT7 significantly differed with age in layer 6 tissue homogenates (Table 2). These comparisons suggest that developmental shifts in the expression of some gene products regulating dendritic spines are especially prominent in layer 3 pyramidal neurons relative to other cell types.
Table 2.
Summary of changes across development between the neonatal and adult age groups for each of the three samples.
| Transcript | Layer 3 Pyramidal Cells | Layer 3 | Layer 6 | ||||
|---|---|---|---|---|---|---|---|
| % Change from Neonatal to Adult Age Groups | p-value | Age Group when Adult Levels Reached | % Change from Neonatal to Adult Age Groups | p-value | % Change from Neonatal to Adult Age Groups | p-value | |
|
|
|
|
|||||
| CDC42 | −35.1% | <0.001 | Prepubertal | −28.0% | 0.178 | −21.5% | 0.32 |
| CDC42EP3 | −46.0% | 0.004 | Infant | −22.4% | 0.368 | −27.3% | 0.085 |
| Cofilin | −17.9% | 0.1 | Infant | −11.4% | 0.843 | −16.2% | 0.372 |
| RhoA | −28.6% | 0.03 | Infant | −5.5% | 0.93 | 29.0% | 0.069 |
| PAK1 | 58.1% | 0.002 | Infant | 99.3% | <0.001 | 111.8% | <0.001 |
| N-cadherin | 57.3% | 0.001 | Prepubertal | 46.1% | 0.349 | 15.2% | 0.871 |
| LIMK1 | 107.5% | <0.001 | Adult | 107.8% | 0.011 | 69.7% | 0.064 |
| CDC42EP4 | −43.6% | 0.186 | Neonatal | 27.9% | 0.471 | 45.4% | 0.391 |
| ARHGDIA | −19.2% | 0.721 | Neonatal | −19.4% | 0.984 | −7.9% | 1 |
| SEPT7 | 8.2% | 0.806 | Neonatal | 38.5% | 0.422 | 77.1% | 0.008 |
Discussion
In schizophrenia, DLPFC layer 3 pyramidal cells have lower spine densities (Glausier and Lewis, 2013) and show altered expression of multiple transcripts whose protein products contribute to the regulation of dendritic spine density (Arion et al., 2015; Datta et al., 2017, 2015a). In this study, we examined the developmental trajectories of these transcripts in a cell type- and layer-specific fashion in monkey DLPFC. Three main findings emerged. First, in layer 3 pyramidal cells, the expression of many of the transcripts examined (cofilin-1, RhoA, CDC42, N-cadherin, CDC42EP3, and PAK1) changed early in development, whereas only one transcript (LIMK1) changed late in development. Second, some transcripts were upregulated with age (PAK1, N-cadherin, and LIMK1), whereas others were downregulated with age (CDC42, RhoA, cofilin-1, and CDC42EP3) (Fig. 5). Third, the changes present in layer 3 pyramidal cells were, with two exceptions (PAK1 and LIMK1), not observed in either layer 3 or 6 tissue homogenates, suggesting that many of the developmental transcript changes may be specific to or enriched in layer 3 pyramidal cells.
Figure 5.
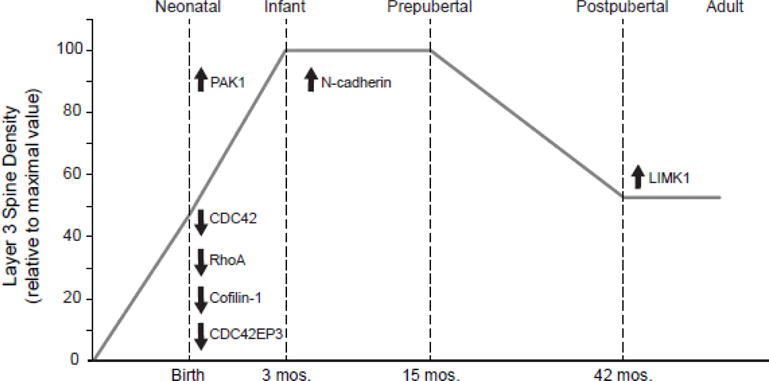
Summary of the timing and direction of change in mRNA expression in layer 3 pyramidal cells during development of the monkey DLPFC. Transcripts are shown in the context of the developmental trajectory of dendritic spine density in layer 3 of macaque monkey DLPFC. Note that most of the transcripts examined exhibited shifts in expression during early postnatal development.
Relationship between Transcript Expression and Dendritic Spine Density over Postnatal Development
In monkey and human DLPFC, layer 3 pyramidal cells exhibit characteristic developmental changes in spine density (Anderson et al., 1995; Petanjek et al., 2011). In macaque monkeys, spine density increases rapidly from birth to ~3 months of age, remains at an elevated plateau until ~15 months of age, and then declines during adolescence until stable adult levels are achieved at ~45 months of age (Anderson et al., 1995). Here we found that these changes in spine density may reflect shifts in the expression of different molecular regulators of dendritic spines during discrete time periods of postnatal development (Fig. 5).
The rapid increase in spines during early postnatal development appears to be a continuation of the exuberant spinogenesis occurring during the late prenatal period in layer 3 pyramidal cells (Bourgeois et al., 1994). In the present study, CDC42, CDC42EP3, and, to a smaller degree, CDC42EP4 transcripts were most highly expressed in neonatal animals. Expression levels declined between the neonatal and infant age groups, suggesting that these gene products play a more significant role in spinogenesis than in the maintenance of existing spines. Consistent with this interpretation, CDC42 induces rapid formation of filopodia, the precursors of dendritic spines (Nobes and Hall, 1995), and suppression of CDC42 inhibits endogenous spine formation (Irie and Yamaguchi, 2002). However, neither the activation nor suppression of CDC42 alters the morphology or density of existing spines (Govek et al., 2004; Tashiro et al., 2000). Similarly, expression of CDC42EP3 and CDC42EP4, the downstream effectors of CDC42, induce the formation of pseudopodia rich in F-actin (Hirsch et al., 2001).
In contrast, PAK1 expression increased between the neonatal and infant age groups, when spine number is markedly increasing. Other labs also report a similar increase in PAK1 mRNA and protein in developing mouse cortex (Hayashi et al., 2007). This relationship might be consistent with findings that upregulation of PAK1 in developing neurons causes thinner and more numerous spines (Hayashi et al., 2007). However, PAK1 also appears to contribute to the maintenance of existing spines, as constitutively active or dominant negative PAK1 alters spine morphology in mature neurons (Hayashi 2004, 2007). This latter role of PAK1 might explain its continued high levels of expression into adulthood.
RhoA and cofilin-1 were also most highly expressed in the neonatal animals and their expression levels decreased between the neonatal and infant age groups, remaining stable thereafter. Both transcripts have roles in destabilizing F-actin and the resulting spine loss. Inhibition of RhoA activity caused an increase in spine density, whereas constitutively active RhoA resulted in large decreases or absence of spines on some dendrites (Tashiro et al., 2000). Similarly, constitutively active cofilin-1 led to an immature spine profile, while constitutively inactive cofilin-1 resulted in greater spine density and a more mature spine profile (Rust, 2015). Therefore, we did not expect to find that both these transcripts were most highly expressed during the period of rapid spinogenesis. Interestingly, although low concentrations of cofilin-1 promote disassembly of actin, high concentrations instead favor assembly of large numbers of actin filaments (Andrianantoandro and Pollard, 2006). Thus, the high expression of cofilin-1 during the neonatal period may lead to actin assembly and dendritic spine growth, rather than disassembly of actin. Furthermore, the normal increase in spine volume by uncaging of glutamate onto single spines is prevented by inhibiting RhoA (Murakoshi et al., 2011), suggesting that RhoA may be crucial for dendritic spine growth in response to activation. Thus, the high expression of these transcripts early in development may aid in spinogenesis.
The developmental period between infancy and puberty is marked by a stable density of dendritic spines. During this period, expression of N-cadherin increases, just prior to the onset of massive spine elimination. Competition between spines for N-cadherin molecules is critical for dendritic spine pruning (Bian et al., 2015); therefore, expression of N-cadherin may increase just prior to puberty to mediate spine pruning and the concurrent maturation of remaining spines.
After the pubertal period and throughout adulthood, the density of dendritic spines remains stable. LIMK1 was the only transcript studied to change in expression between postpubertal and adult animals. LIMK1 is dendritically translated (George et al., 2015) and contributes to long-term dendritic spine stability through inhibition of cofilin-1 (Arber et al., 1998). Therefore, its higher levels of expression during adulthood may aid in the relative stability of dendritic spines after puberty. Consistent with this idea, deletion of LIMK1 results in most spines exhibiting an immature spine profile (Meng et al., 2002).
In concert, these findings indicate that many spine-related transcripts in layer 3 pyramidal cells exhibit specific patterns of changes in expression during certain periods of postnatal development that could contribute to the distinctive developmental trajectories of spine density on these neurons (Fig. 5).
Implications for the Developmental Blunting Hypothesis of Schizophrenia
Prior work suggested that a blunting of the normal developmental trajectories of inhibitory components of DLPFC circuitry could give rise to the cognitive deficits characteristic of schizophrenia (Fish et al., 2013; Hoftman et al., 2015; Hyde et al., 2011). In these studies, markers of the GABA system that showed increases or declines across development were found to be lower or higher, respectively, in schizophrenia. These findings suggest that normal developmental trajectories might be blunted or arrested in individuals with schizophrenia. Consistent with this hypothesis, two of the transcripts studied here (CDC42EP3 and PAK1) were downregulated over development and upregulated in schizophrenia. However, the five other transcripts that showed postnatal changes in expression were changed in the same direction as in the illness, suggesting that developmental expression of these transcripts might be exaggerated, rather than blunted, in schizophrenia. These differences between spine-related transcripts and GABA-related transcripts suggest that the developmental blunting hypothesis of schizophrenia might apply to the linear changes with age seen with GABA system markers (Fung et al., 2010; Hoftman et al., 2015), but not to markers of pyramidal cell dendritic spines which show a more complex pattern of change with age (Fig. 5). Furthermore, developmental changes in cortical GABA system transcripts occur over a protracted period, continuing through adolescence (Caballero and Tseng, 2016; Gonzalez-Burgos et al., 2015). In contrast, most of the pyramidal cell spine-related transcripts examined here stabilized very early in development, as do other markers (Datta et al., 2015b) or measures (Gonzalez-Burgos et al., 2008) of excitatory neurotransmission. Moreover, a recent study of the developing primate transcriptome seems to support the idea that transcripts associated with synapse and dendrite development are enriched earlier in development (Bakken et al., 2016). Thus, the developmental blunting of the normal development of GABA signaling may be an event that occurs later in development during the pathogenesis of schizophrenia.
Implications for the Developmental Nature of the Spine Deficit in Schizophrenia
The deficit in dendritic spines (and presumably the excitatory synapses that they receive) has been suggested to reflect either an early developmental failure in spine production or an excessive pruning of spines during adolescence (Feinberg, 1982; McGlashan and Hoffman, 2000; Weinberger, 1987). Our finding of a predominance of spine-related transcript changes early in postnatal development might be consistent with the view that schizophrenia involves a genetically-driven disturbances in spinogenesis, an interpretation supported by findings that certain cognitive deficits are evident during childhood in individuals later diagnosed with schizophrenia (Reichenberg et al., 2010). Consistent with this idea, genetic variants in regulators of dendritic spines are risk factors for schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014) and de novo mutations associated with schizophrenia are overrepresented at loci containing genes whose products regulate actin filament dynamics (Fromer et al., 2014). Of course, it is possible that alterations in both the regulation of spinogenesis and spine pruning are required for the emergence of the clinical features of schizophrenia.
Limitations
Interpretation of the results of the present study need to take into account the following limitations. First, we focused on a select number of transcripts that are known both to regulate dendritic spines and to exhibit altered expression in DLPFC layer 3 pyramidal neurons in schizophrenia. However, multiple other gene products regulate dendritic spines, and thus future studies of their developmental trajectories and expression patterns in schizophrenia provide opportunity to test the interpretations of the present study. Second, except for one male, all of the animals used in this study were female. Given that males tend to have an earlier onset of schizophrenia than females (Szymanski et al., 1995), it will be interesting to investigate whether sex differences exist in the timing of spine pruning or the molecular regulators of pruning. Third, although the developmental trajectories of the macaque monkey and human DLPFC are similar in multiple respects, additional studies are needed to confirm if the findings of the present study hold true for developing human DLPFC. Finally, this study focused on alterations in mRNA transcripts that regulate dendritic spines, and not the cognate proteins. Understanding how these proteins may be changing within dendritic spines themselves over development requires further investigation.
Conclusions
In summary, our findings reveal changes over postnatal development in the expression of markers of the CDC42 signaling pathway and other regulators of dendritic spine formation and stability in the monkey DLPFC. These changes occur in a manner that appears to be enriched in layer 3 pyramidal cells (as such changes were much less common in tissue homogenates of layer 3 or layer 6), consistent with the more pronounced developmental changes in dendritic spine density on layer 3 pyramidal cells relative to those in deeper cortical layers. The finding that these expression changes are most common in early postnatal development suggests that the schizophrenia-associated changes in these transcripts might occur during that same developmental period and thus the spine deficits on DLPFC layer 3 pyramidal cells in schizophrenia may arise due a disturbance in spinogenesis. Further testing of this idea will require experimental manipulations of these developmental patterns of expression.
Supplementary Material
Highlights.
Expression of transcripts regulating dendritic spines change early in development.
These changes are enriched in layer 3 pyramidal cells.
Alterations in these early changes may contribute to the pathogenesis of schizophrenia.
Acknowledgments
We are grateful to Kelly Rogers and Mary Brady for their excellent technical assistance. This work was supported by the National Institute of General Medical Sciences Training Grant (GM08208 to S.J.D) and the National Institute of Health Grant (MH051234 to D.A.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Lewis currently receives grant/research support from Pfizer. All other authors have declared no conflicts of interest.
References
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous Development of Pyramidal Neuron Dendritic Spines and Parvalbumin-Immunoreactive Chandelier Neuron Axon Terminals in Layer III of Monkey Prefrontal Cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Andrianantoandro E, Pollard TD. Mechanism of Actin Filament Turnover by Severing and Nucleation at Different Concentrations of ADF/Cofilin. Mol Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A, Cacace AM, Zaczek R, Albright CF, Tseng G, Lewis DA. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015;20:1397–1405. doi: 10.1038/mp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken TE, Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Dalley RA, Royall JJ, Lemon T, Shapouri S, Aiona K, Arnold J, Bennett JL, Bertagnolli D, Bickley K, Boe A, Brouner K, Butler S, Byrnes E, Caldejon S, Carey A, Cate S, Chapin M, Chen J, Dee N, Desta T, Dolbeare TA, Dotson N, Ebbert A, Fulfs E, Gee G, Gilbert TL, Goldy J, Gourley L, Gregor B, Gu G, Hall J, Haradon Z, Haynor DR, Hejazinia N, Hoerder-Suabedissen A, Howard R, Jochim J, Kinnunen M, Kriedberg A, Kuan CL, Lau C, Lee ChK, Lee F, Luong L, Mastan N, May R, Melchor J, Mosqueda N, Mott E, Ngo K, Nyhus J, Oldre A, Olson E, Parente J, Parker PD, Parry S, Pendergraft J, Potekhina L, Reding M, Riley ZL, Roberts T, Rogers Br, Roll K, Rosen D, Sandman D, Sarreal M, Shapovalova N, Shi S, Sjoquist N, Sodt AJ, Townsend R, Velasquez L, Wagley U, Wakeman WB, White C, Bennett C, Wu J, Young R, Youngstrom BL, Wohnoutka P, Gibbs RA, Rogers J, Hohmannn JG, Hawrylycz MJ, Hevner RF, Molnar Z, Phillips JW, Dang C, Jones AR, Amaral DG, Bernard A, Lein ES. A comprehensive transcriptional map of primate brain development. Nature. 2016;535:367–375. doi: 10.1038/nature18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. ADF/Cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian WJ, Miao WY, He SJ, Qiu Z, Yu X. Coordinated Spine Pruning and Maturation Mediated by Inter-Spine Competition for Cadherin/Catenin Complexes. Cell. 2015;162:808–822. doi: 10.1016/j.cell.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Bianchi S, Stimpson CD, Duka T, Larsen MD, Janssen WGM, Collins Z, Bauernfeind AL, Schapiro SJ, Baze WB, McArthur MJ, Hopkins WD, Wildman DE, Lipovich L, Kuzawa CW, Jacobs B, Hof PR, Sherwood CC. Synaptogenesis and development of pyramidal neuron dendritic morphology in the chimpanzee neocortex resembles humans. Proc Natl Acad Sci USA. 2013;110:10395–10401. doi: 10.1073/pnas.1301224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the Prefrontal Cortex of Rhesus Monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Caballero A, Tseng KY. GABAergic Function as a Limiting Factor for Prefrontal Maturation during Adolescence. Trends Neurosci. 2016;39:441–448. doi: 10.1016/j.tins.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Arion D, Corradi JP, Lewis DA. Altered Expression of CDC42 Signaling Pathway Components in Cortical Layer 3 Pyramidal Cells in Schizophrenia. Biol Psychiatry. 2015a;78:775–785. doi: 10.1016/j.biopsych.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Arion D, Lewis DA. Developmental Expression Patterns of GABAA Receptor Subunits in Layer 3 and 5 Pyramidal Cells of Monkey Prefrontal Cortex. Cereb Cortex. 2015b;25:2295–2305. doi: 10.1093/cercor/bhu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Arion D, Roman KM, Volk DW, Lewis DA. Altered Expression of ARP2/3 Complex Signaling Pathway Genes in Prefrontal Layer 3 Pyramidal Cells in Schizophrenia. Am J Psychiatry. 2017;174:163–171. doi: 10.1176/appi.ajp.2016.16020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: Caused by a Fault in Programmed Synaptic Elimination during Adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA. Parvalbumin-Containing Chandelier and Basket Cell Boutons Have Distinctive Modes of Maturation in Monkey Prefrontal Cortex. J Neurosci. 2013;33:8352–8358. doi: 10.1523/JNEUROSCI.0306-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O’Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Soares C, Montersino A, Beique JC, Thomas GM. Palmitoylation of LIM Kinase-1 ensures Spine-Specific actin polymerization and morphological plasticity. Elife. 2015;4:e06327. doi: 10.7554/eLife.06327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased Dendritic Spine Density on Prefrontal Cortical Pyramidal Neurons in Schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic Spine Pathology in Schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular Basis of Working Memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional Maturation of Excitatory Synapses in Layer 3 Pyramidal Neurons During Postnatal Development of the Primate Prefrontal Cortex. Cereb Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Miyamae T, Pafundo DE, Yoshino H, Rotaru DC, Hoftman G, Datta D, Zhang Y, Hammond M, Sampson AR, Fish KN, Ermentrout GB, Lewis DA. Functional Maturation of GABA Synapses During Postnatal Development of the Monkey Dorsolateral Prefrontal Cortex. Cereb Cortex. 2015;25:4076–4093. doi: 10.1093/cercor/bhu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Chang TC, Boretto J, Azuma T, Chavrier P. Differential properties of D4/LyGDI versus RhoGDI: Phosphorylation and rho GTPase selectivity. FEBS Lett. 1998;422:269–273. doi: 10.1016/s0014-5793(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Akerman CJ, Cross JR, Van der Veken L, Van Aelst L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat Neurosci. 2004;7:364–372. doi: 10.1038/nn1210. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohshima T, Hashimoto M, Mikoshiba K. Pak1 Regulates Dendritic Branching and Spine Formation. Dev Neurobiol. 2007;67:655–669. doi: 10.1002/dneu.20363. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S. Altered Cortical Synaptic Morphology and Impaired Memory Consolidation in Forebrain-Specific Dominant-Negative PAK Transgenic Mice. Neuron. 2004;42:773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hirsch DS, Pirone DM, Burbelo PD. A New Family of Cdc42 Effector Proteins, CEPs, Function in Fibroblast and Epithelial Cell Shape Changes. J Biol Chem. 2001;276:875–883. doi: 10.1074/jbc.M007039200. [DOI] [PubMed] [Google Scholar]
- Hoftman GD, Datta D, Lewis DA. Layer 3 Excitatory and Inhibitory Circuitry in the Prefrontal Cortex: Developmental Trajectories and Alterations in Schizophrenia. Biol Psychiatry. 2017;81:862–873. doi: 10.1016/j.biopsych.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered Cortical Expression of GABA-Related Genes in Schizophrenia: Illness Progression vs Developmental Disturbance. Schizophr Bull. 2015;41:180–191. doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic Density in Human Frontal Cortex - Developmental Changes and Effects of Aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE. Expression of GABA Signaling Molecules KCC2, NKCC1, and GAD1 in Cortical Development and Schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide M, Lewis DA. Altered Cortical CDC42 Signaling Pathways in Schizophrenia: Implications for Dendritic Spine Deficits. Biol Psychiatry. 2010;68:25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42, and N-WASP. Nat Neurosci. 2002;5:1117–1118. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- Joberty G, Perlungher RR, Sheffield PJ, Kinoshita M, Noda M, Haystead T, Macara IG. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat Cell Biol. 2001;3:861–866. doi: 10.1038/ncb1001-861. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Keefe RSE. Schizophrenia Is a Cognitive Illness: Time for a Change in Focus. JAMA Psychiatry. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14:536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-Specific Reductions in Dendritic Spine Density in the Prefrontal Cortex of Subjects with Schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal Cortical Dendritic Spine Pathology in Schizophrenia and Bipolar Disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a Disorder of Neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a Disorder of Developmentally Reduced Synaptic Connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, Jia Z. Abnormal Spine Morphology and Enhanced LTP in LIMK-1 Knockout Mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases Regulate the Assembly of Multimolecular Focal Complexes Associated with Actin Stress Fibers, Lamellipodia, and Filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Ohashi K. Roles of cofilin in development and its mechanisms of regulation. Dev Growth Differ. 2015;57:275–290. doi: 10.1111/dgd.12213. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RSE, Murray RM, Poulton R, Moffitt TE. Static and Dynamic Cognitive Deficits in Childhood Preceding Adult Schizophrenia: A 30-Year Study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust MB. ADF/cofilin: a crucial regulator of synapse physiology and behavior. Cell Mol Life Sci. 2015;72:3521–3529. doi: 10.1007/s00018-015-1941-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Zecevic N. Schizophrenia : a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. doi: 10.1038/tp.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski S, Lieberman JA, Alvir JM, Mayerhoff D, Loebel A, Geisler S, Chakos M, Koreen A, Jody D, Kane J, Woerner M, Cooper T. Gender Differences in Onset of Illness, Treatment Response, Course, and Biologic Indexes in First-Episode Schizophrenic Patients. Am J Psychiatry. 1995;152:698–703. doi: 10.1176/ajp.152.5.698. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of Dendritic Spine Morphology by the Rho family of Small GTPases: Antagonistic Roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of Normal Brain Development for the Pathogenesis of Schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


