Abstract
Tyrosine kinase inhibitors (TKIs), including imatinib, dasatinib and nilotinib, are effective forms of therapy for various types of solid cancers and Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia. A number of TKIs have been known to have strong effects on T cells, particularly cluster of differentiation (CD) 4+CD25+ T cells, also known as regulatory T cells (Tregs). There is currently a deficit in the available clinical data regarding this area of study. In the present study, a total of 108 peripheral blood samples were collected from patients with chronic myeloid leukemia (CML) at diagnosis (n=31), and at 3 and 6 months following treatment with TKI [imatinib (n=12), dasatinib (n=11) and nilotinib groups (n=8)] and healthy controls (n=15). Peripheral blood mononuclear cells were collected from the patients prior to and following TKI treatment. The subtype and number of T lymphocytes in patients and healthy donors were analyzed using flow cytometry. Additionally, flow cytometry and ELISA were used to detect the proliferation and suppression of Tregs. Expression of cytokines and other molecules [forkhead box P3 (FOXP3), glucocorticoid-induced tumor necrosis factor receptor (GITR) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)] were also analyzed at 3 and 6 months following treatment with TKIs. It was indicated that, at diagnosis, a similar number of lymphocytes were detected in patients and control. However, following treatment with a TKI, the number of total T cells, Tregs, CD4+ T and CD8+ T cells decreased to various degrees in patients. Furthermore, the decrease in the number of Tregs was more significant with time. Although treatment with imatinib, dasatinib and nilotinib demonstrated similar inhibitory effects on the quantity of Tregs in vivo, the TKIs exhibited differential effects on the function of Tregs in vitro. Proliferation, suppression and expression of cytokines [interleukin (IL)-4, IL-10 and transforming growth factor (TGF)-β] and molecules (FOXP3, GITR and CTLA-4) decreased significantly in treatment groups with imatinib and dasatinib. The decrease was not significant in the nilotinib treatment group. Imatinib and dasatinib may exert more marked inhibitory roles compared with nilotinib on regulating the number and function of Tregs. These results suggest that personalized treatment and follow-up of CML patients during TKI treatment, particularly for those who received post-transplant TKI treatment may be beneficial.
Keywords: chronic myeloid leukemia, tyrosine kinase inhibitors, regulatory T cells
Introduction
The development of tyrosine kinase inhibitors (TKIs) targeting the breakpoint cluster region (BCR)-Abelson kinase 1 (ABL1) oncoprotein has changed the landscape of treatment of chronic myeloid leukemia (CML). In addition to targeting BCR-ABL1, TKIs also affect other important signaling pathways including the c-ABL, cellular mast/stem cell growth factor receptor Kit and platelet-derived growth factor receptor signaling pathways. Compared with imatinib, dasatinib, as a second-generation TKI, inhibits a broader range of tyrosine kinases, including proto-oncogene tyrosine protein kinase Src (Src), tyrosine protein kinase Tec, ephrin type-A receptor 1, and other TKIs have important roles in regulating various cellular functions (1,2). Leukocyte C-terminal Src kinase (Lck) and tyrosine protein kinase Fyn (two members of the Src kinase family) are involved in the early steps of T-cell receptor (TCR) activation. A previous in vitro study has suggested that Lck is more important in TCR signaling (3). Therefore, it is not surprising that TKIs are able to affect immune reconstitution as well as proliferation, function and activation of T cells.
T lymphocytes are intimately involved in the pathophysiology of autoimmune diseases, graft-vs. -host disease (GVHD) and the graft-versus leukemia (GVL) effect. Cluster of differentiation (CD) 4+CD25+ T cells (regulatory T cells or Tregs) are a subset of T lymphocytes, which have a crucial role in homeostasis for peripheral T-cells as well as the maintenance of immune tolerance, particularly following allogeneic hematopoietic stem cell transplantation (allo-HSCT) (4–6). The modulation of Tregs may be a novel means for treating autoimmune diseases, including GVHD and GVL, as well as tumors (7–10). There are two therapeutic options available to patients with CML, who relapse following allo-HSCT: Donor lymphocyte infusion and treatment with TKIs (11,12). The combination of these treatments has yielded contradictory results in clinical studies (13). An improved understanding of the effect of TKIs on the biological characteristics of Tregs is important for the development of clinical applications. Recent studies have indicated that the mechanism of suppression performed by Tregs can be divided primarily into two aspects: i) Cell-cell contact dependent mechanism; and ii) regulation by secretion of suppressive cytokines (14). A number of vital surface molecules are involved in the suppressive function of Tregs, including forkhead box P3 (FOXP3), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), tumor necrosis factor receptor (GITR), transforming growth factor (TGF)-β, latency-associated peptide, CD4-related lymphocyte-activation-gene-3, galectin-1 and CD39. Furthermore, Tregs are also able to exhibit an immune suppressive role via the production of interleukin (IL)-10, TGF-β, IL-4 and other cytokines.
In vitro studies have demonstrated that treatment with imatinib, dasatinib and nilotinib have inhibitory effects on proliferation, suppressive capacity and cytokine secretion of Tregs from healthy donors (15–17). However, deficits in our knowledge remain concerning the effects of imatinib, dasatinib and nilotinib treatment on Tregs in patients with CML, particularly on the changes in Tregs in vivo and on functional analysis of Tregs during long-term treatment with TKIs.
To address these issues, in the present study, the quantity and function of Tregs in patients with chronic-phase CML (CML-CP) at the time of diagnosis and during treatment with TKIs were evaluated.
Patients and methods
Patients
The inclusion criteria for the present study were: i) Diagnosis of CML-CP, patients undergoing treatment with one type of TKI (imatinib, dasatinib or nilotinib); ii) patients in the novel diagnostic-phase and not under treatment of CML-associated drugs, including hydroxyurea or TKIs; iii) preserved functioning of major organs (lung, liver, heart and kidney) in patients; iv) patients not undergoing treatment with immunomodulators; and v) written informed consent from patients. The exclusion criteria were: i) Presence of multiple tumors; ii) pregnant women and juveniles (age <18 years); and iii) exclusion from enrollment at the discretion of the physician.
The present study was performed in accordance with a protocol approved by the Ethics Committee of Nanfang Hospital (Guangzhou, China) according to The Declaration of Helsinki. Written informed consent was obtained from each participant prior to sample collection. A total of 108 peripheral blood (PB) samples were obtained from participants between July 2014 and July 2015. Samples were taken from patients at the time of diagnosis (n=31), and at 3 and 6 months while treated with a TKI. TKI-treated patients with CML were divided into three groups: Imatinib (400 mg/day; n=12), dasatinib (100 mg/day; n=11) and nilotinib (300 mg twice daily; n=8). All TKIs were administered orally. The doses of TKIs were decreased according to any side effects or toxicity. A series of PB samples were taken from healthy volunteers (n=15) to serve as the control group.
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) from patients and healthy donors were isolated using the Ficoll-Biocoll separation solution (Biochrom, Ltd., Cambridge, UK) and stored in liquid nitrogen until further use. CD4+CD25+ T cells and CD4+CD25− T cells were isolated from total PBMCs using the CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec, Inc., Cambridge, MA, USA), according to the manufacturer's protocol. The cells were cultured in RPMI-1640 (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and supplemented with 10% human AB serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 2 mmol/l L-glutamine and 100 U/ml penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). The cells were subsequently incubated at 37°C with 5% CO2 and at 95% humidity.
Cell proliferation assay
Prior to stimulation, purified CD4+CD25+ T cells (1×106 cells/ml) were labeled with vital dye carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, the cells were cultured in 96-well U-bottomed plates coated with 5 µg/ml anti-CD3 antibody (cat. no. 564001; BD Biosciences, San Jose, CA, USA), 2 µg/ml soluble anti-CD28 antibody (cat. no. 556620; BD Biosciences) and 300 U/ml IL-2 (R&D Systems Inc., Minneapolis, MN, USA) with or without TKI stimulation [500 nM imatinib (Novartis International AG, Basel, Switzerland), 10 nM dasatinib (Bristol-Myers Squibb, New York, NY, USA) or 10 µM nilotinib (Novartis International AG, Basel, Switzerland)]. After 4 days, proliferation was analyzed by flow cytometry (MACSQuant Analyzer 10, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Non-stimulated T cells served as a negative control in all of the experiments.
Suppression assay
In the suppression assay, CD4+CD25− T cells (i.e., responder T cells; 1×105) and CD4+CD25+T cells (i.e., Tregs; 1×105) were co-cultured in 96-well flat bottom plates, in triplicate. After 72 h of anti-CD3 and anti-CD28 treatment as previously described, the percentages of CFSE positive cells were calculated by MACS Quant flow cytometry. Isolated CD4+CD25− T cells were labeled with CFSE as aforementioned. CD4+CD25+ T cells were stimulated with 300 U/ml IL-2 for 24 h. Subsequently, the CD4+CD25+ T cells were incubated with CD4+CD25− T cells for 4 days in the presence of anti-CD3 and anti-CD28 antibodies, as previously described, at a ratio of 1:1.
Cytokine analysis
Purified CD4+CD25+ T cells were incubated with anti-CD3 antibody, anti-CD28 antibody and IL-2, as previously described, for 4 days. On the last day of culture, the supernatants were collected and stored at −70°C. The levels of IL-4, IL-10 and TGF-β were measured using ELISA kits (Human IL-4 ELISA kit, cat. no, 550614; human IL-10 ELISA set, cat. no. 555157; human TGF-β1 ELISA set cat. no. 559119; all from BD Pharmingen, San Diego, CA, USA), according to the manufacturer's protocol.
Flow cytometric analysis of GITR and FOXP3 in CD4+CD25+ T cells. CD4+CD25+ T cells were stimulated with anti-CD3, anti-CD28 and IL-2, as previously described, with and without TKI stimulation (500 nM imatinib, 10 nM dasatinib or 10 µM nilotinib). Following stimulation, the cells were stained with CD4-phycoerythrin (PE) -cyanine 7 (Cy7; dilution, 1:200; cat. no. FAB3791P), CD25-allophycocyanin (APC) -Cy7 (dilution, 1:200; cat. no. FAB1020A), and GITR-PE (dilution, 1:200; cat. no. FAB689P; R&D Systems, Inc.). Following surface staining, the cells were fixed in 2% paraformaldehyde at 37°C for 15 min and stained with FOXP3-PE (dilution, 1:200; cat. no. 12-4776-41; eBioscience; Thermo Fisher Scientific, Inc.) and CTLA-4-APC (dilution, 1:100; cat. no. 555855; BD Pharmingen), according to the manufacturer's protocol. Flow cytometric analysis was performed and analyzed using MACS Quant with CellQuest software.
Statistical analysis
The data are presented as the mean ± standard deviation. The statistical analysis of the data was performed using GraphPad Prism software (version 5.0 for Windows; GraphPad Software, Inc., La Jolla, CA, USA). The statistical significance of differences in continuous variables was assessed by a non-parametric analysis of variance using the Kruskal-Wallis test, and in the case of a significant main effect, pairwise comparisons of patient groups were calculated using Dunn's multiple comparison test. P<0.05 (two-sided) was considered to indicate a statistically significant difference.
Results
Patient characteristics
Between July 2012 and July 2014, 31 patients with CML-CP were enrolled into the study at the diagnostic phase (n=31; mean age, 40 years, range, 25–56 years; males/females, 15/16) and during treatment with a TKI (imatinib, n=12; mean age, 38 years, range, 25–54 years; males/females, 7/5; total duration of therapy, 9 months, range, 7–12 months; dasatinib, n=11; mean age, 43 years, range, 28–56 years; males/females, 5/6; total duration of therapy, 9 months, range, 7–14 months; nilotinib, n=8; mean age, 43 years, range, 28–56 years; males/females, 3/5; total duration of therapy, 9 months, range, 6–12 months). Additionally, 15 healthy donors (n=15, mean age, 30 years, range, 23–37 years; males/females, 9/6) were enrolled. The clinical characteristics of the patients are presented in Table I.
Table I.
Characteristics of patients and healthy donors.
| Parameter | At diagnosis (no therapy) | Imatinib treatment group | Dasatinib treatment group | Nilotinib treatment group | Healthy donors |
|---|---|---|---|---|---|
| Group size, n | 31 | 12 | 11 | 8 | 25 |
| Age, years | |||||
| Median | 40 | 38 | 43 | 43 | 30 |
| Range | 25–56 | 25–54 | 28–56 | 28–56 | 23–37 |
| Males/females, n | 15/16 | 7/5 | 5/6 | 3/5 | 9/6 |
| Number of samples | 31 | 24 | 22 | 16 | 15 |
All patients in the imatinib treatment group (n=12) were only treated with imatinib. By contrast, 5 of the patients in the dasatinib treatment group (n=11) were resistant or intolerant to imatinib and switched to dasatinib. The remaining 6 patients were only treated with dasatinib. In the nilotinib treatment group (n=8), 4 patients switched to nilotinib treatment due to resistance or intolerance to imatinib, and the remaining 4 patients were treated only with nilotinib. All patients were in complete cytogenetic remission [as defined by European LeukemiaNet (ELN) recommendations (18)] at the time of sampling.
Analysis of lymphocytes in patients during treatment with TKIs
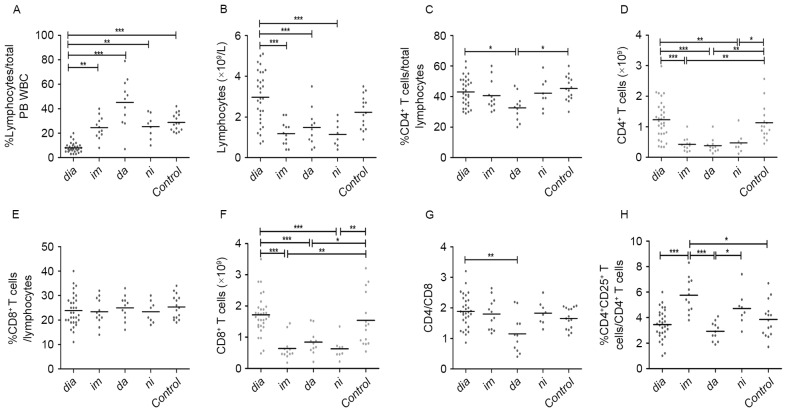
At the time of diagnosis, the patients from all treatment groups exhibited a significantly lower median proportion of lymphocytes compared with healthy controls (8 vs. 29%; P<0.0001; Fig. 1A). There were no significant differences in median absolute lymphocyte numbers between diagnostic-phase patients and healthy controls (Fig. 1B). Patients in the TKI treatment groups had an increased proportion of lymphocytes compared with newly diagnosed patients with CML (25,45 and 25 vs. 8%, for imatinib, dasatinib and nilotinib, respectively, P<0.0001, Fig. 1A). However, following TKI treatment, the median lymphocyte counts in the TKI treatment groups were significantly decreased compared with diagnostic-phase patients (1.18, 1.48 and 1.15 vs. 2.97×109 cells/l, for imatinib, dasatinib and nilotinib, respectively, P<0.0001, Fig. 1B). Additionally, there were no significant differences between the three TKI treatment groups in terms of median proportion and absolute numbers of lymphocytes (Fig. 1A and B).
Figure 1.
Analysis of peripheral blood lymphocytes from patients with CML at diagnosis and during treatment with tyrosine kinase inhibitors. (A) Percentage and (B) absolute numbers of lymphocytes. (C) Percentage and (D) absolute numbers of CD4+ T cells. (E) Percentage and (F) absolute numbers of CD8+ T cells. (G) CD4/CD8 ratio and (H) percentage of CD4+CD25+ T cells. Statistical significance of differences of continuous variables was assessed by a non-parametric analysis of variance using the Kruskal-Wallis test. In the case of a significant main effect, pairwise comparisons of patient groups were calculated using Dunn's multiple comparison test. *P<0.05. CML, chromic myeloid leukemia; CD, cluster of differentiation; dia, diagnosis; im, imatinib treatment; da, dasatinib treatment; ni, nilotinib treatment; Control, control group; PB, peripheral blood; WBC, white blood cells. *P<0.05, **P<0.01 and ***P<0.0001.
Analysis of CD4+ T cells, CD8+ T cells and CD4/CD8 in patients treated with TKIs
The (median) proportion of CD4+ T cells in lymphocytes were comparable among the diagnostic-phase patients, healthy donors, and imatinib and nilotinib treatment groups (Fig. 1C). By contrast, the median proportion of CD4+ T cells in patients in the dasatinib treatment groups was markedly decreased compared with newly diagnosed patients or healthy controls (33, 43 and 45%, for dasatinib, at diagnosis and control, respectively; P=0.0167). The proportions of CD4+ T cells were similar among the three TKI treatment groups (Fig. 1C). In terms of median absolute counts of CD4+ T cells, patients in the imatinib, nilotinib and dasatinib treatment groups exhibited significantly decreased CD4+ T cells compared with diagnostic-phase patients and controls (0.43, 0.38, 0.48, 1.23 and 1.13×109 cells/l, for imatinib, dasatinib, nilotinib, at diagnosis and control, respectively; P<0.0001; Fig. 1D). Notably, the (median) proportions of CD8+ T cells in lymphocytes were similar among all groups (Fig. 1E). The median CD8+ T cells counts of the patients in the three TKI treatment groups were decreased compared with patients at diagnosis and in the control groups (0.64, 0.84, 0.63, 1.72 and 1.54×109 cells/l, for imatinib, dasatinib, nilotinib, at diagnosis and control, respectively; P<0.0001; Fig. 1F). With the exception of the decreased CD4/CD8 ratio of the dasatinib treatment group, the CD4/CD8 ratios of other groups were similar and close to normal levels [normal levels, 1.82±0.39 (1.18–2.6); 1.15 vs. 1.89, 1.66, for dasatinib, at diagnosis and control, respectively; P=0.0197; Fig. 1G].
Analysis of CD4+CD25+ T cells in CML patients treated with TKIs
To assess whether imatinib, dasatinib, nilotinib have different effects on CD4+CD25+ T cells, the (median) proportions and absolute numbers of CD4+CD25+ T cells in patients in the TKI treatment groups were analyzed. The imatinib and nilotinib treatment groups exhibited increased (median) proportions of CD4+CD25+ T cells compared with the dasatinib treatment group, the newly diagnosed group and the control (5.8, 4.7, 2.9, 3.5 and 3.9%, for imatinib, nilotinib, dasatinib, at diagnosis and control, respectively; P<0.0001; Fig. 1H).
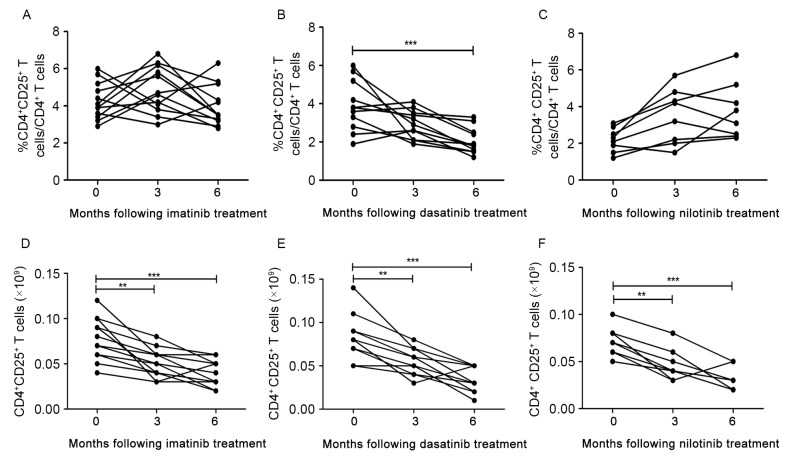
For patients in the imatinib treatment group, the median proportion of CD4+CD25+ T cells increased at 3 months following treatment and subsequently decreased to diagnostic level at 6 months (Fig. 2A). However, for patients in the dasatinib treatment group, a decrease in the median CD4+CD25+ T cells proportion was observed at 3 months following treatment, and the decrease was significant at 6 months (3.9 vs. 2.0%, for at diagnosis and 6 months, respectively; P=0.0009; Fig. 2B). No significant increase was identified in the median proportion of CD4+CD25+ T cells in the nilotinib treatment group between 3 and 6 months following treatment (Fig. 2C).
Figure 2.
Analysis of CD4+CD25+ T cells from peripheral blood of patients with chronic myeloid leukemia at diagnosis and during treatment with tyrosine kinase inhibitors. Percentage of CD4+CD25+T cells in (A) imatinib, (B) dasatinib and (C) nilotinib. Absolute numbers of CD4+CD25+T cells in (D) imatinib, (E) dasatinib and (F) nilotinib treatment groups. CD, cluster of differentiation. *P<0.05, **P<0.01 and ***P<0.0001.
Furthermore, median absolute numbers of CD4+CD25+ T cells in the three TKI treatment groups decreased significantly between 3 and 6 months following treatment compared with at diagnosis (P<0.0001, P<0.0001 and P=0.0004, for imatinib, dasatinib and nilotinib, respectively; Fig. 2D-F).
Proliferation and suppressive capacity of CD4+CD25+ T cells in patients treated with TKIs
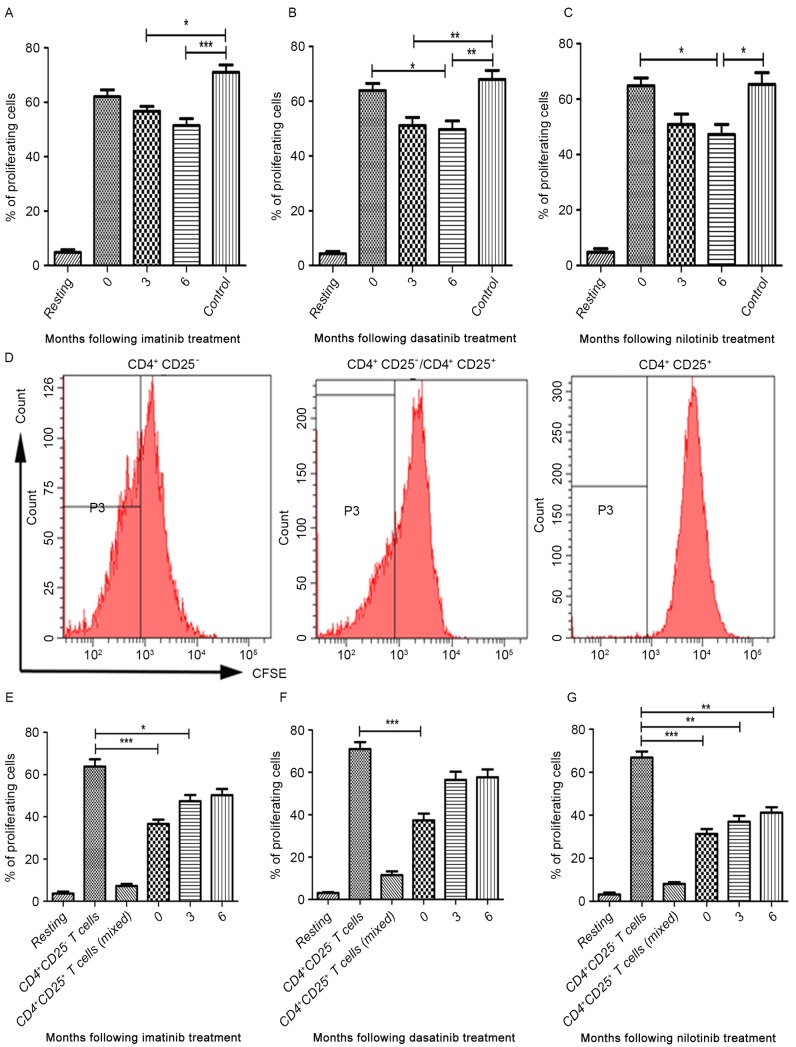
As demonstrated by proliferation assays, the proportion of proliferating CD4+CD25+ T cells in the three TKI treatment groups and healthy controls were increased following stimulation with anti-CD3, anti-CD28 and IL-2 (Fig. 3A-C). No differences were observed in the median proportion of proliferating CD4+CD25+ T cells between diagnostic-phase CML patients and controls (Fig. 3A-C). In the imatinib treatment group, the median proportion of proliferating CD4+CD25+ T cells at 3 and 6 months following treatment were decreased compared with healthy donors (P=0.0003; Fig. 3A). However, no significant differences were identified in the median proportion of proliferating CD4+CD25+ T cells between patients in the imatinib treatment group and at the time of diagnosis (Fig. 3A). In the dasatinib treatment group, the median proportion of proliferating CD4+CD25+ T cells at 3 months following treatment was significantly decreased compared with the control (P<0.0001). Furthermore, the median proportion of proliferating CD4+CD25+ T cells at 6 months following treatment was significantly decreased compared with the diagnostic-phase group and the control (P=0.0004; Fig. 3B). A similar trend was observed in the nilotinib treatment group (P=0.0004; Fig. 3C). However, no significant differences were identified in the median proportion of proliferating CD4+CD25+ T cells between 3 and 6 months following treatment in all three TKI treatment groups (Fig. 3A-C).
Figure 3.
Proliferative and suppressive capacity of CD4+CD25+ T cells isolated from peripheral blood in patients with chronic myeloid leukemia from three different tyrosine kinase inhibitor treatment groups at 3 and 6 months following treatment. ‘Resting’ is a negative control without CFSE. Treatment with (A) imatinib, (B) dasatinib and (C) nilotinib. (D) Proliferation of CD4+CD25- T cells decreased when incubated with CD4+CD25+ T cells. (E) imatinib (F) dasatinib and (G) nilotinib. CD4+CD25- T cells and CD4+CD25+ T cells were co-cultured either alone or at a 1:1 ratio. CD, cluster of differentiation; CFSE, carboxyfluorescein diacetate succinimidyl ester. *P<0.05, **P<0.01 and ***P<0.0001.
To further investigate the effect of imatinib, dasatinib and nilotinib treatment on the suppressive capacity of the CD4+CD25+ T cells, CD4+CD25+ T cells from patients with CML treated with TKIs were incubated in the presence of IL-2 prior to being co-cultured with CD4+CD25− T cells at a ratio of 1:1. Following incubation, the median proliferation proportion of CD4+CD25− T cells was analyzed using flow cytometry (Fig. 3D). In the imatinib treatment group, imatinib treatment was able to inhibit the suppressive capacity of the CD4+CD25+ T cells at 6 months following treatment (P<0.0001; Fig. 3E). In dasatinib treatment group, there was a significant inhibitory effect of dasatinib between 3 and 6 months following treatment (P=0.0003; Fig. 3F). Notably, nilotinib did not abrogate the suppressive capacity of CD4+CD25+ T cells (Fig. 3G).
Analysis of cytokines secreted by CD4+CD25+ T cells from patients treated with TKIs
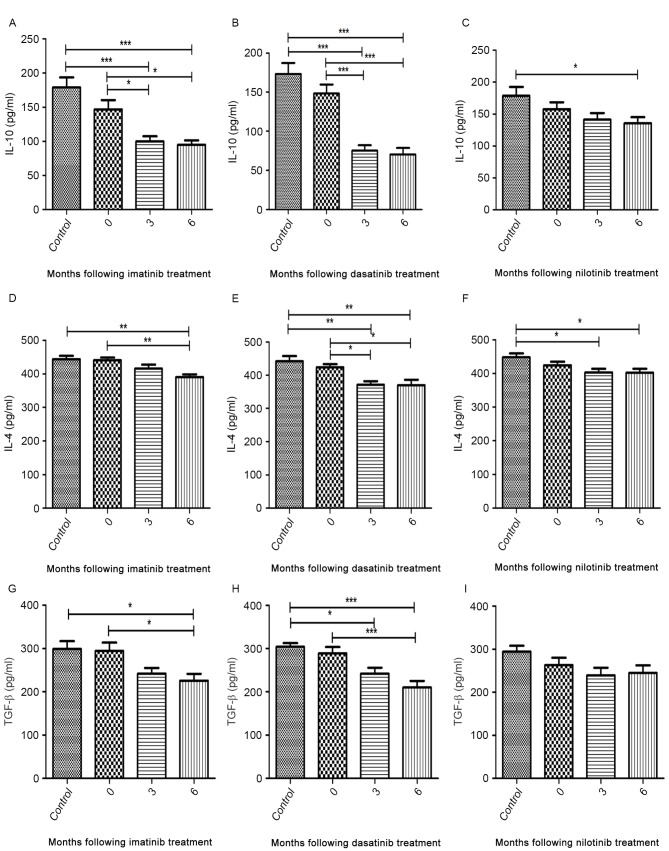
The median concentrations of IL-4, IL-10 and TGF-β secreted by CD4+CD25+ T cells from diagnostic-phase patients were comparable with those of healthy controls (Fig. 4A-I). During imatinib and dasatinib treatment, the production of cytokines was significantly inhibited to various degrees (Fig. 4A, B, D, E, G and H). Nilotinib treatment was able to significantly inhibit the secretion of IL-10 and IL-4 but not that of TGF-β (Fig. 4C, F and I). Additionally, no significant difference was identified in the median cytokine concentration detected between 3 and 6 months following treatment (Fig. 4A-I).
Figure 4.
ELISA of IL-10, IL-4 and TGF-β in CD4+CD25+ T cells. A decrease in the level of IL-10, IL-4 and TGF-β was detected at 3 and 6 months following treatment with tyrosine kinase inhibitors. Levels of IL-10 following treatment with (A) imatinib (B) dasatinib and (C) nilotinib; IL-4 following treatment with (D) imatinib (E) dasatinib and (F) nilotinib; TGF-β. following treatment with (G) imatinib (H) dasatinib and (I) nilotinib. CD, cluster of differentiation; IL, interleukin; TGF-β, transforming growth factor-β. *P<0.05, **P<0.01 and ***P<0.0001.
Expression of CD4+CD25+ T cell-specific molecules in patients treated with TKIs
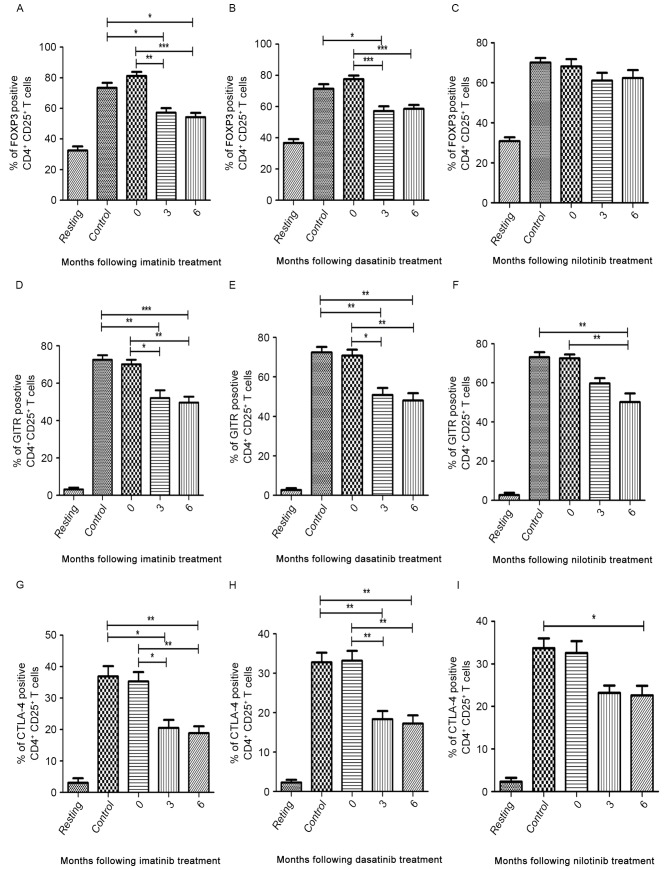
The median expression levels of FOXP3, GITR and CTLA-4 in patients at the time of diagnosis were similar to those of the controls (Fig. 5A-I). In the imatinib and dasatinib treatment groups, the median expression of FOXP3, GITR and CTLA-4 decreased between 3 and 6 months following treatment compared with patients at diagnosis and healthy volunteers (Fig. 5A, B, D, E, G and H). In the nilotinib treatment group, the median expression of GITR and CTLA-4 significantly decreased at 6 months following treatment compared with diagnostic-phase patients (P<0.0001 and P=0.004, respectively; Fig. 5F and I). However, nilotinib did not significantly inhibit the expression of FOXP3 (Fig. 5C).
Figure 5.
Flow cytometric analysis of FOXP3, GITR and CTLA-4 in CD4+CD25+ T cells in three TKI treatment groups. ‘Resting’ is a negative control without antibodies. Percentage of FOXP3-positive cells in (A) imatinib, (B) dasatinib and (C) nilotinib treatment groups. Percentage of GITR-positive cells in (D) imatinib, (E) dasatinib and (F) nilotinib treatment groups. Percentage of CTLA-4-positive cells in (G) imatinib, (H) dasatinib and (I) nilotinib treatment groups. FOXP3, forkhead box P3; GITR, tumor necrosis factor receptor; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; CD, cluster of differentiation; TKI, tyrosine kinase inhibitor. *P<0.05, **P<0.01 and ***P<0.0001.
Discussion
The clinical benefits obtained with TKIs in the treatment of solid cancers and Philadelphia-positive (Ph+) CML have led to a new era in oncology, creating possibilities for advances in tumor-targeted therapies (19). TKIs are usually tolerable to the patient (relative to other forms of therapy), and therapy discontinuation due to side effects is uncommon. Although for the majority of the time TKIs are well-tolerated, they are not entirely selective for oncogenic kinases (20). Therefore, long-term off-target effects in normal cells and tissues may occur. Recently, a number of in vitro studies have investigated the inhibitory effects of TKIs on immune response. Treatment with imatinib and dasatinib has been demonstrated to reversibly inhibit T-cell proliferation in vitro, and the effect of dasatinib treatment is greater than that of imatinib (21,22). Treatment with dasatinib was able to inhibit LCK at low concentrations. By contrast, higher concentrations of imatinib were required to be effective (23). Furthermore, treatment with nilotinib inhibited T cells approximately twice as markedly as imatinib (24). Therefore, dasatinib and nilotinib have been suggested to be potent T cell activation inhibitors.
In the present study, patients with CML had similar absolute numbers of lymphocytes compared with healthy donors. During treatment with TKIs, the absolute number of total T cells, CD4+ T and CD8+ T cells were decreased compared with diagnostic-phase patients. Absolute numbers and proportion of lymphocytes did not differ significantly between TKI treatment groups. The results of the present study were consistent with the idea that TKIs have immune suppressive effects, and the clinical complications induced by these effects require further study with larger samples and longer follow-up periods.
Tregs constitute a naturally occurring subpopulation of T cells with immunosuppressive capacity. Consistently, the results of the present study indicated that imatinib dasatinib and nilotinib attenuate the quantity of Tregs from patients with CML. In the present study, a number of patients in the imatinib and nilotinib treatment groups exhibited increased proportion and absolute numbers of Tregs compared with those in the dasatinib treatment group. Previous studies have indicated that increased numbers of Tregs in tumor tissue are associated with an improved prognosis (25). TKIs have been reported to be able to affect the quantity of Tregs and their function. As an indispensable molecule, which mediates suppression, FOXP3 is highly expressed in Tregs (26). When FOXP3 genes mutate, the probability of the occurrence of autoimmune diseases and/or allergies increases (27,28). In humans, FOXP3highCD25highCD4+ T cells have marked suppression, and this is accompanied by high levels of CTLA-4 expression, which may be promoted by FOXP3 (29). Results from prior experiments have demonstrated that anti-GITR antibodies may reverse the suppressive effects of Tregs in vitro and indicated that GITR exerts an important effect on the function of Tregs (30). Apart from being dependent on cell-to-cell contact, Tregs also exhibit immunosuppressive activity by secreting suppressive cytokines, including IL-4, IL-10 and TGF-β. Inhibition of the IL-10 receptor and the neutralization of TGF-β are also able to abolish Treg-mediated inhibition of autoimmune disease (31). Taken together, cytokines and molecules expressed on the cell surface of Tregs synergistically contribute to their suppressive functions (32). Therefore, it is necessary to detect suppressive cytokines and key suppressive-associated molecules in order to comprehensively evaluate the effect of TKIs on functions of Tregs.
In the present study prior to treatment with TKIs, Tregs from patients with CML-CP have similar levels of proliferation, suppression, concentrations of suppressive cytokines (IL-4, IL-10 and TGF-β) and cell-specific molecules (FOXP3, GITR and CTLA-4) compared with healthy donors. During treatment with TKIs, cytokines and the expression of cell-specific molecules of Tregs in the imatinib and dasatinib treatment groups decreased to different degrees. The inhibitory effect of imatinib and dasatinib treatment on Tregs was observed for 3 months after treatment. The results of the present study have demonstrated that the inhibitory effects of imatinib and dasatinib treatment remain for up to 6 months. By contrast, the inhibitory effect of nilotinib treatment on Tregs was not significant.
The results of the present study differ from a previous study, which demonstrated that nilotinib treatment was more potent compared with imatinib treatment in its inhibition of T cells (24). The discrepancy may be due to factors, including, but not limited to, differences in the treatment dosage and duration of TKIs, individual drug metabolism and the in vivo environment. The inhibitory effects of imatinib and dasatinib treatment may impair the immunosuppressive role of Tregs, which in turn may induce a relative immune hyper-reactivity. It should be noted that careful follow-up is warranted in patients with impaired Tregs to prevent the possibility of autoimmune diseases.
In conclusion, TKIs exhibit different off-target effects in T lymphocytes, particularly Tregs in patients with CML-CP. The results of the present study have demonstrated that imatinib and dasatinib treatments have more marked inhibitory effects on the number and functions of Tregs compared with nilotinib treatment. These results indicate that personalized treatment and follow-up are warranted. The immune system is relevant for infection control and for the mediation of tumor cell expansion. In the near future, strategies may be developed for controlling CML by combining TKIs with adoptive immunotherapy.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81170521) and the Science Foundation of Nanfang Hospital (grant no. 2012Z013). The abstract of the present study was presented at the 57th American Society of Hematology Annual Meeting, 5–8 December 2015, Orlando, FL, USA and was published as Abstract no. 4036 in Blood (126), 2015.
References
- 1.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 2.Rix U, Hantschel O, Dürnberger G, Rix LL Remsing, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 3.van Oers NS, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meignin V, de Latour R Peffault, Zuber J, Régnault A, Mounier N, Lemaître F, Dastot H, Itzykson R, Devergie A, Cumano A, et al. Numbers of Foxp3-expressing CD4+CD25high T cells do not correlate with the establishment of long-term tolerance after allogeneic stem cell transplantation. Exp Hematol. 2005;33:894–900. doi: 10.1016/j.exphem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, Bellucci R, Alyea EP, Antin JH, Soiffer RJ, Ritz J. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theil A, Tuve S, Oelschlägel U, Maiwald A, Döhler D, Oßmann D, Zenkel A, Wilhelm C, Middeke JM, Shayegi N, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy. 2015;17:473–486. doi: 10.1016/j.jcyt.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Bluestone JA, Trotta E, Xu D. The therapeutic potential of regulatory T cells for the treatment of autoimmune disease. Expert Opin Ther Targets. 2015;19:1091–1103. doi: 10.1517/14728222.2015.1037282. [DOI] [PubMed] [Google Scholar]
- 8.Demirkiran A, Hendrikx TK, Baan CC, van der Laan LJ. Impact of immunosuppressive drugs on CD4+CD25+FOXP3+ regulatory T cells: Does in vitro evidence translate to the clinical setting? Transplantation. 2008;85:783–789. doi: 10.1097/TP.0b013e318166910b. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savani BN, Montero A, Kurlander R, Childs R, Hensel N, Barrett AJ. Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;36:1009–1015. doi: 10.1038/sj.bmt.1705167. [DOI] [PubMed] [Google Scholar]
- 12.Weisser M, Tischer J, Schnittger S, Schoch C, Ledderose G, Kolb HJ. A comparison of donor lymphocyte infusions or imatinib mesylate for patients with chronic myelogenous leukemia who have relapsed after allogeneic stem cell transplantation. Haematologica. 2006;91:663–666. [PubMed] [Google Scholar]
- 13.Chunduri S, Dobogai LC, Bruno A, Kadkol S, Rondelli D. Does post-transplant treatment with imatinib mesylate inhibit graft-versus-leukemia? Leukemia. 2005;19:456–457. doi: 10.1038/sj.leu.2403656. [DOI] [PubMed] [Google Scholar]
- 14.de Rezende LC, Silva IV, Rangel LB, Guimarães MC. Regulatory T cell as a target for cancer therapy. Arch Immunol Ther Exp (Warsz) 2010;58:179–190. doi: 10.1007/s00005-010-0075-0. [DOI] [PubMed] [Google Scholar]
- 15.Larmonier N, Janikashvili N, LaCasse CJ, Larmonier CB, Cantrell J, Situ E, Lundeen T, Bonnotte B, Katsanis E. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. J Immunol. 2008;181:6955–6963. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Götz M, Döhner H, Bunjes D, Schmitt M. Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells. Br J Haematol. 2009;144:195–205. doi: 10.1111/j.1365-2141.2008.07433.x. [DOI] [PubMed] [Google Scholar]
- 17.Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J, Götz M, Bunjes D, Schmitt M. Effects of nilotinib on regulatory T cells: The dose matters. Mol Cancer. 2010;9:22. doi: 10.1186/1476-4598-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah K, Parikh S, Rawal R. Tyrosine kinase inhibitors in Ph+ chronic myeloid leukemia therapy: A review. Asian Pac J Cancer Prev. 2016;17:3025–3033. [PubMed] [Google Scholar]
- 20.Hughes TP, Hochhaus A, Branford S, Müller MC, Kaeda JS, Foroni L, Druker BJ, Guilhot F, Larson RA, O'Brien SG, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: An analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116:3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seggewiss R, Loré K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105:2473–2479. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 22.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111:1366–1377. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das J, Chen P, Norris D, Padmanabha R, Linc J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, et al. 2-Aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carbox-amide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem. 2006;49:6819–6832. doi: 10.1021/jm060727j. [DOI] [PubMed] [Google Scholar]
- 24.Blake SJ, Lyons AB, Hughes TP. Nilotinib inhibits the Src-family kinase LCK and T-cell function in vitro. J Cell Mol Med. 2009;13:599–601. doi: 10.1111/j.1582-4934.2009.00500_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustjoki S, Lundán T, Knuutila S, Porkka K. Appearance of bone marrow lymphocytosis predicts an optimal response to imatinib therapy in patients with chronic myeloid leukemia. Leukemia. 2007;21:2363–2368. doi: 10.1038/sj.leu.2404807. [DOI] [PubMed] [Google Scholar]
- 26.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 27.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: Regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 28.Geiger TL, Sharyn T. Nature and nurture in Foxp3(+) regylatory T cell development, stability, and function. Hum Immunol. 2012;73:232–239. doi: 10.1016/j.humimm.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 31.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+)CD25(+) T cells. J Autoimmun. 2001;16:115–123. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Du Y, Lin X, Qian Y, Zhou T, Huang Z. CD4+CD25+ regulatory T cells in tumor immunity. Int Immunopharmacol. 2016;34:244–249. doi: 10.1016/j.intimp.2016.03.009. [DOI] [PubMed] [Google Scholar]