Abstract
The estrogen receptors (ERs) ERα and ERβ are important factors in breast cancer progression. Nevertheless, the molecular interplay between ERα and ERβ and its clinical significance in breast cancer is controversial. The establishment of a clear association is required; therefore, the current study analyzed the expression patterns of ERα and ERβ in 32 breast tumor tissues using reverse transcription-quantitative polymerase chain reaction. Furthermore, human epidermal growth factor receptor 2 (HER2) and the Ki-67 status were detected by immunohistochemistry. The results revealed that the ERα and ERβ expression rates recorded were 68 and 65%, respectively. The ERα:ERβ ratio exhibited a decline along with disease progression. ERα and ERβ were found to be negatively correlated with HER2 status but positively correlated with Ki-67. Co-expression of ERα and ERβ was associated with breast cancer aggressiveness, including higher histological grade and positive nodal status, which commonly occur following the menopause. In addition, in cases where ERβ was coexpressed with ERα, HER2 expression was frequently found to be negative, whereas the Ki-67 index was upregulated. These data suggest that ERα and ERβ co-expression may be an indicator of tumor aggressiveness and the sensitivity of hormonal therapy via the downregulation of HER2.
Keywords: estrogen receptor α, estrogen receptor β, breast cancer, human epidermal growth factor receptor 2, Ki-67
Introduction
Estrogen regulates numerous physiological processes including normal cell growth, the central nervous and skeletal systems and the development and regulation of tissue-specific genes in the genital tract (1–3). Estrogen also affects the pathological process of numerous hormone-dependent diseases including breast, endometrial and ovarian cancer (1). The biological actions of estrogen are mediated by the binding to one of two specific estrogen receptors (ERs), ERα or ERβ, which belong to the nuclear receptors superfamily (3). The binding of estrogen to its receptor leads to a conformational change in the structure of the ER and to the formation of estrogen receptor dimers that bind estrogen response elements as homo- or hetero-dimers within the regulatory sequences of estrogen-dependent genes (4). This conformational change, occurring as a result of ligand binding, facilitates the association of coactivator receptors and stabilizes the estrogen receptor complex with estrogen response elements (4). This promotes gene transcription and supports the stimulation of cell growth in various tissues (5–7). ERα was the first estrogen receptor to be isolated and cloned in 1980 from a cell line of human breast cancer (MCF7) (8,9). In the presence of estradiol (E2), this receptor may induce cell proliferation via the regulation of certain genes including Myc, cyclinD and Wnt11 (10). These genes have several effects, including interfering with cadherins, stimulating the cell cycle, promoting the transition from G1 to S phase and altering apoptosis (10,11). This leads to an increase in cell division, which may cause errors in replication and promote cancer development (11).
In the 1990s, ERβ was the second estrogen receptor discovered and identified in the rat prostate and ovaries, encoded by 485 amino acids (1996) (12). In the same year, ERβ was also isolated from human tissues, in this case encoded by 477 amino acids (13). The role of ERβ in breast cancer remains to be established (14,15). The majority of previous studies have shown that ERβ functions as a negative modulator of ERα and is associated with a good prognosis and prolonged disease-free survival (16,17).
Previous studies have revealed that the expression of ERβ is correlated with a poor prognosis, including accelerated cell proliferation and distant metastasis (18,19). However, Speirs et al (20) have identified that co-expression of ERα and ERβ is associated with high-grade tumors and metastases. In addition, Grober et al (21) demonstrated that ERβ is able to interfere with ERα in the regulation of target genes. It is therefore necessary to understand the role of ERβ in breast cancer and to elucidate the nature of its association with ERα. In this context, the present study was conducted to investigate the expression the of estrogen receptors ERα and ERβ in a series of breast cancer tumors. This was achieved by comparing the results with clinical and pathological parameters, as well as the expression of the oncoprotein human epidermal growth factor receptor 2 (HER2) and the proliferation index Ki-67. The objective of this study was also to analyze the ERα and ERβ subgroups according to the aforementioned parameters.
Materials and methods
Patients and samples
The tissue of malignant mammary tumors was excised during tumorectomy from 32 females (mean age, 58.5 years; range, 32–85 years) was analyzed. Healthy tissues collected from patients during the tumorectomy were used as controls. The patients all had invasive ductal carcinoma and did not receive radiotherapy or chemotherapy prior to surgery. The samples were subject to a histological examination by a pathology specialist to determine the presence of malignant cells. Each diagnosed sample was divided into two portions: One portion was immediately processed for immunohistochemistry and the other portion was frozen and maintained at −80°C until RNA extraction. All pathological, clinical and personal data were anonymized and separated from any personal identifiers. All the procedures followed were examined and approved by the Saleh Azaiez Oncology Institute (Tunis, Tunisia).
Total RNA isolation and reverse transcription
Total RNA was extracted from breast specimens using a mechanical stirrer in the presence of a lysis buffer [4.5 M guanidine-HCl, 50 ml Tris-Hcl, 30% Triton X-100 (w/v) pH 6.6 (25°C)], prior to the use of total RNA isolation and high pure RNA isolation kits (Roche Diagnostics, Basel, Switzerland). Equal amounts of total RNA (1 µg) were reverse transcribed. cDNA synthesis was carried out using the PrimeScript™ 1st strand cDNA Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China).
Primers and quantitative polymerase chain reaction (qPCR)
All PCR reactions were performed using an ABI Prism 7700 Sequence Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA was amplified using the SYBR1-Green PCR Core Reagents kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction mixes used for qPCR were as follows: 10 µl SYBR-Green (Applied Biosystems; Thermo Fisher Scientific, Inc.), 6 µl water; 1 µl forward primer; 1 µl reverse primer; and 2 µl cDNA. The primers used to amplify ERα were as follows: forward, 5′-TGCCAAGGAGACTCGCTA-3′; reverse, 5′-TCAACATTCTCCCTCCTC-3′. For ERβ, the primer sequences were forward, 5′-TGTTACGAAGTGGGAATGTGA-3′ and reverse, 5′-TCTTGTTCTGGACAGGGATG-3 (40 cycles of: 94°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec for both ERα and ERβ). 18S was used as an endogenous control. The primer sequences used to amplify 18S were forward, 5′GTAACCCGTTGAACCCCATT-3′ and reverse, 5′-CCATCCAATCGGTAGTAGCG-3′ (40 cycles of: 94°C for 30 sec, 56°C for 30 sec and 72°C for 30 sec). Relative mRNA levels were calculated based on Cq values and corrected for the 18S expression according to the equation 2−ΔΔCq (22). Relative mRNA levels in the control tissue were equated to 1 and other values were expressed relative to this.
All primer pairs were initially validated by testing them for equal amplification efficiencies. The amplification efficiency was close to 2 under these conditions. Experiments were performed in triplicate for each data point.
Immunohistochemical staining
The immunohistochemistry expression of the oncoprotein Her2/neu and the proliferation index Ki-67 were tested on the same set of tumors. The primary antibodies used were as follows: Mouse anti-human Her2 (#CB11) and mouse anti-human Ki-67 (#MM1) (Novocastra; Leica Biosystems GmbH, Wetzlar, Germany).
A total of 32 tissue samples were fixed for 24 h at room temperature in 0.1 M phosphate buffered 10% formaldehyde, dehydrated in a graded ethanol series of increasing concentrations (70, 85, 90 and 100% for 10 min each), impregnated with xylene and embedded in paraffin. Sections (4 µm thick) were processed following the NovoLink™ Polymer Detection systems (Novocastra; Leica Biosystems GmbH) method. Sections were deparaffinized by an overnight incubation at 59°C, and subsequently placed in a xylene bath for 15 min at room temperature. Sections were subsequently hydrated, incubated for 30 min in 1% hydrogen peroxide to block endogenous activity, and then antigen retrieval was performed by incubating the sections in a 0.01 M citrate buffer (Epitope Retrieval Solution pH 6.0; Leica Microsystems GmbH, Wetzlar, Germany) for 30 min at 98°C. Subsequently, the primary antibodies were applied for 1 h at 4°C, with a dilution of 1:40 for Her2 and 1:200 for Ki-67. The sections were then incubated at room temperature with Post Primary Block for 30 min to block non-specific polymer binding. The sections were incubated with a NovoLink™ Polymer for 30 min at room temperature, followed by incubations with 3,3′-diaminobenzidine (DAB) working solution for 5 min at room temperature to develop peroxidase activity. The slides were counterstained with hematoxylin and mounted. Staining specificity was checked using negative controls. Negative controls were obtained by replacing the primary antibody with an antibody of the same unrelated isotope during the immunohistochemistry technique or by omission of the primary antibody. Primary breast tissues were incubated in blocking peptides (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) instead of primary antibodies.
The Ki-67 assessment and the Her2 status tests were performed by two experienced breast pathologists. The percentage of positively stained cells obtained is an average following counting of the stained cells and the total number of cells were counted in four high-magnification fields using a light microscope (magnification, ×400; Media Cybernetics, Inc., Rockville, MD, USA). The staining of Ki-67 was scored for the percentage of positive cells (0, 0–5%; 1, 6–25%; 2, 26–50%; 3, >50%). The optimal cutoff value was identified as 1 for the low Ki-67 expression level and >1 for high expression (23). The scoring of Her2 was performed on a 0–3 scale (24). Positive (3+) was defined as intense complete membranous staining in >30% of the tumor cell population; borderline (2+) was defined as moderate membranous staining in >10% of tumor cells; 1+ was defined as either weak or barely perceptible membranous staining in >10% of the tumor cells. Furthermore, a chromogenic in situ hybridization (CISH) analysis was performed, as described previously (25) for Her2/neu gene amplification in all 2+ cases, as defined by IHC. Scores of 0, 1+ and 2+ according to IHC but negative following CISH were considered as negative for the Her2/neu expression, whereas 3+ scores and 2+ cases defined as positive by CISH were considered as positive for Her2/neu expression.
Statistical analysis
The analysis of the results involving the expression of the estrogen receptors ERα and ERβ together with the various histological and clinical parameters was performed using the χ2 test with R (i386 3.2.1) software. Data are presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of mRNA ERα and ERβ in malignant human breast tissues and their association with clinical parameters
In total, 32 breast tumor samples were analyzed and compared for the expression of the two ER isoforms by reverse transcription-qPCR. The expression of estrogen receptors in breast cancer tumors had a positivity of ~68% for ERα and 65% for ERβ. As presented in Table I, no significant difference was identified between the levels of expression of the two ER isoforms (P>0.05).
Table I.
Association between mRNA ERα and ERβ levels and standard clinicopathological factors and molecular settings.
| ERα | ERβ | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total population | Negative (%) | Positive (%) | P-value | Negative (%) | Positive (%) | P-value |
| Total | 32 (100%) | 10 (31.28) | 22 (68.75) | 11 (34.37) | 21 (65.62) | >0.05 | |
| Age | |||||||
| ≤50 years | 12 (37.5) | 4 (40) | 8 (36.36) | 5 (45.45) | 7 (33.33) | ||
| >50 years | 20 (62.5) | 6 (60) | 14 (63.63) | 0.13 | 6 (54.54) | 14 (66.66) | 0.06 |
| Grade | |||||||
| SBRI | 7 (21.87) | 4 (40) | 3 (13.63) | 4 (36.36) | 3 (14.28) | ||
| SBRII | 17 (53.12) | 3 (30) | 14 (63.63) | 5 (45.45) | 12 (57.1) | ||
| SBRIII | 8 (25) | 3 (30) | 5 (22.72) | 0.01 | 2 (18.18) | 6 (28.57) | 0.01 |
| Lymph node status | |||||||
| Positive | 17 (53.12) | 6 (60) | 11 (50) | 4 (36.36) | 13 (61.90) | ||
| Negative | 15 (46.87) | 4 (40) | 11 (50) | >0.05 | 7 (63.63) | 8 (38.09) | 0.20 |
| Tumor size | |||||||
| ≤30 mm | 22 (68.75) | 6 (60) | 16 (72.72) | 8 (72.72) | 14 (66.66) | ||
| >30 mm | 10 (31.25) | 4 (40) | 6 (27.27) | 0.006 | 3 (27.27) | 7 (33.33) | 0.06 |
| HER2 status | |||||||
| Positive | 8 (25) | 3 (30) | 5 (22.72) | 6 (72.72) | 2 (57.14) | ||
| Negative | 24 (75) | 7 (70) | 17 (77.27) | 0.0009 | 5 (27.27) | 19 (42.85) | 7.905−7 |
| Ki-67 | |||||||
| ≤5% | 9 (28.12) | 3 (44) | 6 (27.27) | 3 (27.27) | 6 (28.57) | ||
| >5% | 23 (71.87) | 7 (56) | 16 (72.72) | 0.0066 | 8 (72.72) | 15 (71.42) | 0.01 |
P-values calculated using the χ2 test in R. SBR, Scarff-Bloom-Richardson; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor.
In order to determine whether the expression of each ER isoform in the breast tumors was associated with clinical parameters, associations between tumor grade and size, lymph node metastasis and menopausal status were examined (Table I). When compared with tumor grade, there was a significant association between ERα and ERβ expression and tumor grade (P=0.01; Table I). Analysis of the mRNA expression of the receptors ERα and ERβ revealed a significant increase of ERα in tumors that were grade 2 compared with grades 1 and 3. However, the highest ERβ expression was found in the Scarff-Bloom-Richardson (SBR)3 grade (Fig. 1). According to the histopathological grade progress, the ERα: ERβ ratio declined from SBR1 to SBR2 and SBR3 (Fig. 2).
Figure 1.
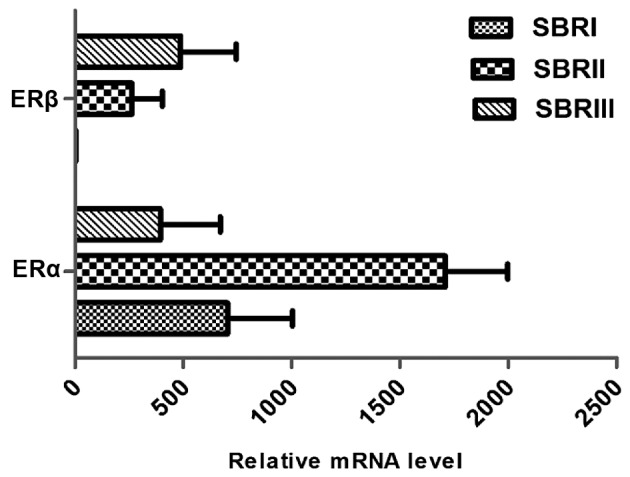
ERα and ERβ mRNA expression level according to SBR grade. ER, estrogen receptor; SBR, Scarff-Bloom-Richardson.
Figure 2.
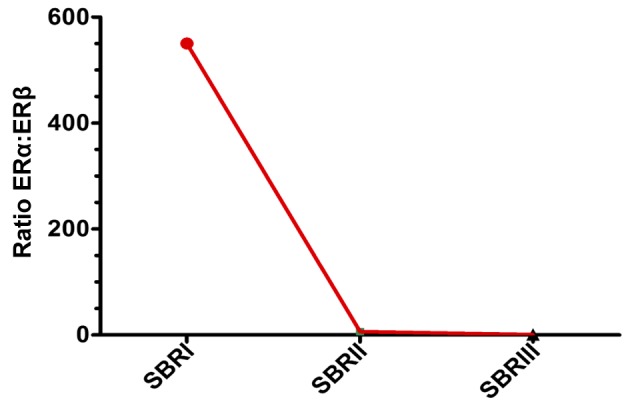
ERα:ERβ ratio according to SBR grade. ER, estrogen receptor; SBR, Scarff-Bloom-Richardson.
Tumor size was correlated with ERα (P=0.006) expression and did not correlate with ERβ expression (P=0.06; Table I). In addition, there was no significant difference in ERα and ERβ expression according to lymph node metastasis status (P>0.05; Table I). As presented in Fig. 3, the expression of the two ER isoforms differed with menopausal status. The amount of mRNA ERα expression was 1.5X higher in premenopausal breast tumors compared with postmenopausal breast tumors. Inversely, mRNA expression of ERβ increased between the premenopausal and postmenopausal status (Fig. 3). Furthermore, a shift in the ERa:ERβ ratio was noted and this was revealed to decline in the postmenopausal vs. premenopausal status group (7.66 to 3.36) (Fig. 4).
Figure 3.
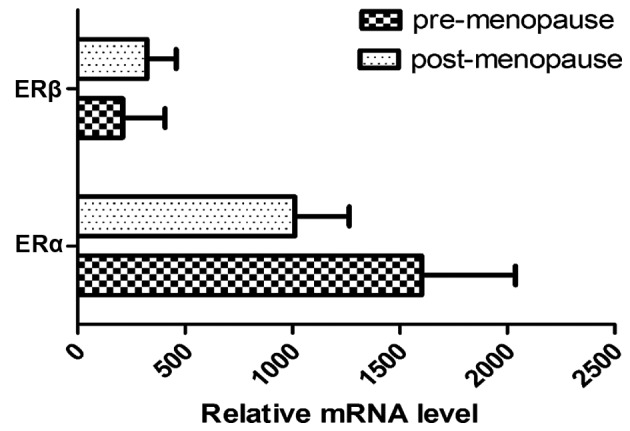
mRNA expression level of ERα and ERβ according to menopausal status. ER, estrogen receptor; SBR, Scarff-Bloom-Richardson.
Figure 4.
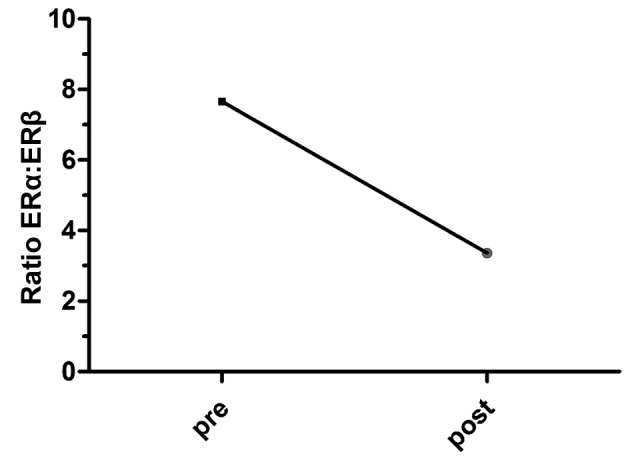
ERα:ERβ ratio according to menopausal status. ER, estrogen receptor; pre, premenopausal; post, postmenopausal.
HER2/neu and Ki-67 in ER-positive vs. ER-negative cases
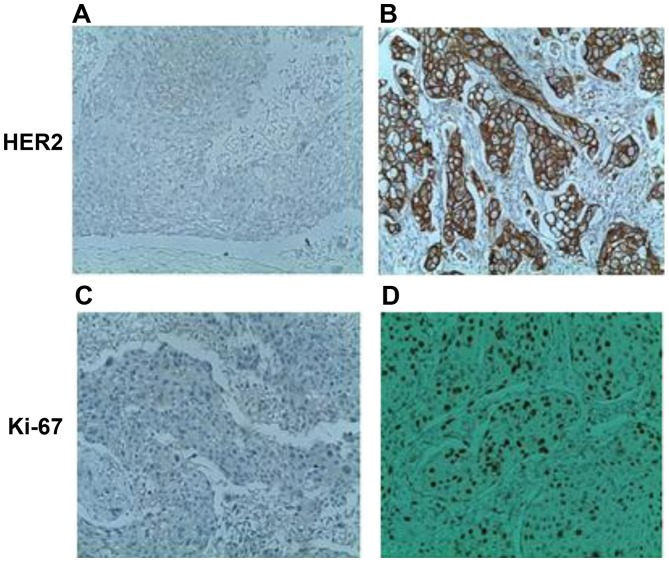
Differences in the HER2 and Ki-67 status between ER-positive and ER-negative cases were further analyzed. Immunohistochemical analysis was performed to assess the expression of HER2 and Ki-67 in breast cancer samples. Fig. 5 reveals membrane localization of the HER2 protein in malignant breast cells. Ki-67 was primarily localized to the nucleus of breast neoplastic cells (Fig. 5). As summarized in Table I, a marked negative association between the two estrogen receptors and the oncoprotein HER2/neu was observed. In total, 17/22 ERα positive cases were negative for HER2. Similarly, 19/21 ERβ positive cases were negative for HER2. This negative association was more significant for ERβ (P=7.905×10−7) than ERα (P=0.0009; Table I).
Figure 5.
Immunochemical staining with anti-HER2 and anti-Ki-67 antibodies in breast tumors. (A) Absence of overexpression of HER2 oncoprotein (HER2 score, 0; original magnification, ×250). (B) Presence of overexpression of HER2 oncoprotein (HER2 score, 3; original magnification, ×400). (C) Proliferation index estimated at 2% and (D) Ki-67 proliferation index estimated at 70% (original magnification, ×250).
For Ki-67, there was a discrepancy in the prognostic importance of this factor between the ER-positive and ER-negative cases. A high Ki-67 index of ≥5% was associated with the ER-positive subgroup (P=0.006 for ERα and P=0.01 for ERβ; Table I).
Association between ERα and ERβ breast cancer subgroups and clinical information
The expression of ER subtypes within the tumor group was further analyzed. According to positive or negative expression of the hormone receptors ERα and ERβ, ERα+ and ERβ+ was significantly the most expressed ER subgroup in patients with breast cancer (P=0.01; Fig. 6). A total of 43.75% of the malignant breast samples co-expressed the two ER subtypes, compared with only 25 and 21.87% of the breast tumors which expressed either (ERα+, ERβ- or ERα-, ERβ+ subgroups, respectively). A small number of breast cancer samples were in the ERα-, ERβ- subgroup (9.37%; Fig. 6).
Figure 6.
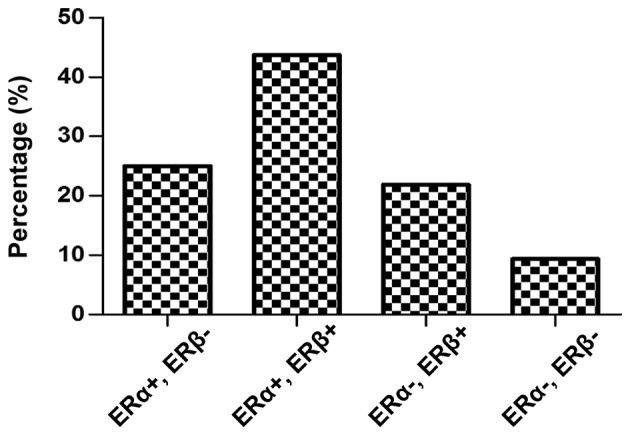
ERα and ERβ breast cancer subgroups. ER, estrogen receptor.
The distribution of ERα and ERβ expression groups according to the menopausal status exhibited a significant difference for the ERα+, ERβ+ subgroup (P=0.05). As presented in Table II, 71.42% of the ERα and ERβ co-expression cases occurred following the menopause stage, whereas only 28.57% occur prior to menopause. The other ER subgroups studied were distributed approximately homogeneously prior to and following the menopause phase. When compared with tumor grade, there was a significant association between the ERα+, ERβ+ subgroup and SBR grade (P=0.005). This was not observed for the other ER subgroups, but there was a non-significant association between the ERα+, ERβ-subgroups and the primary stages of cancer (P=0.08). Similarly, depending on the nodal status, there was an association between the ERα+, ERβ+ subgroup with the infiltration of the lymphatic ganglion (P=0.05; Table II).
Table II.
Association between ERα and ERβ breast cancer subgroups with clinicopathological and molecular parameters.
| Clinicopathological and molecular parameters | ERα+, ERβ- (n=8) | ERα+, ERβ+ (n=14) | ERα-, ERβ+ (n=7) | ERα-, ERβ- (n=3) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Menopausal status | ||||
| Premenopausal | 4 (50) | 4 (28.57) | 3 (42.85) | 1 (33.33) |
| Postmenopausal | 4 (50) | 10 (71.42) | 4 (57.14) | 2 (66.66) |
| P-value | >0.05 | 0.05 | >0.05 | >0.05 |
| Histological grade | ||||
| SBRI | 2 (25) | 1(7.14) | 2 (28.57) | 2 (66.66) |
| SBRII | 5 (62.5) | 9 (64.28) | 3 (42.85) | 0 |
| SBRIII | 1 (12.5) | 4 (28.57) | 2 (28.57) | 1 (33.33) |
| P-value | 0.08 | 0.01 | 0.80 | 0.22 |
| Lymph node status | ||||
| Positive | 2 (25) | 10 (71.42) | 4 (57.14) | 2 (66.66) |
| Negative | 6 (75) | 4 (28.57) | 3 (42.85) | 1 (33.33) |
| P-value | 0.13 | 0.05 | >0.05 | >0.05 |
| HER2/neu | ||||
| Positive | 4 (50) | 1 (7.14) | 1 (14.28) | 2 (66.66) |
| Negative | 4 (50) | 13 (92.85) | 6 (85.71) | 1 (33.33) |
| P-value | >0.05 | 3.22×10–5 | 0.03 | >0.05 |
| Ki-67 | ||||
| ≤5% | 6 (75) | 3 (21.42) | 3 (42.85) | 1 (33.33) |
| >5% | 2 (25) | 11 (78.57) | 4 (57.14) | 2 (66.66) |
| P-value | 0.10 | 0.01 | >0.05 | >0.05 |
P-values calculated using the χ2 test in R. SBR, Scarff-Bloom-Richardson; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor.
Subset analyses of HER2/neu and Ki-67 in ERa, ERβ breast cancer subgroups
As presented in Table II, the complex associations between various pathological variables were considered in the current study. Using this analysis, it is possible to visualize the association of biological factors (ERα, ERβ, HER2 and Ki-67) with ER subgroups and study their associations with conventional pathological factors. Breast tumors with an ERα+, ERβ+ profile were characterized by negative HER2 status. A statistically significant relationship between the co-expression of estrogen receptors and HER2 negative was identified in the tumor samples (P=3.21×10−5). A statistically significant negative association was also observed between the ERα-, ERβ+ profile and HER2 expression (P=0.03; Table II).
Using a Ki-67 cut-off value of 5% (Table II), only the ERα+, ERβ+ breast cancer subgroup was significantly associated with a Ki-67 index >5% (P=0.008).
Discussion
Previous studies have been conducted to decipher the role of ERs in breast carcinogenesis (16,18,19). Nonetheless, the role of the ERβ isoform in this malignancy and its association with ERα remains to be elucidated and the results of earlier studies are somewhat contradictory (26,27). The results of the current study indicate that ERα and ERβ expression was retained in a majority of breast cancer cases (68 and 65%, respectively). The associations between ERα and ERβ expression and menopausal status revealed that each isoform was more significantly expressed in postmenopausal patients than in premenopausal ones (Table I), primarily for ERβ which was ~2 times higher in post-menopausal cases (P=0.06). These data are concordant with previous studies demonstrating that the two ER isoforms are frequently positive in postmenopausal patients (28–30). However, considering the relative amount of mRNA, the ERα expression was found to be higher in premenopausal phases (1.5-fold) compared with postmenopausal breast tumors in the present study. Inversely, the mRNA expression level of ERβ was often higher in postmenopausal patients. Consequently, the ERα:ERβ ratio decreases from 7.66 to 3.36 (Fig. 4), which translates into the ERβ activity increasing in malignant breast tumors of post-menopausal patients. Furthermore, the expression levels of ERα and ERβ were revealed to be associated with smaller tumors and significantly associated with the SBR histopathological grade (P=0.01; Table I). In fact, ERβ was more highly expressed than ERα in the SBRIII grade. In addition, the ERα mRNA level reached a maximum in the SBRII grade and decreased in SBR grade III. However, the ERβ mRNA level was associated with the advancement of the SBR grade. ERβ expression reached its maximum in the SBR III grade and was higher than that of ERα. Consequently, the ERα:ERβ ratio was inversely associated with SBR grade advancement, and the ratio tended to 0 at the SBR III grade (Fig. 2).
These results demonstrate a slight association between ERβ expression (but not ERα) and node-positive breast cancer (P=0.06), which is corroborated by the findings of Hou et al (31), which demonstrated that ERβ exerts stimulatory effects on breast cancer development and metastasis. Furthermore, Yan et al (23) revealed that the expression of ERβ2 is also correlated with high-grade tumors, distant metastasis and breast cancer mortality (23).
Estrogen-ER signaling is important in normal mammary gland development and breast carcinogenesis (32). Due to the crosstalk between the ER and epidermal growth factor receptor (EGFR)/HER2 signaling pathways and/or their downstream effectors, the majority of patients develop resistance to endocrine therapy (predominantly tamoxifen) (33). In fact, the relation of ERα and ERβ with HER2 is of particular interest. In the current study, the HER2-negative status was associated with positive ERα and ERβ expression. This result is concordant with previous data that demonstrated a significant negative correlation between the expression of the hormone receptor and HER2/neu amplification (24,34,35). The combination of HER2 overexpression and a high Ki-67 index has been suggested to be a prognostic molecular marker for breast cancer (36). The present study revealed that positive ER receptors (α and β) were associated with a high Ki-67 index of ≥5% (P=0.006 and P=0.01 respectively) for breast cancer (Table I). These results are concordant with previous reports (18,37), and imply that ERα and ERβ may have a role in breast cancer development, metastasis and proliferation.
ERβ may be expressed alone (ERα-, ERβ+) or co-expressed with ERα (ERα+, ERβ+); therefore, a comparison of the previously cited clinical and molecular parameters referring to the (ERα, ERβ) breast cancer subgroups is required. The present study identified that ERα and ERβ co-expression was retained in the majority of breast cancer cases (P=0.01; Fig. 6), hypothesizing an interaction between the two nuclear receptors. In this context, Järvinen et al (38) and Leung et al (39) reported that positive ERβ expression is associated with positive ERα in breast cancer tumors. Given that ERα and ERβ were coexpressed in the majority of breast tumors, this expression may occur in the form of their heterodimers. ERα and ERβ heterodimers may also serve a significant role in breast cancer, but this remains to be established (40).
The present study demonstrated that the co-expression of ERα and ERβ occurs frequently following menopause, which may be associated with the in situ synthesis of steroid hormones (the epithelium of the mammary gland) from the steroid precursors dehydroepiandrosterone (DHEA) and DHEA-sulfate. Through an intracrinology mechanism, E2 may be synthesized locally by the aromatization of the androgen by aromatase (41). Depending on the presence of ERα and ERβ in certain cells, the receptors form functional homo- or heterodimers on promoter elements (40). The results of the current study reveal that the progression of breast cancer may be dependent on the expression levels of estrogenic receptors, particularly the co-expression of the ERα and ERβ isoforms (ERα+, ERβ+ subgroup). Indeed, ERα and ERβ co-expression is associated with high-grade tumors (grade II and III; P=0.005) and potentially lymph node infiltration (P=0.05). This finding is concordant with the findings of Speirs et al (20), which demonstrated that tumors that expressed ERα and ERβ were node-positive and tended to be of a higher grade. This implies that ERα and ERβ may cooperate to generate a tumor phenotype with a higher metastatic potential. The present study hypothesizes that there is a synergic effect between ERα and ERβ, which exerts stimulative effects on breast cancer development and metastasis.
The potential stimulative effect exerted by the co-expression of the ERα and ERβ receptors is supported by the significantly high proliferation rate estimated by the Ki-67 index in the (ERα+, ERβ+) breast cancer subgroup. Notably, it has been reported that an increase in the proliferation rate occurs during the progression towards invasive ductal carcinoma, implying a significant role for Ki-67 in breast tumorigenesis (42). However, the (ERα+, ERβ-) subgroup is associated with primary cancer grade (SBRI and II), non-infiltrated lymph nodes and a lower proliferation index (<5%). This reveals that the expression of ERα alone may be a marker of non-aggressive tumors, as has been suggested by a previous study (28). Our results demonstrate a negative association between the (ERα-, ERβ +) subgroup and HER2. Such results are corroborated by a study by Marotti et al (34), which demonstrated that the co-expression of ERa and ERb was associated with a negative status of HER2. In addition, Lindberg et al (35) revealed that ERβ is able to increase phosphatase and tensin homolog levels and decrease HER2/HER3 signaling, thereby reducing protein kinase B signaling. The co-expression of ERβ and ERα (ERα+, ERβ+ subgroup) was negatively associated with HER2 expression in the present study. These findings are consistent with a previous report, which concluded that ERβ is significantly associated with ERα expression and inversely associated with HER2 over-expression (34). As these two receptor systems (ER isoforms and HER2) have the capacity to activate one another (30), the present study hypothesizes a synergistic effect between ERα and ERβ to abrogate HER2 activation as a part of the crosstalk between ER and growth factor receptors. In this context, ER-induced signaling pathways, demonstrated through in vitro cellular models, were found to induce EGFR ligands, such as transforming growth factor α (43) and lead to downregulation of EGFR (44) and HER2 (45). This growth factor receptor, HER2/neu, is not typically over-expressed in normal or benign breast lesions (46). However, a significantly lower level of HER2/neu expression in invasive carcinoma has been previously reported (47). The results of the current study imply that the molecular phenotype defined by the presence of ERa, ERβ and the absence of HER2 may be a precursor for the development of a more aggressive and malignant invasive ductal carcinoma. Furthermore, the coexpression of ERα and ERβ may be a marker of hormonal sensitivity in association with downregulation of HER2 expression.
In conclusion, the results of the current study suggest an important role for ERβ in breast cancer development, proliferation and metastasis, particularly when coexpressed with ERα. The ERα+, ERβ+ subgroup is associated with high tumor grade, metastasis and a high proliferation index. Therefore, the co-expression of ERα and ERβ may be an indicator of aggressive tumors. In addition, the ERα+, ERβ+ subgroup is significantly associated with an HER2-negative status, and this may indicate a sensitivity towards hormonal therapy due to the downregulation of HER2 expression. Given that the ERα-ERβ balance is influenced by the tumor microenvironment, including cytokines and growth factors, decrypting signaling pathways elicited by those components and their crosstalk with ER signaling may be investigated further in future studies.
Acknowledgements
The authors would like to thank the Histology and Immunocytology lab members at the Saleh Azeiz Cancer Institute (Tunis, Tunisia). The present study was funded by The IMEC unit, The Sciences Faculty of Bizerte, The University of Carthage and The Tunisian ministry of higher education.
References
- 1.Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 3.Pettersson K, Gustafsson J. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol. 2001;63:165–192. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 4.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 5.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 6.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/S0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 8.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, et al. Cloning of the human estrogen receptor cDNA; Proc Natl Acad Sci USA; 1985; pp. 7889–7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human estrogen receptor cDNA: Sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, Reierstad S, Huang CC, Bulun SE. Novel estrogen receptor-alpha binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res. 2007;67:5017–5024. doi: 10.1158/0008-5472.CAN-06-3696. [DOI] [PubMed] [Google Scholar]
- 11.Finn R, Dering J, Conklin D, Kalous O, Cohen D, Desai A, Ginther C, Atefi M, Chen I, Fowst C, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary; Proc Natl Acad Sci USA; 1996; pp. 5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosselman S, Polman J, Dijkema R. ER beta: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- 14.Leung YK, Lee MT, Lam HM, Tarapore P, Ho SM. Estrogen receptor-beta and breast cancer: Translating biology into clinical practice. Steroids. 2012;77:727–737. doi: 10.1016/j.steroids.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox EM, Davis RJ, Shupnik MA. ERbeta in breast cancer-onlooker, passive player, or active protector? Steroids. 2008;73:1039–1051. doi: 10.1016/j.steroids.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 17.Lazennec G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006;231:151–157. doi: 10.1016/j.canlet.2005.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen EV, Cheng G, Palmieri C, Saji S, Mäkelä S, van Noorden S, Wahlström T, Warner M, Coombes RC, Gustafsson JA. Estrogen receptors and proliferation markers in primary and recurrent breast cancer; Proc Natl Acad Sci USA; 2001; pp. 15197–16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer. 2006;95:616–626. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speirs V, Parkes AT, Kerin MJ, Walton DS, Carleton PJ, Fox JN, Atkin SL. Coexpression of estrogen receptor alpha and beta: Poor prognostic factors in human breast cancer? Cancer Res. 1999;59:525–528. [PubMed] [Google Scholar]
- 21.Grober OM, Mutarelli M, Giurato G, Ravo M, Cicatiello L, De Filippo MR, Ferraro L, Nassa G, Papa MF, Paris O, et al. Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genomics. 2011;12:36. doi: 10.1186/1471-2164-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(−Delta Delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Yan M, Rayoo M, Takano EA, kConFab Investigators and Fox SB Nuclear and cytoplasmic expressions of ERβ1 and ERβ2 are predictive of response to therapy and alters prognosis in familial breast cancers. Breast Cancer Res Treat. 2011;126:395–405. doi: 10.1007/s10549-010-0941-9. [DOI] [PubMed] [Google Scholar]
- 24.Arafah M. Correlation of hormone receptors with Her-2 Neu protein expression and the histological grade in invasive breast cancers in a cohort of Saudi Arabia. Turk Patoloji Dergisi. 2012;28:38–43. doi: 10.5146/tjpath.2012.01095. [DOI] [PubMed] [Google Scholar]
- 25.Isola J, Tanner M. Chromogenic in situ hybridization in tumor pathology. Methods Mol Med. 2004;97:133–144. doi: 10.1385/1-59259-760-2:133. [DOI] [PubMed] [Google Scholar]
- 26.Jonsson P, Katchy A, Williams C. Support of a bi-faceted role of estrogen receptor β (ERβ) in ERα-positive breast cancer cells. Endocr Relat Cancer. 2014;21:143–160. doi: 10.1530/ERC-13-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang B, Warner M, Gustafsson JÅ. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol 418 Pt. 2015;3:240–244. doi: 10.1016/j.mce.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Bièche I, Parfait B, Laurendeau I, Girault I, Vidaud M, Lidereau R. Quantification of estrogen receptor alpha and beta expression in sporadic breast Cancer. Oncogene. 2001;20:8109–8115. doi: 10.1038/sj.onc.1204917. [DOI] [PubMed] [Google Scholar]
- 29.Miller WR. Aromatase and the breast: Regulation and clinical aspects. Maturitas. 2006;54:335–341. doi: 10.1016/j.maturitas.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Qui WS, Yue L, Ding AP, Sun J, Yao Y, Shen Z, Fan LH. Co-expression of ER-beta and HER2 associated with poorer prognosis in primary breast cancer. Clin Invest Med. 2009;32:E250–E260. doi: 10.25011/cim.v32i3.6114. [DOI] [PubMed] [Google Scholar]
- 31.Hou YF, Yuan ST, Li HC, Wu J, Lu JS, Liu G, Lu LJ, Shen ZZ, Ding J, Shao ZM. ERbeta exerts multiple simulative effects on human breast carcinoma cells. Oncogene. 2004;23:5799–5806. doi: 10.1038/sj.onc.1207765. [DOI] [PubMed] [Google Scholar]
- 32.Oladimeji P, Skerl R, Rusch C, Diakonova M. Synergistic activation of ERα by estrogen and prolactin in breast cancer cells requires tyrosyl phosphorylation of PAK1. Cancer Res. 2016;76:2600–2611. doi: 10.1158/0008-5472.CAN-15-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison G, Fu X, Shea M, Nanda S, Giuliano M, Wang T, Klinowska T, Osborne CK, Rimawi MF, Schiff R. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast Cancer Res Treat. 2014;144:263–272. doi: 10.1007/s10549-014-2878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marotti JD, Collins LC, Hu R, Tamimi RM. Estrogen receptor-beta expression in invasive breast cancer in relation to molecular phenotype: Results from the Nurses' Health Study. Mod Pathol. 2010;23:197–204. doi: 10.1038/modpathol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindberg K, Helguero LA, Omoto Y, Gustafsson JÅ, Haldosén LA. Estrogen receptorb β represses Akt signaling in breast cancer cells via downregulation of HER2/ HER3 and upregulation of PTEN: Implications for tamoxifen sensitivity. Breast Cancer Res. 2011;13:R43. doi: 10.1186/bcr2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knoop AS, Lænkholm AV, Jensen MB, Nielsen KV, Andersen J, Nielsen D, Ejlertsen B, Danish Breast Cancer Cooperative Group Estrogen receptor, Progesterone receptor, HER2 status and Ki67 index and responsiveness to adjuvant tamoxifen in postmenopausal high-risk breast cancer patients enrolled in the DBCG 77C trial. Eur J Cancer. 2014;50:1412–1421. doi: 10.1016/j.ejca.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Jia M, Qin X, Hu J, Zhang X, Zhou G. Harmful effect of ERβ on BCRP-mediated drug resistance and cell proliferation in ERα/PR-negative breast cancer. FEBS J. 2013;280:6128–6140. doi: 10.1111/febs.12533. [DOI] [PubMed] [Google Scholar]
- 38.Järvinen TA, Pelto-Huikko M, Holli K, Isola J. Estrogen receptor beta is coexpressed with ERalpha and PR and associated with nodal status, grade and proliferation rate in breast cancer. Am J Pathol. 2000;156:29–35. doi: 10.1016/S0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung YK, Lee MT, Lam HM, Tarapore P, Ho SM. Estrogen receptor-beta and breast cancer: Translating biology into clinical practice. Steroids. 2012;77:727–737. doi: 10.1016/j.steroids.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M. Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways. Mol Cell Biol. 2004;24:7681–7694. doi: 10.1128/MCB.24.17.7681-7694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNamara KM, Sasano H. The intracrinology of breast cancer. J Steroid Biochem Mol Biol. 2015;145:172–178. doi: 10.1016/j.jsbmb.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, Ortmann O. Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancerregistry. Breast Cancer Res Treat. 2013;139:539–552. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stope MB, Popp SL, Knabbe C, Buck MB. Estrogen receptor alpha attenuates transforming growth factor-beta signaling in breast cancer cells independent from agonistic and antagonistic ligands. Breast Cancer Res Treat. 2010;120:357–367. doi: 10.1007/s10549-009-0393-2. [DOI] [PubMed] [Google Scholar]
- 44.Yarden RI, Wilson MA, Chrysogelos SA. Estrogen suppression of EGFR expression in breast cancer cells: A possible mechanism to modulate growth. J Cell Biochem Suppl. 2001;36(Suppl):S232–S246. doi: 10.1002/jcb.1142. [DOI] [PubMed] [Google Scholar]
- 45.Subik K, Lee JF, Baxter L, Strzepek T, Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG, Tang P. The expression patterns of ER PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in-breast cancer cell lines. Breast Cancer (Auckl) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 46.Stark A, Hulka BS, Joens S, Novotny D, Thor AD, Wold LE, Schell MJ, Melton LJ, III, Liu ET, Conway K. HER-2/neu amplification in benign breast disease and the risk of subsequent breast cancer. J Clin Oncol. 2000;18:267–274. doi: 10.1200/JCO.2000.18.2.267. [DOI] [PubMed] [Google Scholar]
- 47.Allred DC, Clark GM, Molina R, Tandon AK, Schnitt SJ, Gilchrist KW, Osborne CK, Tormey DC, McGuire WL. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992;23:974–979. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]