Abstract
High-density lipoproteins (HDL) are important endogenous inhibitors of inflammatory responses. Functional impairment of HDL might contribute to the excess mortality experienced by patients with liver disease, but the effect of cirrhosis on HDL metabolism and function remain elusive. To get an integrated measure of HDL quantity and quality, we assessed several metrics of HDL function using apolipoprotein (apo) B-depleted sera from patients with compensated cirrhosis, patients with acutely decompensated cirrhosis and healthy controls. We observed that sera of cirrhotic patients showed reduced levels of HDL-cholesterol and profoundly suppressed activities of several enzymes involved in HDL maturation and metabolism. Native gel electrophoresis analyses revealed that cirrhotic serum HDL shifts towards the larger HDL2 subclass. Proteomic assessment of isolated HDL identified several proteins, including apoA-I, apoC-III, apoE, paraoxonase 1 and acute phase serum amyloid A to be significantly altered in cirrhotic patients. With regard to function, these alterations in levels, composition and structure of HDL were strongly associated with metrics of function of apoB-depleted sera, including cholesterol efflux capability, paraoxonase activity, the ability to inhibit monocyte production of cytokines and endothelial regenerative activities. Of particular interest, cholesterol efflux capacity appeared to be strongly associated with liver disease mortality. Our findings may be clinically relevant and improve our ability to monitor cirrhotic patients at high risk.
Keywords: Liver disease, HDL-function, HDL-proteome, cholesterol efflux, paraoxonase, LCAT
Introduction
Alterations in serum lipids and lipoproteins in cirrhotic patients are secondary to complex abnormalities in lipoprotein synthesis, secretion and catabolism [1, 2]. A decrease below the normal level of serum cholesterol is often observed in advanced liver disease or in severe acute liver disease [3]. Alterations in lipid metabolism were shown to promote inflammation, fibrosis and proliferation in a mouse model of chronic cholestatic liver injury [4]. Interestingly, high-density lipoprotein (HDL)-cholesterol levels predict mortality in cirrhotic patients [5], suggesting that HDL particles modulate inflammatory responses [6]. However, steady-state assessment of circulating HDL concentrations may incompletely reflect in vivo functionality; therefore, direct measures of HDL function are needed [6–9]. Strategies to measure cholesterol efflux capacity have been used successfully in clinical studies, revealing inverse correlations between cholesterol efflux capacity and prevalent coronary artery disease, independently of the HDL-cholesterol levels [10, 11]. Moreover, recent data have clearly shown that inflammation markedly modifies composition and function of HDL, generating dysfunctional or even pro-inflammatory forms of HDL [7, 9, 12–14]. Dysfunctional HDL might contribute to the serious complications experienced by patients with acute and chronic liver disease. In the present study, we investigated whether cirrhosis affects HDL composition, structure and metabolism and whether these changes translate into impaired cholesterol efflux capability, anti-inflammatory and endothelial protective activities of apoB-depleted serum.
Methods
Subjects
The study population of this explorative cross sectional study comprised 59 consecutive patients with clinical and radiological evidence of cirrhosis, and/or biopsy proven cirrhosis. Patients with a Child-Pugh score > 11, abstinence from alcohol for < 2 weeks, clinical evidence of active infection, antibiotic treatment within 7 days prior to enrolment (except for primary or secondary prophylaxis of spontaneous bacterial peritonitis), gastrointestinal hemorrhage within the previous 2 weeks, use of immune modulating agents within one month (steroids etc.), renal failure (such as hepatorenal syndrome), creatinine >1.5x ULN, hepatic encephalopathy II to IV, pancreatitis, other organ failure, hepatic or extra-hepatic malignancy, and pregnancy were excluded. In addition, 21 age- and sex-matched consecutive cirrhotic patients who were admitted with acute decompensation of their liver disease were included. There were no restrictions regarding etiology of cirrhosis or reason for decompensation. Furthermore, 20 sex-matched healthy controls were included after they passed following exclusion criteria: any history of cardiovascular disease, pregnancy, obesity, dyslipidemia, liver disease, renal disease, diabetes or clinical signs of inflammation. Control subjects were free of lipid-lowering medication and anti-inflammatory drugs. Blood was sampled from patients and healthy volunteers in serum tubes (Greiner, Kremsmünster, Austria) after obtaining written informed consent. The study protocol was approved by the Ethics Committee of the Medical University of Graz and informed consent was obtained in accordance with the Declaration of Helsinki (No. 23-056 ex. 10/11, No. 23-285 ex 10/11 and No. 21-523 ex. 09/10).
Determination of serum lipid composition
Levels of total cholesterol and phospholipids (Diasys, Holzheim, Germany) were measured enzymatically. Low-density lipoprotein (LDL) cholesterol was calculated according to the Friedewald equation using HDL cholesterol values measured in the supernatant of the phosphotungstic acid/MgCl2 precipitation.
Preparation of apolipoprotein B (apoB)-depleted serum
ApoB-depleted serum was prepared by addition of 40 μL polyethylenglycol (20% in 200 mmol/L glycine buffer) to 100 μL serum [15]. Serum was incubated at room temperature for 20 minutes and the supernatant recovered after centrifugation (10.000 rpm, 20 minutes, 4°C).
Serum enzyme activities
Commercial kits were used to assess phospholipid transfer protein (PLTP) (Eubio, Vienna, Austria), cholesteryl-ester transfer protein (CETP) (Eubio, Vienna, Austria), lecithin-cholesterol acyltransferase (LCAT) (Merck, Darmstadt, Germany) and SN1-lipolytic activity (Cayman Europe, Tallinn, Estonia) in sera according to the manufactures instructions.
HDL particle size analysis
ApoB-depleted serum (2 µL) was separated by native gradient gel electrophoresis (4–16% NativePage; Life Technologies, Vienna, Austria). Gels were run for 120 min at constant voltage of 150 V, in NativePage running buffer (Life Technologies, Vienna, Austria). Gels were subsequently either fixed (with 25% isopropanol/10% acetic acid for 10 min) and neutral lipids stained with Sudan black (Thermo Scientific, Rockford, USA), or proteins were blotted onto a polyvinylidene difluoride (PVDF) membrane (for 45 min at 100 V). Membranes were probed using a primary monoclonal apoA-I antibody (Novus Biologicals, Littleton, USA) overnight at 4°C and secondary goat anti-mouse IgG (Thermo Fischer, Waltham, USA) for 120 min at room temperature. Size distribution of HDL was analyzed using ImageJ software. Intensity blots of individual samples were obtained and the peak areas of HDL2, HDL3 and small HDL3 particles identified using standard proteins (NativeMark, Life Technologies, Vienna, Austria).
HDL cholesterol efflux capability
Cholesterol efflux capacity was assessed using an established assay [11, 16]. RAW264.7 macrophages, maintained in DMEM with 10% fetal bovine serum were plated on 48-well plates (300.000 cells/well). Cells were labeled for 24 hours with 1 μCi/mL [3H]-cholesterol (Perkin Elmer, Boston, MA, USA). To upregulate ATP-binding cassette transporter A1 (ABCA1), cells were stimulated for 6 hours with serum-free DMEM containing 0.3 mmol/L 8-(4-chlorophenylthio)-cyclic AMP (Sigma, Darmstadt, Germany). After labeling, cells were washed and [3H]-cholesterol efflux was determined by incubating cells for 4 hours with 2.8 % apoB-depleted serum or 50 µg/mg HDL protein. Cholesterol efflux was expressed as the radioactivity in the medium relative to total radioactivity in medium and cells. All steps were performed in the presence of 2 μg/mL of the acyl coenzyme A cholesterol acyltransferase inhibitor Sandoz 58-035 (Sigma, Darmstadt, Germany).
Paraoxonase activity
Arylesterase activity was determined with a photometric assay using phenylacetate as substrate as described [15, 17, 18].
Determination of nuclear factor-κB (NF-κB) expression and cytokine releases
U937 monocytic cells containing a 5x NF-κB green fluorescence protein reporter cassette [19] were cultivated in RPMI 1640 containing 7.5% fetal bovine serum. The cells were pretreated for 1 ½ hours with reconstituted HDL (1-50 µg/mL), lipoprotein deficient serum or 7% apoB-depleted serum. Subsequently, the cells were stimulated for 24 hours with lipopolysaccharide (LPS) (50 ng/mL) (Sigma, Darmstadt, Germany), collected by centrifugation at 400 g for 7 minutes and fixed with 100 μL BD CellFIX solution (BD Biosciences, Franklin Lakes, NJ, USA). The expression of NF-κB was assessed by flow cytometry and cytokines were quantified using a multiplex bead-based immunoassay (eBioscience, San Diego, CA, USA).
Endothelial barrier measurements
Impedance measurements at 4,000 Hz were performed using the Electric Cell-substrate Impedance Sensing system (Applied Biophysics, Troy, NY) as described [20]. Human coronary artery endothelial cells (Lonza, Basel, Switzerland) (25,000 cells per chamber) were grown to confluence on 1% gelatin-precoated 96W1E+ polycarbonate arrays containing gold microelectrodes (Applied Biophysics, Troy, NY). Cells were serum starved for 2 hours, subsequently electrically wounded (4 seconds at 2,400 μA and 48 kHz) and incubated with HDL (50 µg/mL), 3% apoB-depleted serum or 3% lipoprotein deficient serum (LPDS) to assess repopulation of the wound and barrier promoting capability. Impedance was continuously monitored.
Isolation of HDL and lipoprotein deficient sera
Serum density was adjusted with potassium bromide (Sigma, Vienna, Austria) to 1.24 g/ml, and a two-step density gradient was generated in centrifuge tubes (16 × 76 mm, Beckman) by layering the density-adjusted serum underneath a NaCl density solution (1.063 g/ml) as described [21]. Tubes were sealed and centrifuged at 60.000 rpm for 5 h in a 90Ti fixed angle rotor (Beckman Instruments, Krefeld, Germany). After centrifugation, the HDL-containing band and the lipoprotein deficient sera (bottom fraction) were collected, and desalted via PD10 columns (GE Healthcare, Vienna, Austria).
LC-MS/MS Analysis
Proteomic profiling of HDL was performed as previously described with minor modifications [17, 21, 22]. HDL was digested with trypsin, and the resulting peptides were separated after online desalting by nano-HPLC (Dionex Ultimate 3000, Thermo Scientific, Waltham, MA) using a flow rate of 300 nl/min and a 2 hour gradient. The samples were ionized in the nanospray source equipped with nanospray tips and analyzed in an Orbitrap velos pro mass spectrometer (Thermo Scientific, Waltham, MA) in positive ion mode, applying alternative full scan MS (m/z 380-2000) at 60000 resolution and MS/MS by collision induced dissociation of the 20 most intense peaks in the ion trap with dynamic exclusion enabled. Data were analysed by searching the human SwissProt public database downloaded on Jul. 13, 2012, with Proteome Discoverer 1.4 (Thermo Scientific, Waltham, MA) and Mascot 2.3 (Matrixscience, London, UK). Detailed search criteria were as follows: trypsin; maximum missed cleavage sites = 2; fixed modification = carbamidomethylation at cysteine; variable modification = oxidized methionine; precursor mass tolerance = ±0.05 Da; and product mass tolerance = ±0.7 Da. Protein hits were subjected to automatic validation by Mascot using a decoy search and an FDR of 1%. Changes in HDL proteome (by comparison of relative protein abundances of the same protein between groups) were evaluated by label free quantitation from areas under the curve of precursor ions (i.e. mean areas of extracted ion chromatograms of the individual peptides matched to a protein) normalized to the total area under the curve of all proteins in each sample.
Statistical analysis
Comparison of normal distributed groups was performed with One-Way ANOVA and Bonferroni post-hoc test and non-parametric groups were compared with Kruskal-Wallis test. Correlations were determined using the Pearson product-moment estimates. Group differences were considered statistically significant for *P < 0.05, **P < 0.01 and ***P < 0.001. Statistical analyses were performed using GraphPad Prism (Version 4.0, GraphPad Software) or SPSS Statistics Version 20.
Results
Clinical characteristics
The clinical characteristics of study subjects are given in Table 1. Patients with acutely decompensated cirrhosis (n = 21) showed significantly decreased levels of total cholesterol, low-density lipoprotein cholesterol, HDL-cholesterol and apolipoprotein A-I (apoA-I), whereas C-reactive protein levels were increased. Compensated cirrhotic patients (n = 59) exhibited diminished HDL-cholesterol and apoA-I levels when compared to controls (n = 20).
Table 1. Clinical characteristics of study subjects.
| cirrhosis |
|||
|---|---|---|---|
| control | compensated | decompensated | |
| n | 20 | 59 | 21 |
| Age (y) | 67 (66-69) | 58 (53-64) | 58 (50-66) |
| Male/female | 15/5 | 42/17 | 16/5 |
| Total cholesterol (mg/dL) | 169 (152-188) | 173 (134-209) | 86 (64-123)***†† |
| Triglycerides (mg/dL) | 103 (70-145) | 81 (60-105) | 77 (62-123) |
| LDL-cholesterol (mg/dL) | 78 (60-102) | 107 (72-133) | 63 (42-74)†† |
| HDL-cholesterol (mg/dL) | 65 (55-80) | 44 (25-61)* | 10 (6-19)***†† |
| Apolipoprotein A-I (mg/dL) | 79 (57-113) | 37 (27-52)*** | 17 (15-24)***† |
| CRP (mg/L) | 2 (1-3) | 3 (1-5) | 25 (12-46)***†† |
| Child-Pugh Score | - | 5 (5-6) | 10 (8-12) |
| MELD Score | - | 10 (8-14) | 17 (15-19) |
| Etiology of liver disease (%) | |||
| Alcohol | - | 49.1 | 95.2 |
| Viral infection | - | 21.1 | 4.8 |
| Other | - | 29.8 | - |
| Albumin$ (g/dL) | - | 4.0 ± 0.6 | 2.9 ± 0.7† |
| Bilirubin$ (mg/dL) | - | 1.7 ± 1.6 | 5.3 ± 4.7† |
| Creatinine$ (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.6 |
Values are given as medians with the interquartile range. $Values are given as mean ± SD. CRP, C-reactive protein. *P < 0.05, ***P < 0.001 vs. control. †P < 0.01, ††P < 0.001 vs. compensated.
Cirrhosis affects HDL metabolism and subclass distribution
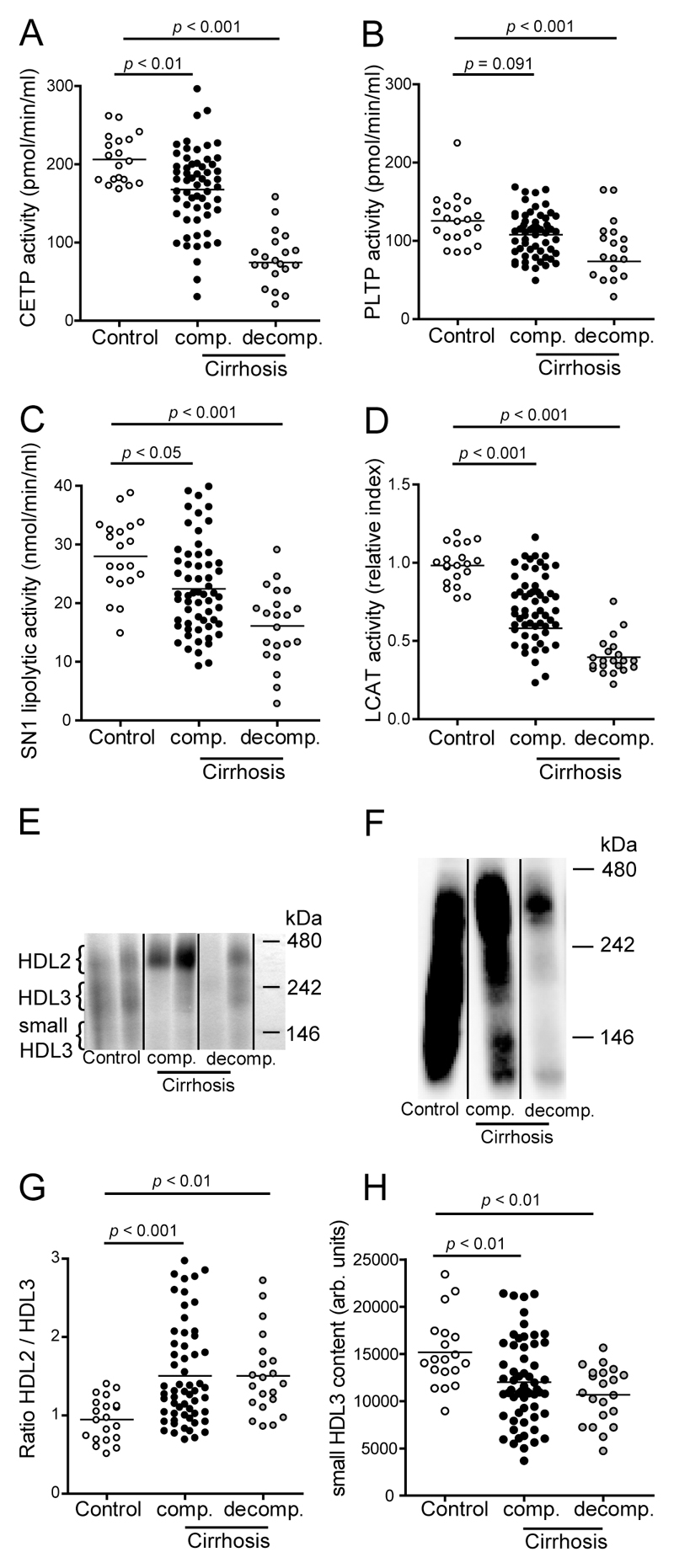
We first assessed activities of serum enzymes modulating HDL maturation and metabolism. CETP (Fig. 1A), SN1-lipolytic (Fig. 1C) and LCAT (Fig. 1D) activities were all significantly decreased in sera of patients with compensated cirrhosis and even more profoundly reduced in patients with acute decompensation. PLTP activity was significantly reduced in patients with acute decompensation, and tended to be lower in compensated cirrhotic patients (Fig. 1B). Native gel analysis revealed a marked shift towards the larger HDL2 subclass in compensated as well as in acutely decompensated patients, resulting in an increased HDL2/HDL3 ratio (Fig. 1E, 1F, 1G). Additionally, we observed a significant decrease of small HDL3 content in cirrhotic patients (Fig. 1E, 1F, 1H).
Figure 1. Cirrhosis alters serum enzymes involved in HDL metabolism.
Sera of healthy subjects (control, n = 20), patients with compensated (comp., n = 59) and acutely decompensated (decomp., n = 21) cirrhosis were examined for activities of (A) cholesteryl ester transfer protein (CETP), (B) phospholipid transfer protein (PLTP), (C) SN1-lipolytic activity and (D) lecithin-cholesterol acyltransferase (LCAT). (E) Apolipoprotein B (apoB)-depleted sera were separated by native gradient gel electrophoresis and lipids were stained with Sudan Black. (F) Western Blot analysis of apoB-depleted sera. Pooled apoB-depleted sera were separated by native gradient gel electrophoresis and blotted onto a PVDF membrane. ApoA-I was detected using a monoclonal apoA-I antibody. (G,H) Intensity blots of individual samples were obtained and HDL2/HDL3 ratios (G) and small HDL3 content (H) were calculated.
Sera of cirrhotic patients display an impaired ability to suppress the production of cytokines by lipopolysaccharide (LPS) stimulated monocytes
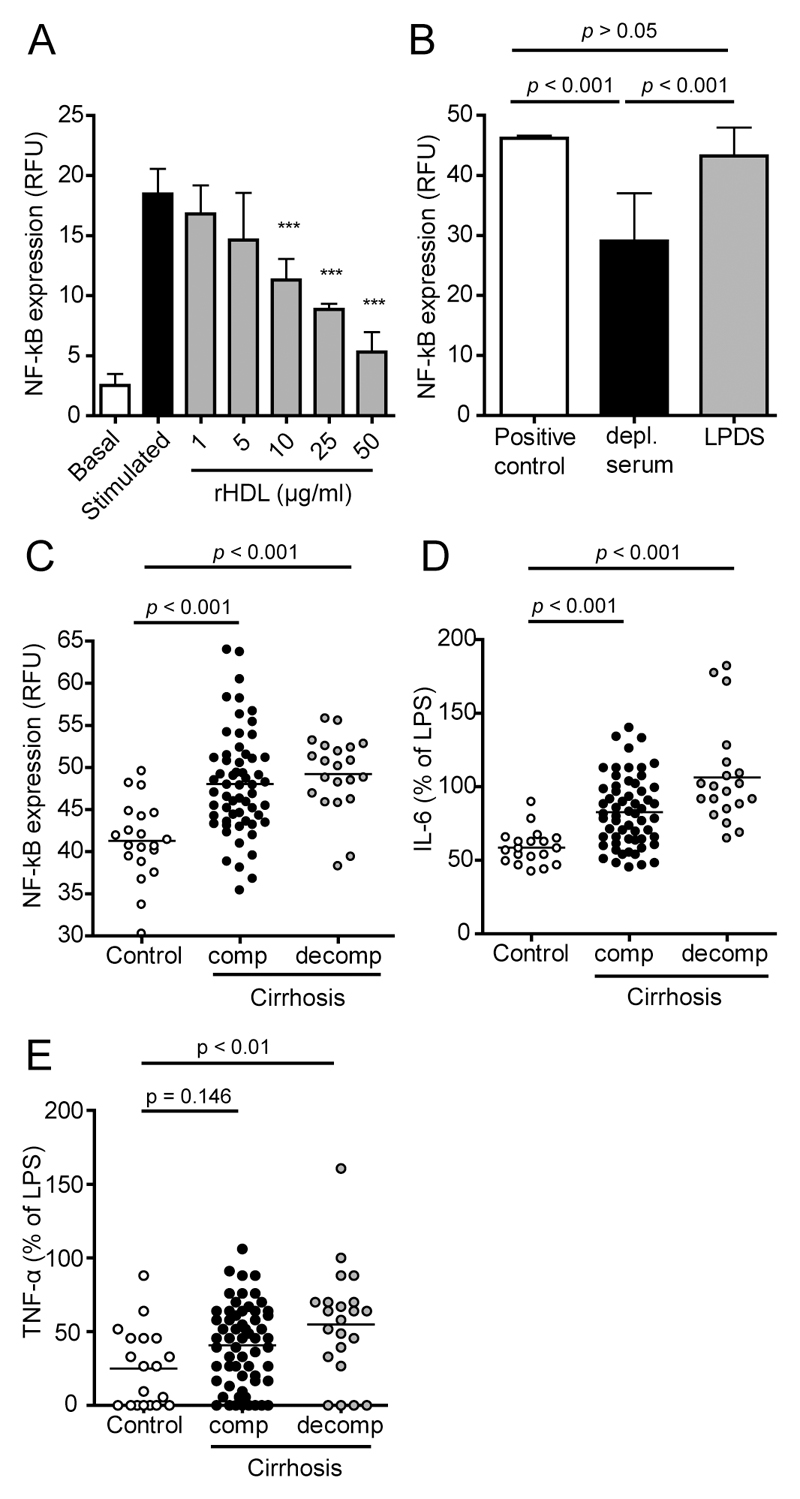
Several lines of evidence suggest that HDL acts as an endogenous inhibitor of inflammatory responses [6]. Therefore, we assessed the capacity of sera to inhibit LPS induced activation of the pro-inflammatory transcription factor NF-κB in monocytes. Addition of reconstituted HDL (containing human apoA-I and phosphatidylcholine as sole constituents) effectively and dose-dependently inhibited LPS induced NF-κB activation (Fig. 2A) whereas lipoprotein deficient serum showed no inhibitory activity (Fig. 2B). These data clearly suggest that HDL is required to suppress LPS-induced activation of NF-κB in monocytes. In the majority of cases, apoB-depleted serum of cirrhotic patients showed a reduced ability to suppress LPS-induced activation of NF-κB, when compared to controls (Fig. 2C). In good agreement with the NF-κB inhibitory activity, apoB-depleted sera of cirrhotic patients showed impaired ability to reduce production of the inflammatory cytokines interleukin-6 (IL-6) (Fig. 2D) and tumor necrosis factor-α (TNF-α) (Fig. 2E).
Figure 2. Cirrhosis is associated with reduced anti-inflammatory capacity of serum and a reduced ability to suppress the production of inflammatory cytokines.
Apolipoprotein B (apoB)-depleted sera of healthy subjects (control, n = 20), of patients with compensated (comp., n = 59) cirrhosis and of cirrhotic patients with acute decompensation (decomp., n = 21) were analyzed for their ability to inhibit lipopolysaccharide (LPS)-induced nuclear factor-κB (NF-κB) activation in monocytes. U937 monocytes containing a reporter cassette for NF-κB, were pretreated with (A) increasing concentrations (1-50 µg/mL) of reconstituted HDL (rHDL), (B) 10% lipoprotein deficient sera (LPDS) or (B,C) 7% apoB-depleted sera. After 1 ½ hours cells were stimulated with LPS (50 ng/mL) for 24 hours, followed by assessment of GFP expression by flow cytometry. (D,E) The supernatants of LPS stimulated monocytes which were pretreated with 7% apoB-depleted sera of patients or controls were analyzed for (D) interleukin-6 (IL-6) and (E) tumor necrosis factor-α (TNF-α) concentrations using flow cytometry. All values shown represent means of two independent experiments measured in duplicates. RFU, relative fluorescence units.
Sera of patients with acute decompensation depict severely impaired endothelial regenerative activities
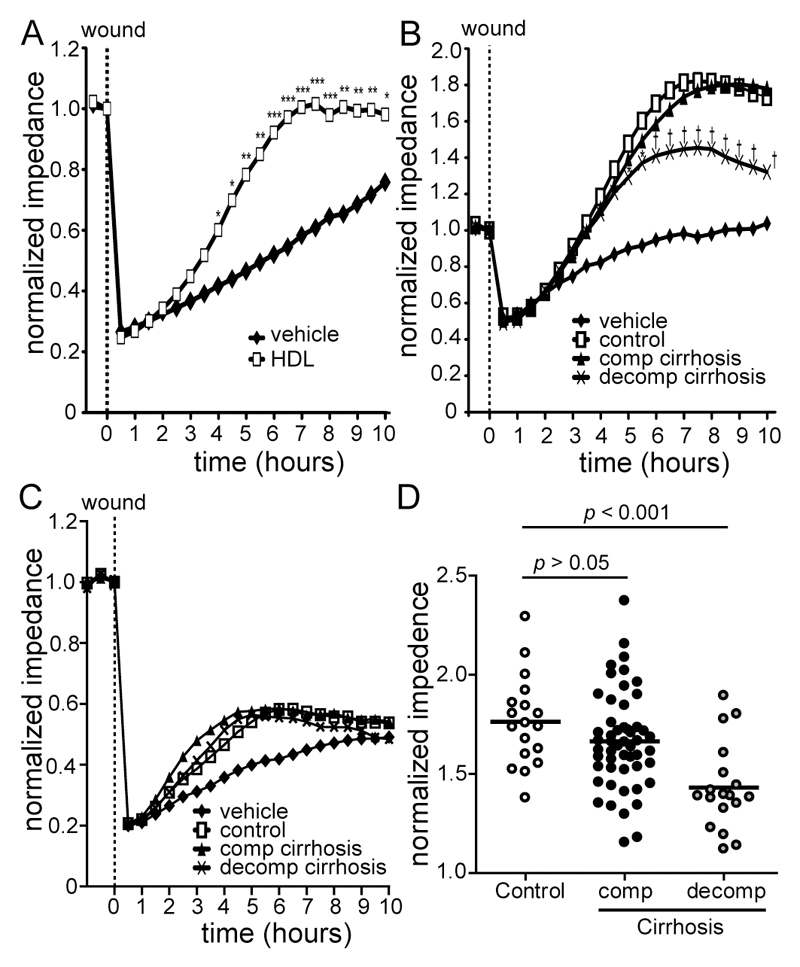
HDL is known to promote endothelial regenerative activities [23]. We assessed whether endothelial regenerative and barrier promoting activities of cirrhotic sera were affected using an electric cell-substrate impedance sensing system. As expected, addition of HDL effectively promoted barrier function as compared to vehicle treated cells (Fig. 3A). Lipoprotein deficient serum (LPDS) was generally less effective in promoting endothelial barrier function and similar across groups (Fig. 3C). Strikingly, apoB-depleted sera of cirrhotic patients with acute decompensation showed a significantly impaired endothelial regenerative activity (Fig. 3B, 3D).
Figure 3. Apolipoprotein B-depleted sera of patients with acute decompensation show impaired endothelial regenerative activities.
Human coronary artery endothelial cells were serum-starved for 2 hours and a stable baseline (8-10 kΩ) for normalization was recorded. Cells were electrically wounded using the electric cell-substrate impedance sensing system. Immediately after wounding, cells were treated with (A) HDL (50 µg/mL), (B) 3% apolipoprotein B (apoB)-depleted sera of healthy subjects (control, n = 18), patients with compensated (comp., n = 52) and acutely decompensated (decomp., n = 18) cirrhosis or (C) 3% of lipoprotein deficient sera (pooled fractions) of healthy subjects (n = 5), compensated (n = 5) and decompensated (n = 5) cirrhotic subjects. Re-endothelialization was monitored over time. (D) Scatter blot of apoB-depleted sera induced re-endothelialization at 6 ½ hours. Values shown represent means of two individual experiments measured in duplicates. *P < 0.05 vs. control, †P < 0.001 vs. control.
HDL efflux capacity and liver disease mortality
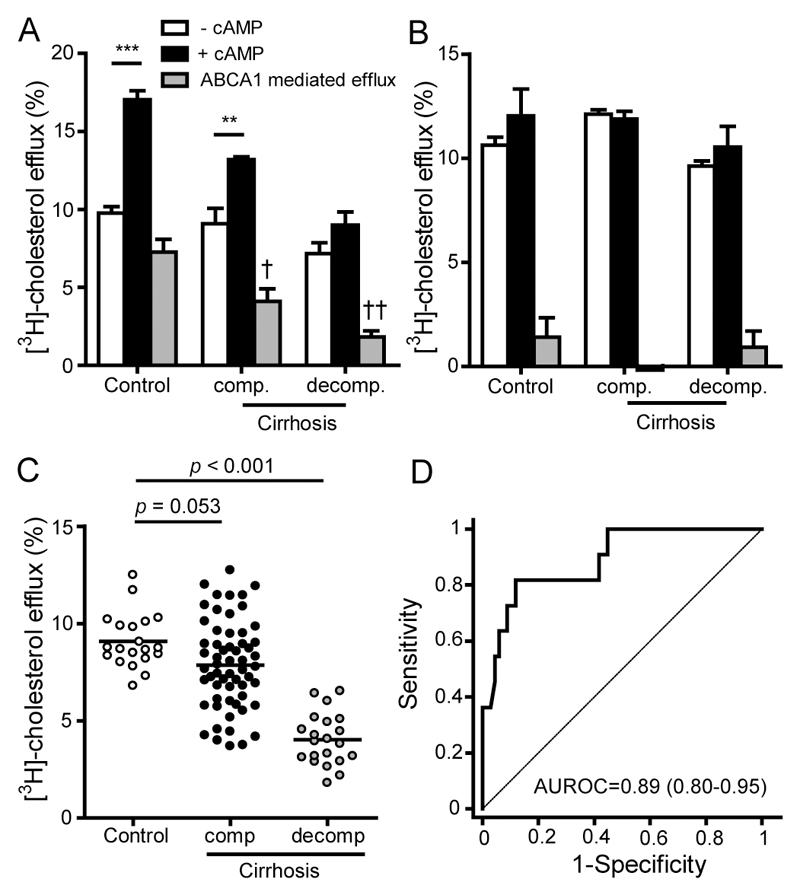
Removing excess membrane cholesterol from monocytes, lipid laden macrophages or endothelial cells is considered to be a main protective activity of HDL. Cholesterol efflux capacity of apoB-depleted serum using cAMP stimulated macrophages (to induce ABCA1 expression) inversely predicts the risk of coronary artery disease [10, 11]. We observed that the efflux potential of apoB-depleted serum was lower in compensated cirrhotic patients and was profoundly decreased in patients with acute decompensation (Fig. 4A, Fig. 4C). ApoB-depleted sera of controls showed the highest ABCA1 mediated efflux (44 % of total efflux), whereas sera of compensated and decompensated showed suppressed ABCA1 dependent efflux capacity (26 % and 23 % of total efflux) (Fig 4A). Interestingly, cholesterol efflux capacity of isolated HDL from liver disease patients was not altered (Fig. 4B). ABCA1 mediated efflux of isolated HDL was very low, in line with the notion that a large fraction of pre-beta HDL is stripped of during the ultracentrifugation procedure to isolate HDL. Prompted by the marked cirrhosis-induced alterations in the functionality of apoB-depleted serum, we tested whether metrics of function associate with survival of cirrhotic patients (Table 2). We observed that all metrics of HDL-mediated function significantly associated with one-year liver disease mortality. After adjustment for LDL-cholesterol, total cholesterol, triglycerides, age and sex, and further adjustment for HDL-cholesterol, cholesterol efflux capability remained significantly associated with 1-year mortality (Table 2). Receiver operating characteristics (ROC) analysis revealed excellent diagnostic accuracy of cholesterol efflux capacity to predict one-year mortality (area under ROC curve = 0.89) (Fig. 4D).
Figure 4. HDL cholesterol efflux potential and 1-year mortality.
Pooled fractions of sera of healthy subjects (control, n = 10), patients with compensated (comp., n = 10) and acutely decompensated (decomp., n = 10) cirrhosis were used to isolate HDL and to generate apolipoprotein (apo) B-depleted sera. ApoB-depleted sera (A) or HDL (B) were examined for their ability to promote [3H]-cholesterol efflux from macrophages. [3H]-cholesterol-labeled RAW264.7 macrophages were incubated, in the absence and presence of cAMP, with either 2.8% apoB-depleted sera (A) or 50 µg/mL HDL-protein (B) for 4 hours. Cells were either exposed to 2.8% apoB-depleted sera (A) or 50 µg/mL HDL-protein (B) for 4 hours. ABCA1 mediated efflux was calculated by subtracting effluxed [3H]-cholesterol of macrophages not exposed to cAMP from effluxed [3H]-cholesterol of cAMP stimulated cells. Cholesterol efflux is expressed as radioactivity in the supernatant relative to total radioactivity (in supernatant and cells). The values represent means of two independent experiments measured in duplicates. †P < 0.05, ††P < 0.01 vs. control.
ApoB-depleted sera of healthy subjects (control, n = 20), patients with compensated (comp., n = 59) and acutely decompensated (decomp., n = 21) cirrhosis were examined for their ability to (C) promote [3H]-cholesterol efflux from macrophages. [3H]-cholesterol-labeled and cAMP stimulated RAW264.7 macrophages were exposed to 2.8% apoB-depleted sera for 4 hours. Cholesterol efflux is expressed as radioactivity in the supernatant relative to total radioactivity (in supernatant and cells). The values represent means of two independent experiments measured in duplicates. (D) Receiver operating characteristics (ROC) curves illustrating the performances of [3H]-cholesterol efflux capability of apoB-depleted sera of patients with compensated (n = 59, 5 deceased) and acutely decompensated cirrhosis (n = 21, 11 deceased) for prediction of one-year mortality. AUROC, area under ROC curve.
Table 2. Correlation of one-year mortality and metrics of HDL function.
| One-year mortality |
||||||
|---|---|---|---|---|---|---|
| adjusted* | adjusted† | |||||
| r | p | r | p | r | p | |
| Cholesterol efflux | -0.458 | < 0.001 | -0.456 | < 0.001 | -0.321 | 0.010 |
| Paraoxonase activity | -0.382 | < 0.001 | -0.273 | 0.020 | -0.210 | 0.076 |
| NF-κB expression | 0.290 | 0.009 | 0.161 | 0.204 | 0.107 | 0.403 |
| Barrier regenerative activity | -0.344 | 0.004 | -0.268 | 0.032 | -0.242 | 0.056 |
| Small HDL3 particle content | -0.255 | 0.022 | -0.227 | 0.053 | -0.171 | 0.150 |
| HDL2/HDL3 ratio | 0.148 | 0.192 | 0.204 | 0.107 | 0.196 | 0.124 |
adjusted for LDL-cholesterol, total-cholesterol, triglycerides, age and sex.
adjusted for LDL-cholesterol, total-cholesterol, triglycerides, age, sex and HDL-cholesterol.
HDL from cirrhotic patients carries a distinct protein cargo
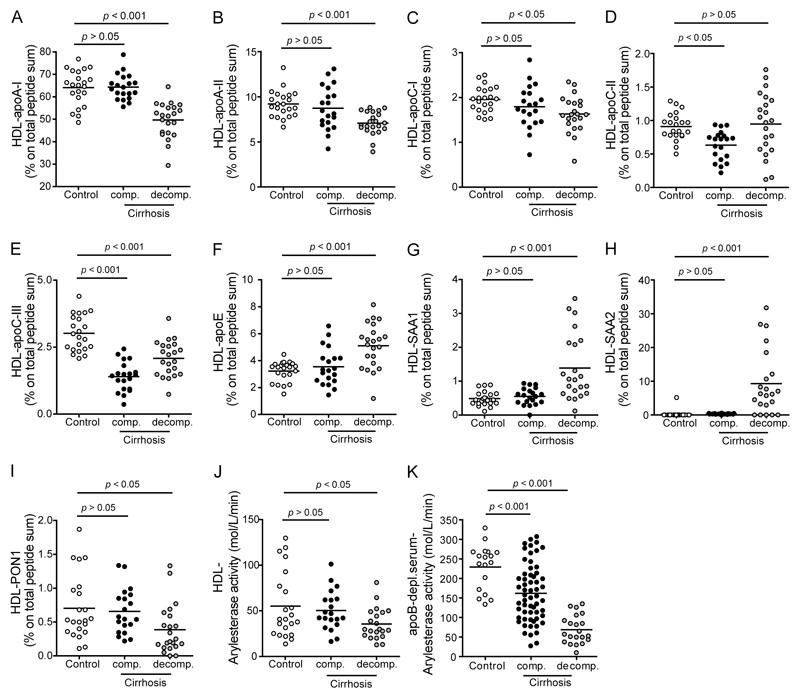
It is now well accepted that inflammation markedly alters HDL composition and function [7, 12]. Therefore, we also assessed possible alterations in the HDL protein composition of cirrhotic patients. HDL was digested and the resulting peptides were analyzed by tandem mass spectrometry. A list of identified proteins is shown in the Supplementary Table 1; all major HDL associated apolipoproteins that were significantly altered in cirrhotic patients are depicted in Figure 5. As expected, the most abundant proteins on HDL particles from healthy controls and cirrhotic patients were apoA-I and apoA-II (Fig. 5). After statistical analysis, we identified apoC-II and apoC-III to be significantly decreased on HDLs of patients with compensated cirrhosis when compared to healthy controls. We observed a significant decrease of apoA-I, apoA-II, apoC-I, apoC-III and PON1 while apoE, serum amyloid A1 (SAA1) and SAA2 were significantly increased on HDLs of patients with decompensated cirrhosis (Fig. 5A-I). In line with the reduced levels of HDL associated PON1 identified by mass spectrometry (Fig. 5I), we observed that arylesterase activity of isolated HDL was significantly lower in decompensated cirrhotic patients when compared with controls (Fig. 5J). Arylesterase activity of apoB-depleted sera was even more profoundly suppressed, which is explained by low levels of HDL seen in cirrhotic patients, especially in acutely decompensated patients (Fig. 5K).
Figure 5. Identification of proteins in HDL isolated from healthy subjects and patients with cirrhosis.
(A-I) HDL was isolated from healthy subjects (control, n = 22), patients with compensated (comp., n = 20) and acutely decompensated (decomp., n = 22) cirrhosis. The HDL proteome was analyzed on a LC-MS/MS system. Data were analyzed by searching the human SwissProt public database with Proteome Discoverer 1.4 (Thermo Scientific) and Mascot 2.3 (MatrixScience) and label free quantitation of precursor ion chromatograms. Values shown represent percentage of total peptide sum. (J) HDL-associated PON activity of healthy subjects, patients with compensated and acutely decompensated cirrhosis. (K) PON activity of apolipoprotein B-depleted sera of healthy subjects (control, n = 20), patients with compensated (comp., n = 59) and acutely decompensated (decomp., n = 21) cirrhosis. Paraoxonase activity was measured using phenylacetate as a substrate and was calculated from the slope of the kinetic chart of two independent experiments.
To gain further insight into the relationship between HDL protein composition and serum HDL-mediated functions, a detailed correlation analysis was performed (Tab. 3). We observed that those proteins that were significantly altered in cirrhotic patients, such as apoA-I, apoE, apoC-III and SAA, were the strongest predictors of functionality of apoB-depleted sera, suggesting that HDL composition is linked to functionality of apoB-depleted serum. We found that HDL associated apoA-I and PON-1 strongly associated with functionality of apoB-depleted sera, whereas HDL associated SAA1, SAA2 and apoE were linked to impaired functionality of sera. Interestingly, we found a negative correlation between the HDL2/HDL3 ratio and the paraoxonase activity as well as anti-inflammatory capacity of apoB-depleted sera. In line with a previous study showing that small HDL3 particles are major acceptors of ABCA1 mediated efflux [24], we found that small HDL3 particle content correlated strongly with the cholesterol efflux potential, but also with paraoxonase activity and the anti-inflammatory capacity of apoB-depleted sera. As expected, levels of HDL (apoA-I and cholesterol) strongly correlated with metrics of function of apoB-depleted sera.
Table 3. Correlation of HDL-composition, -distribution and -levels with metrics of function of apoB-depleted serum.
| Cholesterol efflux | Paraoxonase activity | NF-κB expression | Barrier regenerative activity | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| HDL-composition | ||||||||
| apoA-I | 0.629 | < 0.001 | 0.565 | < 0.001 | -0.240 | 0.070 | 0.381 | < 0.01 |
| apoE | -0.519 | < 0.001 | -0.484 | < 0.001 | 0.362 | < 0.01 | -0.142 | 0.310 |
| apoC-III | 0.337 | 0.010 | 0.323 | 0.015 | -0.565 | < 0.001 | 0.145 | 0.301 |
| SAA1 | -0.495 | < 0.001 | -0.480 | < 0.001 | 0.122 | 0.363 | -0.218 | 0.117 |
| SAA2 | -0.524 | < 0.001 | -0.508 | < 0.001 | 0.156 | 0.243 | -0.240 | 0.083 |
| PON1 | 0.395 | < 0.01 | 0.468 | < 0.001 | -0.089 | 0.507 | 0.211 | 0.129 |
| HDL-distribution | ||||||||
| HDL2/HDL3 ratio | -0.160 | 0.114 | -0.329 | 0.001 | -0.352 | < 0.001 | -0.100 | 0.358 |
| small HDL3 particles | 0.371 | < 0.001 | 0.432 | < 0.001 | -0.449 | < 0.001 | 0.106 | 0.329 |
| HDL-levels in apoB-depleted serum | ||||||||
| HDL-cholesterol | 0.874 | < 0.001 | 0.590 | < 0.001 | -0.506 | < 0.001 | 0.293 | < 0.01 |
| apoA-I | 0.678 | < 0.001 | 0.569 | < 0.001 | -0.597 | < 0.001 | 0.219 | 0.073 |
apo, apolipoprotein; SAA, serum amyloid A; PON1, paraoxonase 1
Discussion
The liver plays a central role in several stages of lipid synthesis, transport and metabolism. In the present study, we observed that activities of enzymes involved in HDL maturation and metabolism, such as PLTP, LCAT, CETP and serum SN1-lipolytic activity are markedly reduced in cirrhotic patients. Native gel analysis revealed a marked shift towards the larger HDL2 subclass in cirrhotic patients and a marked reduction in small HDL3 particles. Based on previous results we assume that low CETP as well as SN1 lipase activities appear to hinder the formation of small HDL3 [25]. This may be of critical importance, given that certain HDL subspecies play distinct functional roles, not only in lipid homeostasis but also in innate immunity [7, 12]. Small, dense HDL subfractions are evidenced by relatively low lipid/cholesterol content but are superior in mobilizing cellular cholesterol [24]. In line with that concept, we observed that low levels of small HDL3 particles in liver disease subjects were associated with decreased cholesterol efflux capacity and anti-inflammatory activities of apoB-depleted serum. Pre-β-HDL (lipid-poor HDL) acts as an efficient initial acceptor of cholesterol in an ABCA1 dependent pathway. We observed that ABCA1 mediated cholesterol efflux was severely suppressed in cirrhotic patients. ABCA1 mediated about 44 % of total cholesterol efflux of apoB-depleted serum of controls, whereas only 26 % and 23 % of total cholesterol efflux was mediated by ABCA1 in compensated and decompensated liver disease patients, respectively. Interestingly, liver disease did not alter cholesterol efflux capacity of isolated HDL, which is largely ABCA1 independent [26], given that a large fraction of lipid-poor HDL is stripped off during the ultracentrifugation procedure required to isolate HDL. Summing up, our data clearly suggest that the formation of lipid-poor HDL is profoundly suppressed in liver disease. Cholesterol efflux capacity of apoB-depleted serum, an integrated measure of HDL quantity and quality, has been shown to be inversely related to both atherosclerotic burden and, more recently, incident cardiovascular events in multiple cohorts independent of circulating HDL-cholesterol levels [10, 11]. A very interesting finding of our study is that cholesterol efflux capacity predicted 1-year mortality in cirrhotic patients, even after adjustment for LDL-cholesterol, total-cholesterol, triglycerides, age, sex and HDL-cholesterol. Although our data suggest an inverse relationship between cholesterol efflux capacity and mortality, the causal nature of this relationship is uncertain. HDL regulates the plasma membrane cholesterol content through the ability to remove cholesterol and other lipid species from cells, thereby dampening inflammatory receptor signaling [27]. Therefore, functional HDL is thought to be an important endogenous inhibitor of inflammatory responses. This might be of relevance, given that patients with liver cirrhosis are highly susceptible to bacterial infections that are the most common event of acute-on-chronic liver failure [28]. Previous studies reported that LPS-induced overproduction of pro-inflammatory cytokines by monocytes can be abolished by incubation of whole blood with reconstituted HDL [29, 30]. HDL was found to promote endothelial cell motility, a process that relates to endothelial barrier function in the context of vascular injury [31]. Up to date, no therapeutics has sufficiently addressed the vascular leak of fluid and immune cells from plasma to interstitial space caused by a loss of endothelial barrier function. Of particular interest, we observed that cirrhosis markedly impaired the ability of apoB-depleted serum to suppress NF-kB activation and cytokine production. Moreover, our data indicate that cirrhosis reduces the ability of apoB-depleted serum to promote endothelial regeneration. These are novel and important observations and might explain, at least in part, why cirrhotic patients are highly susceptible to bacterial infections. Recent proteomic studies provided convincing evidence that inflammation alters the protein composition of HDL thereby rendering it dysfunctional [12]. Using shotgun proteomics, we observed that isolated HDL from cirrhotic patients, especially from patients with decompensated cirrhosis, contains a distinct protein cargo, with reduced levels of apoA-I, apoA-II, apoC-I, apoC-II, apoC-III and PON1 whereas apoE, and the pro-inflammatory acute phase proteins SAA1 and SAA2 were markedly enriched. Of particular importance, we found significant correlations of HDL associated proteins and apoB-depleted serum functionality. SAA1 and SAA2 are well known pro-inflammatory mediators that suppress cholesterol efflux capability of HDL and render HDL pro-inflammatory [7, 32–34]. It is well known that in the circulation SAA associates with HDL particles causing HDL remodeling with displacement of apoA-I [33, 35–37]. In line with these previous observations, both, HDL associated SAA1 and SAA2 were negatively associated with cholesterol efflux capacity of apoB-depleted serum whereas apoA-I was strongly positively associated. Interestingly, we found that HDL associated apoE showed a strong negative correlation with cholesterol efflux capacity and was associated with a poor ability of apoB-depleted serum to suppress LPS induced NF-kB activation in monocytes. Our observations are in line with a recent study demonstrating that apoE suppresses ABCA1-specific cholesterol efflux capacity [38]. Another finding of particular interest is that HDL-associated PON1 levels and activities are reduced in cirrhotic patients. There is strong evidence for a mechanistic link between activity of PON1 with systemic oxidative stress and prospective cardiovascular risk, indicating a potential mechanism for the atheroprotective function of PON1 [39]. A reduction of HDL associated PON1 activity as observed in cirrhotic patients is therefore expected to alter antioxidant and cardioprotective properties of HDL. In line with a previous study showing that small HDL3 particles are major acceptors of ABCA1 mediated efflux [24], we found that small HDL3 particle content correlated strongly with the cholesterol efflux potential, but also with paraoxonase activity and the anti-inflammatory capacity of apoB-depleted sera. As expected, levels of HDL (cholesterol and apoA-I) strongly correlated with metrics of function of apoB-depleted sera. Thus, our data provide strong evidence that low HDL quality and quantity impair the functionality of apoB-depleted serum in cirrhotic patients.
This study has limitations. We cannot exclude that other serum components in addition to HDL contribute to some effects observed of apoB-depleted serum. Therefore additional studies are required to establish final evidence.
In conclusion, our studies demonstrate that liver disease alters cholesterol efflux capacity, paraoxonase activity as well as anti-inflammatory and endothelial regenerative activities of apoB-depleted serum. Cholesterol efflux capacity of apoB-depleted serum, an integrated measure of HDL level and quality, appears to be related to liver disease mortality. Consequently, such alterations in HDL levels, structure, metabolism, composition and function may be clinically relevant and improve our ability to monitor cirrhotic patients at high risk.
Supplementary Material
Highlights.
Liver disease suppresses several enzymes involved in HDL maturation and metabolism
Cirrhosis alters composition and structure of HDL, the formation of lipid-poor HDL is profoundly suppressed in liver disease.
Anti-inflammatory activity of apoB-depleted serum is linked to low HDL levels and compositional alterations of HDL
Cholesterol efflux capability of apoB-depleted serum, an integrated measure of HDL quantity and quality, predicts liver disease mortality.
Acknowledgments
The authors would like to thank Barbara Darnhofer, Austrian Center of Industrial Biotechnology, Graz, Austria, for technical assistance in mass spectrometry.
Sources of Funding: This work was supported by the Austrian Science Fund FWF (Grant P22521-B18 to A. Heinemann, P24362-B23 to V.S., P22976-B18 to G.M. and P26074-B19 to R.B.-G.) and the Jubiläumsfond of the Austrian National Bank (14853 to G.M., and 15883 to M.H). L.P. was funded by the FWF within the frame of DK-MOLIN (W1241). W.S. was supported by BioPersMed (COMET K-project 825329) which is funded by the Austrian Federal Ministry of Transport, Innovation and Technology (BMVIT) and the Austrian Federal Ministry of Economics and Labour/the Federal Ministry of Economy, Family and Youth (BMWA/BMWFJ) and the Styrian Business Promotion Agency (SFG).
Abbreviations
- ABCA1
adenosine triphosphate-binding cassette transporter A1
- apo
apolipoprotein
- CETP
cholesteryl-ester transfer protein
- HDL
high-density lipoproteins
- LDL
low-density lipoprotein
- PLTP
phospholipid transfer protein
- LCAT
lecithin-cholesterol acyltransferase
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- ROC
receiver operating characteristics
References
- [1].Cicognani C, Malavolti M, Morselli-Labate AM, Zamboni L, Sama C, Barbara L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. 1997;157:792–796. [PubMed] [Google Scholar]
- [2].McIntyre N. Plasma lipids and lipoproteins in liver disease. Gut. 1978;19:526–530. doi: 10.1136/gut.19.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].D'Arienzo A, Manguso F, Scaglione G, Vicinanza G, Bennato R, Mazzacca G. Prognostic value of progressive decrease in serum cholesterol in predicting survival in Child-Pugh C viral cirrhosis. Scand J Gastroenterol. 1998;33:1213–1218. doi: 10.1080/00365529850172593. [DOI] [PubMed] [Google Scholar]
- [4].Moustafa T, Fickert P, Magnes C, Guelly C, Thueringer A, Frank S, Kratky D, Sattler W, Reicher H, Sinner F, Gumhold J, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology. 2012;142:140–151.e12. doi: 10.1053/j.gastro.2011.09.051. [DOI] [PubMed] [Google Scholar]
- [5].Habib A, Mihas AA, Abou-Assi SG, Williams LM, Gavis E, Pandak WM, Heuman DM. High-density lipoprotein cholesterol as an indicator of liver function and prognosis in noncholestatic cirrhotics. Clin Gastroenterol Hepatol. 2005;3:286–291. doi: 10.1016/s1542-3565(04)00622-6. [DOI] [PubMed] [Google Scholar]
- [6].Pirillo A, Catapano AL, Norata GD. HDL in infectious diseases and sepsis. Handb Exp Pharmacol. 2015;224:483–508. doi: 10.1007/978-3-319-09665-0_15. [DOI] [PubMed] [Google Scholar]
- [7].Birner-Gruenberger R, Schittmayer M, Holzer M, Marsche G. Understanding high-density lipoprotein function in disease: Recent advances in proteomics unravel the complexity of its composition and biology. Prog Lipid Res. 2014;56C:36–46. doi: 10.1016/j.plipres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- [8].Kontush A, Chapman MJ. Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Curr Opin Lipidol. 2010;21:312–318. doi: 10.1097/MOL.0b013e32833bcdc1. [DOI] [PubMed] [Google Scholar]
- [9].Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D'Agostino RBS, Davidson MH, Davidson WS, Heinecke JW, Karas RH, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525. doi: 10.1016/j.jacl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- [10].Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marsche G, Saemann MD, Heinemann A, Holzer M. Inflammation alters HDL composition and function: Implications for HDL-raising therapies. Pharmacol Ther. 2013;137:341–351. doi: 10.1016/j.pharmthera.2012.12.001. [DOI] [PubMed] [Google Scholar]
- [13].Luscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res. 2014;114:171–182. doi: 10.1161/CIRCRESAHA.114.300935. [DOI] [PubMed] [Google Scholar]
- [14].Curcic S, Holzer M, Frei R, Pasterk L, Schicho R, Heinemann A, Marsche G. Neutrophil effector responses are suppressed by secretory phospholipase A2 modified HDL. Biochim Biophys Acta. 2015;1851:184–193. doi: 10.1016/j.bbalip.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holzer M, Wolf P, Inzinger M, Trieb M, Curcic S, Pasterk L, Weger W, Heinemann A, Marsche G. Anti-psoriatic therapy recovers high-density lipoprotein composition and function. J Invest Dermatol. 2014;134:635–642. doi: 10.1038/jid.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Holzer M, Trieb M, Konya V, Wadsack C, Heinemann A, Marsche G. Aging affects high-density lipoprotein composition and function. Biochim Biophys Acta. 2013;1831:1442–1448. doi: 10.1016/j.bbalip.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Holzer M, Schilcher G, Curcic S, Trieb M, Ljubojevic S, Stojakovic T, Scharnagl H, Kopecky CM, Rosenkranz AR, Heinemann A, Marsche G. Dialysis Modalities and HDL Composition and Function. J Am Soc Nephrol. 2015;26:2267–2276. doi: 10.1681/ASN.2014030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jorgl A, Platzer B, Taschner S, Heinz LX, Hocher B, Reisner PM, Gobel F, Strobl H. Human Langerhans-cell activation triggered in vitro by conditionally expressed MKK6 is counterregulated by the downstream effector RelB. Blood. 2007;109:185–193. doi: 10.1182/blood-2006-05-022954. [DOI] [PubMed] [Google Scholar]
- [20].Konya V, Ullen A, Kampitsch N, Theiler A, Philipose S, Parzmair GP, Marsche G, Peskar BA, Schuligoi R, Sattler W, Heinemann A. Endothelial E-type prostanoid 4 receptors promote barrier function and inhibit neutrophil trafficking. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.05.008. [DOI] [PubMed] [Google Scholar]
- [21].Holzer M, Wolf P, Curcic S, Birner-Gruenberger R, Weger W, Inzinger M, El-Gamal D, Wadsack C, Heinemann A, Marsche G. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res. 2012;53:1618–1624. doi: 10.1194/jlr.M027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G. Uremia alters HDL composition and function. J Am Soc Nephrol. 2011;22:1631–1641. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- [24].Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- [25].Murdoch SJ, Breckenridge WC. Influence of lipoprotein lipase and hepatic lipase on the transformation of VLDL and HDL during lipolysis of VLDL. Atherosclerosis. 1995;118:193–212. doi: 10.1016/0021-9150(95)05606-8. [DOI] [PubMed] [Google Scholar]
- [26].de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- [29].Pajkrt D, Doran JE, Koster F, Lerch PG, Arnet B, van der Poll T, ten Cate JW, van Deventer SJ. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Galbois A, Thabut D, Tazi KA, Rudler M, Mohammadi MS, Bonnefont-Rousselot D, Bennani H, Bezeaud A, Tellier Z, Guichard C, Coant N, et al. Ex vivo effects of high-density lipoprotein exposure on the lipopolysaccharide-induced inflammatory response in patients with severe cirrhosis. Hepatology. 2009;49:175–184. doi: 10.1002/hep.22582. [DOI] [PubMed] [Google Scholar]
- [31].Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, Tall AR. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118:3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weichhart T, Kopecky C, Kubicek M, Haidinger M, Doller D, Katholnig K, Suarna C, Eller P, Tolle M, Gerner C, Zlabinger GJ, et al. Serum Amyloid A in Uremic HDL Promotes Inflammation. J Am Soc Nephrol. 2012;23:934–947. doi: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol. 2000;20:763–772. doi: 10.1161/01.atv.20.3.763. [DOI] [PubMed] [Google Scholar]
- [34].Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, Lehmann M, Kopecky C, Saemann MD, Lepper PM, Silbernagel G, et al. Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. Eur Heart J. 2015;36:3007–3016. doi: 10.1093/eurheartj/ehv352. [DOI] [PubMed] [Google Scholar]
- [35].Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986;261:9644–9651. [PubMed] [Google Scholar]
- [36].Artl A, Marsche G, Pussinen P, Knipping G, Sattler W, Malle E. Impaired capacity of acute-phase high density lipoprotein particles to deliver cholesteryl ester to the human HUH-7 hepatoma cell line. Int J Biochem Cell Biol. 2002;34:370–381. doi: 10.1016/s1357-2725(01)00132-7. [DOI] [PubMed] [Google Scholar]
- [37].Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC. HDL remodeling during the acute phase response. Arterioscler Thromb Vasc Biol. 2009;29:261–267. doi: 10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pamir N, Hutchins P, Ronsein G, Vaisar T, Reardon CA, Getz GS, Lusis AJ, Heinecke JW. Proteomic analysis of HDL from inbred mice strains implicates APOE associated with HDL in reduced cholesterol efflux capacity via the ABCA1 pathway. J Lipid Res. 2015 doi: 10.1194/jlr.M063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.