Abstract
Background
The use of marijuana for medical purposes is now legal in some U.S. states and other jurisdictions, such as Canada, and Israel. Despite the widespread legalization of medical marijuana globally, there is limited information on patterns and correlates of medical marijuana use (MMU). We conducted a literature review to assess prevalence, reasons, perceived effects, and correlates of MMU among adolescents and adults.
Methods
We searched peer-reviewed articles in English between January 1996 and August 2016 from several databases (PubMed, Google Scholar, Embase, CINAHL, and PsycINFO) using different combinations of keywords.
Results
A total of 25 articles met the inclusion criteria. In the U.S., national survey estimates of prescribed MMU was 1.1% among 12th graders and 17% among adults who reported past-year marijuana use. The reported prevalence of prescribed MMU ranged from <1.7% in Israeli cancer patients to 17.4% in American health care patients. The reported prevalence of self-medication with marijuana ranged from 15% in Canadian patients with chronic pain to 30% in British patients with multiple sclerosis. Pain was the most frequently endorsed reason for use. MMU appeared to provide symptom relief for a range of pain conditions, sleep disturbance, and anxiety symptoms, but it did not appear to provide sufficient relief of cluster headache symptoms. Non-medical marijuana use was a common factor associated with MMU across studies.
Conclusion
Either MMU or self-medication with marijuana was common, mainly due to pain management. Additional research is needed to evaluate temporal and causal associations of non-medical marijuana use with MMU.
Keywords: Medical marijuana, Medical marijuana laws, Marijuana use, Self-medicate, Pain
1. INTRODUCTION
Marijuana is the most widely used recreational drug in the U.S. The 2015 National Survey on Drug Use and Health (NSDUH) estimated that approximately 22.2 million Americans aged ≥12 years reported marijuana use (MU) in the past 30 days (Center for Behavioral Health Statistics and Quality (CBHSQ), 2016). MU, particularly long-term and heavy use, increases the likelihood of adverse health effects, such as motor vehicle accidents, chronic bronchitis and impaired respiratory function, psychotic symptoms, cognitive impairment, substance use disorder (SUD), and poor school performance (Hall, 2009; Hall and Degenhardt, 2009; Volkow et al., 2014). Of various recreational drugs, marijuana had the highest past-year prevalence of use disorder in the U.S. In 2015, past-year prevalence of MU disorder among Americans aged ≥12 years was 1.5%, which is higher than that of other drug use disorders, such as prescription opioid (0.8%), cocaine (0.3%), and heroin (0.2%) (CBHSQ, 2016). Considering the large population size of individuals who use marijuana recreationally and have MU disorder, problematic MU is an important public health issue that should be monitored to reduce its potential risks (Blanco et al., 2016; Wu et al., 2016).
In the U.S., marijuana is classified as a Schedule I drug under the Controlled Substances Act that has high potential for abuse and its medical use is prohibited (Hoffmann and Weber, 2010). Nevertheless, medical marijuana use (MMU), which refers to using marijuana as a medicine with a physician’s recommendation, is legal in many states. California became the first state to legalize the use of medical marijuana (MM) in 1996, and to date 28 states and District of Columbia passed MM laws—most recently, Florida, North Dakota, Arkansas, and Montana have approved a ballot measure that approves the use of MM. MM laws vary by states in terms of approved conditions but they typically include cancer, glaucoma, HIV/AIDS, cachexia, severe chronic pain, severe nausea, seizures, and severe muscle spasms (Hoffmann and Weber, 2010). While adults with a qualifying disease have legal access to MM, adolescents (under age 18) are only allowed to use MM under limited conditions with consent of a parent or caregiver. The landscape of MM laws is also rapidly changing globally. In Canada, the Marihuana Medical Access Regulations (MMAR) was enacted in 2001, which permitted the use of MM for severe illnesses upon approval by Health Canada. In 2014, the new Marihuana for Medical Purposes Regulations was enacted to replace the MMAR, enabling medical practitioners to prescribe MM regardless of patient’s medical conditions or failure of conventional treatments (Fitzcharles and Jamal, 2015). In Israel, individual patients can obtain a MM license after the Medical Cannabis Unit of the Ministry of Health approves a specialist’s recommendation (Sznitman and Lewis, 2015). In Australia, the New South Wales Government introduced the Terminal Illness Cannabis Scheme in 2014, which permitted the use of MM for adult with a terminal illness that meets the definition by the scheme (Martin and Bonomo, 2016). Recently, the Narcotic Drugs Amendment Bill of 2016 that legalizes the cultivation of marijuana for medical and scientific purposes went into effect on November 2016 (Parliament of Australia, 2016). In the U.K., Sativex® became the first licensed marijuana-based medicine for the treatment of Multiple Sclerosis (MS)-related spasticity in 2010 (Kmietowicz, 2010).
The global movement towards legalizing MM raises concern about its potential impact on public health. Joffe and Yancy (2004) suggested that legalization of MM may potentially reduce the risk perception related to MU and increase the availability of marijuana, which may contribute to MU by adolescents (Bachman et al., 1998; Swaim, 2003). During the MM commercialization period (2009–2011), adolescents (12–17 years) in Colorado showed significantly lower risk perception related to regular MU compared to the pre-MM commercialization period (2006–2008) than adolescents in non-medical marijuana states (NMMS) (Schuermeyer et al., 2014). A national survey of adults aged ≥18 years in the U.S. showed that residents in MM states had higher past-year prevalence of MU than residents in NMMS, although comparison was not made prior to and after implementation of MM laws in MM states and NMMS (Cerdá et al., 2012).
To date, there is limited information that outlines patterns and correlates of MMU. Most of the available studies on MMU in the U.S. were conducted on samples of HIV/AIDS patients. A telephone survey of 180 HIV patients found that nearly 24% used marijuana for medical purposes in the past year, primarily due to nausea, weight loss, or diarrhea (52.8%) (Fairfield et al., 1998). An anonymous survey on 442 HIV/AIDS patients revealed that approximately 33% were those who used marijuana, mainly for improving relaxation/stress (79%), appetite/weight loss (67%), or nausea (66%) (Sidney, 2001). However, none of these studies addressed perceived effects of MM on symptoms, and there are very few studies on patients with other medical conditions. There is also little information about factors associated with MMU. Data from state-administered MM programs revealed that the majority of MM patients were male (75.4% in Arizona and 69% in Colorado) (Bowles, 2012). However, retrospective chart reviews of patients with authorized access to MM for chronic pain in Washington found that males and females were similar in terms of access to MM and duration of use (Aggarwal et al., 2009).
To date, no previous studies have systematically examined characteristics and patterns of MMU across age groups or medical conditions. To fill these research gaps, we conducted a literature review for both adolescents and adults in different countries to (1) assess MMU prevalence, (2) explore reasons and perceived effects of MMU, and (3) examine correlates of MMU.
2. METHOD
We conducted a literature search between August and September 2016 using databases PubMed, Google Scholar, Embase, CINAHL, and PsycINFO. The keywords “medical marijuana”, “medical cannabis”, or “medical cannabinoids” were combined with each of the following: “user” and “patients.” Peer-reviewed articles published in English between January 1996 and August 2016 and those that are clearly relevant to the scope of this review were considered for inclusion. In terms of quality assessment, studies were excluded if they used a less rigorous sampling strategy (self-selected, convenience, or purposive sample), if they did not use appropriate measurement tools for exposure and outcome variables, or if they had a particular source of bias (Sanderson et al., 2007).
3. RESULTS
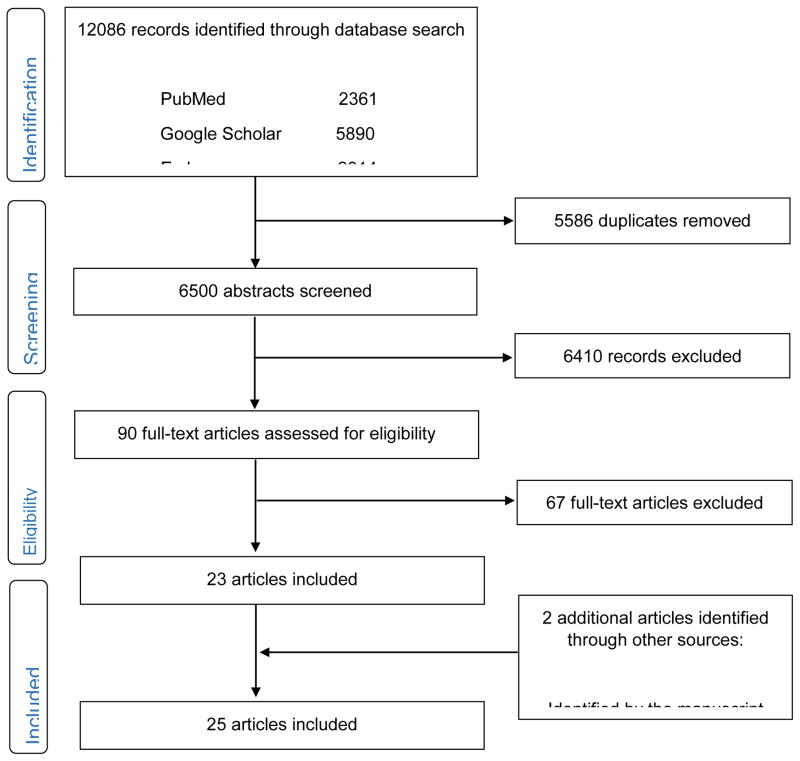
Figure 1 summarizes literature search process. Different combinations of keywords (“medical marijuana user”, “medical marijuana patients”, “medical cannabis user, “medical cannabis patients”, “medical cannabinoids user” and “medical cannabinoids patients”) brought up the same article within search engine or with other search engines. The literature searches yielded 6,502 potentially relevant records, after removing duplicate records. We excluded 6,410 records that did not meet the inclusion criteria or that were inaccessible to full-text, leaving 90 records. Of the 90 articles that were read in full text, 67 articles were excluded due to: outside the scope of this review (35), a lack of descriptive data (8), case-report (7), qualitative study (8), literature review (1), not peer-reviewed articles (2), self-selected or purposive sample (5), and a lack of measures of substance use and mental health variables (1). Two additional articles were identified by the manuscript reviewer. A total of 25 articles were included for this review, providing data on adolescents and adults who used marijuana for medical purposes from seven countries (the U.S., Canada, Australia, the U.K., Spain, France and Israel). Findings are summarized separately for adolescents (Table 1) and adults (Table 2). Findings among adults are listed according to medical conditions.
Figure 1.
The PRISMA diagram of the literature search Figure 1.
Table 1.
Adolescents: prevalence and correlates of Medical Marijuana Use
| Citation | Data Source | Study type | Country | N/Age | Definition of MMU | Key Findings |
|---|---|---|---|---|---|---|
| Boyd et al., 2015 | 2012–2013 Monitoring the Future | Cross-sectional | U.S. | 4394 12th graders (weighted) | MU from respondents’ own marijuana prescription |
|
MMU = Medical Marijuana Use; MU = Marijuana Use; N = Sample size.
Table 2.
Adults: prevalence, reasons for use, perceived effects, and correlates of Medical Marijuana Use
| Citation | Data Source | Study type | Country | N/Age | Definition of MMU | Key Findings |
|---|---|---|---|---|---|---|
| Symptomatically diverse samples | ||||||
| Nunberg et al., 2011 | Medical records filled out by patients and evaluating physicians | Cross-sectional | U.S. | 1655 patients from MM evaluation clinics | Prescribed |
|
| Reinarman et al., 2011 | Medical records filled out by patients and evaluating physicians | Cross-sectional | U.S. | 1746 patients from MM evaluation clinics (≥18 years) | Prescribed |
|
| Ilgen et al., 2013 | Survey in a single MM certification clinic | Cross-sectional | U.S. | 348 MM patients, Mean age: 41.5±12.6 | Prescribed |
|
| Grella et al., 2014 | A mixed approach of focus group & survey | Cross-sectional | U.S. | Individuals from MM dispensaries | Prescribed |
|
| Richmond et al., 2015 | Screening data as a part of SBIRT initiative | Cross-sectional | U.S. | 2030 patients who screened positive for MU (≥18 years) | Prescribed |
|
| Ryan-Ibarra et al., 2015 | California BRFSS 2012 | Cross-sectional | U.S. | 7525 adults (≥18 years) | Having used MM for a serious medical condition |
|
| Roy-Byrne et al., 2015 | Baseline interview and subsequent follow-up at 3, 6, 9, and 12 months | Two-group, RCT | U.S | 868 primary care patients (≥18 years) | Prescribed or self-medicate |
|
| Zolotov et al., 2016 | Questionnaire | Cross-sectional | Israel | 95 patients with a MM license (≥18 years) | Prescribed |
|
| Woodruff and Shillington, 2016 | Baseline measures from a larger study | Cross-sectional | U.S. | 292 patients visiting ED and who use marijuana only (≥18 years) | Prescribed |
|
| Lin et al., 2016 | 2013 NSDUH | Cross-sectional | U.S. | 3200 adults living in MM states and reporting past-year MU (≥18 years) | Prescribed |
|
| Compton et al., 2017 | 2013–2014 NSDUH | Cross-sectional | U.S. | 96100 respondents (≥18 years) | Prescribed |
|
| Patients with psychiatric disorders | ||||||
| Ashrafioun et al., 2015 | Survey | Cross-sectional | U.S. | 433 participants in SUD treatment, Mean age: 34.8 ± 10.7 | MM as treatment received for pain |
|
| Nussbaum et al., 2015 | Discharge survey | Cross-sectional | U.S. | 623 psychiatric inpatients (≥18 years) | Prescribed |
|
| Davis et al., 2016 | Screening measures for a RCT | Cross-sectional | U.S | 841 veterans in SUD treatment, Mean age: 48.21 ±13.34 | Prescribed |
|
| Patients with MS | ||||||
| Clark et al., 2004 | Survey | Cross-sectional | Canada | 205 MS patients | Self-medicate |
|
| Chong et al., 2006 | Questionnaire | Cross-sectional | U.K. | 254 MS outpatients, Median age: 49 years | Self-medicate |
|
| Martínez-Rodríguez et al., 2008 | Questionnaire survey in neurology clinics | Cross-sectional | Spain | 175 MS patients | Self-medicate |
|
| Patients with CNCP | ||||||
| Ware et al., 2003 | Questionnaire survey | Cross-sectional | Canada | 209 CNCP patients | Self-medicate |
|
| Degenhardt et al., 2015 | Pain and Opioids IN Treatment Study | Cohort | Australia | 1,514 individuals who have been prescribed opioids for CNCP (≥18 years) | Prescribed or self-medicate |
|
| Patients with CH attacks | ||||||
| Leroux et al., 2013 | Questionnaire | Cross-sectional | France | 139 patients with CH | Self-medicate |
|
| Patients with IBD | ||||||
| Storr et al., 2014 | Questionnaire | Cross-sectional | Canada | 313 patients with IBD (≥18 years) | Self-medicate |
|
| Patients with HIV/AIDS | ||||||
| Furler et al., 2004 | Survey & chart review | Cross-sectional | Canada | 104 HIV patients, Mean age: 42.7±9.3 | Self-medicate |
|
| Fogarty et al., 2007 | Positive Health Study | Cohort | Australia | 408 HIV/AIDS patients, Mean age: 44 years | Self-medicate |
|
| Patients with cancer | ||||||
| Waissengrin et al., 2015 | Permit application forms, reapplication forms, survey data from medical charts | Cross-sectional | Israel | 17,000 cancer patients | Prescribed |
|
MMU = Medical Marijuana Use; MM = Medical Marijuana; MU = Marijuana Use; N = Sample size; SBIRT = Screening, Brief Intervention, and Referral to Treatment; ED = Emergency Department; RCT = Randomized Clinical Trial.
3.1. Adolescents: prevalence and correlates of MMU (Table 1)
One cross-sectional study investigated the prevalence and correlates of prescribed MMU among 12th grade students in the general U.S. population (Boyd et al., 2015). In the 2012–2013 Monitoring the Future surveys, respondents were asked, “Where did you get the marijuana you used during the last year?” Among 4,394 respondents, 1.1% self-reported that they obtained the marijuana from their own marijuana prescription. They also found that those who used marijuana from their own prescription had greater odds of engaging in substance use behaviors (marijuana, non-medical prescription drug, and illicit drugs other than marijuana) and using marijuana because “I am hooked” (AOR=10.2, 95% CI: 3.25–32.3) compared to those who used marijuana from neither legal nor medical sources. Reasons for use and perceived effects of MM were not examined.
3.2. Adults: prevalence, reasons for use, perceived effects, and correlates of MMU (Table 2)
3.2.1. Prevalence
Two studies provided estimates of prescribed MMU in the general U.S. adult population from the NSDUH (Compton et al., 2017; Lin et al, 2016). Compton et al. (2017) found that 12.9% (95% CI: 12.6–13.2) of 96,100 respondents were those who used marijuana in the past year, of whom 6.2% (95% CI: 5.6–6.9) were those who used prescribed marijuana only. Lin et al. (2016) revealed that among 3,200 individuals who used marijuana in the past year, 17% indicated any use of prescribed marijuana in states where MM is legal.
According to data from the 2012 California Behavioral Risk Factor Surveillance System (BRFSS), about 5% (95% CI: 4.56–5.83) of 7,525 adults in California reported having used MM for a serious medical condition (Ryan-Ibarra et al., 2015). However, they were not asked whether they have a MM recommendation, which could lead to overestimation of MMU in the general adult population of California. Ashrafioun et al. (2015) conducted survey on 433 patients (mean age: 34.8±10.7) at a large SUD treatment center in the Midwestern U.S. and found that 15% reported having received MM as their treatment for pain in the past year. However, they also did not clarify whether it was done with a MM license.
Four studies reported the prevalence of prescribed MMU, including one study on Israeli cancer patients (Waissengrin et al., 2015), two studies on American patients with psychiatric disorders (Davis et al., 2016; Nussbaum et al., 2015), and one study on American health care patients (Richmond et al., 2015). In Israel, Waissengrin et al. (2015) found that among 17,000 cancer patients, less than 1.7% were those having a MM permit. In the U.S., the prevalence of prescribed MMU among individuals with psychiatric disorders ranged from 8% to 15%. Davis et al. (2016) found that among 841 veterans in SUD treatment (mean age: 48.21±13.34), 8% were those who had been issued a MM card. Nussbaum et al. (2015) found that among 397 psychiatric inpatients who gave an answer regarding MMU, 15.1% were those having a MM card. In Colorado, Richmond et al. (2015) found that among 2,030 health care patients who were screened positive for MU, 17.4% were those with a MM card.
Eight studies provided information about the prevalence of self-medication with marijuana. Four studies were from Canada (Clark et al., 2004; Furler et al., 2004; Storr et al., 2014; Ware et al., 2003) and one study each was from Spain (Martínez-Rodríguez et al., 2008), the U.K. (Chong et al., 2006), France (Leroux et al., 2013), and Australia (Degenhardt et al., 2015). In Canada, the prevalence of self-medication with marijuana was modestly similar across medical conditions, except for patients with HIV. An anonymous survey of 209 Canadian patients with chronic non-cancer pain (CNCP) found that 15% reported having used marijuana for pain relief (Ware et al., 2003). Clark et al. (2004) found that 16.5% of 205 Canadian patients with MS were those who have ever used marijuana for medical purposes. In a study of 313 Canadian patients with inflammatory bowel disease (IBD), 18% reported current or past MU for IBD (Storr et al., 2014). Canadian patients with HIV had the relatively higher prevalence of self-medication with marijuana. Furler et al. (2004) found that among 104 Canadian patients with HIV (mean age: 42.7±9.3), 28.8% used marijuana to self-manage their symptoms in the past year. Two studies provided the estimates of self-medication with marijuana among MS patients in different European countries (Chong et al., 2006; Martínez-Rodríguez et al., 2008). In Spain, 17.1% of 175 MS patients reported any recurrent consumption of marijuana to alleviate MS-related symptoms (Martínez-Rodríguez et al., 2008), which was similar to that observed in Canada (Clark et al., 2004). In the U.K., Chong et al. (2006) found that 30% of 254 MS patients were those who started using marijuana because of MS, which was considerably higher than those observed in Canada (Clark et al., 2004) and Spain (Martínez-Rodríguez et al., 2008). In France, Leroux et al. (2013) showed that 19.4% of 139 patients with cluster headache (CH) were those who had tried marijuana to treat CH symptoms. In Australia, Degenhardt et al. (2015) found that, among 1,514 individuals who have been prescribed opioids for pain, nearly 16% (95% CI: 14.0–17.6) reported ever using marijuana for pain. Of them, only 24.5% (95% CI: 19.4–30.4) indicated that their doctor recommended using marijuana to manage pain.
3.2.2. Reasons for use
Pain was the most commonly endorsed reason for either prescribed MMU or self-medication with marijuana. Among 350 individuals who reported ever using MM in California, chronic pain (31.12%, 95% CI: 25.28–36.96) was the most commonly cited condition for which marijuana was used to help, followed by arthritis (10.70%, 95% CI: 6.84–14.55), migraine (8.43%, 95% CI: 4.74–12.11), and cancer (6.75%, 95% CI: 3.31–10.19) (Ryan-Ibarra et al., 2015). Ilgen et al. (2013) found that among 348 individuals seeking MM certification at a MM clinic in Michigan, 87% sough MM for pain. Grella et al. (2014) found that anxiety (60.2%), insomnia/sleep problems (56.4%), and chronic pain (42%) were the most frequently cited reasons for MMU among 181 respondents from MM dispensaries in Los Angeles County. However, these studies lacked the information about study participants’ primary medical conditions.
Five cross-sectional studies suggested that pain was the leading indication for use among diverse patient groups, with the exception of HIV patients (Chong et al., 2006; Davis et al., 2016; Furler et al., 2004; Nussbaum et al., 2015; Waissengrin et al., 2015). Davis et al. (2016) found among 67 veterans who had been issued a MM card in the Midwestern U.S., 78% used MM for severe and chronic pain. Similarly, Nussbaum et al. (2015) found that among 60 psychiatric inpatients in Colorado, 78.6% reported severe pain as the reason why their doctor recommended marijuana. In Israel, Waissengrin et al. (2015) found that pain was the most commonly cited reason for use (78.3%) among 69 cancer patients with a MM license. In the U.K., Chong et al. (2006) found that over 80% used marijuana to alleviate pain (83.7%, n=41/49) and limb spasms (80.4%, n=37/46) among MS patients who reported experience of these symptoms. However, pain was the least frequently cited condition for which HIV patients seek treatment. In Canada, Furler et al. (2004) found that, among 30 HIV patients, only 20% used marijuana to manage pain, while 70% reported using marijuana to improve appetite/weight loss.
Studies relying on medical records data reported similar results. Analyzing medical records from 1,655 individuals seeking a MM recommendation in California (main diagnosis: chronic pain, 58.2%; mental disorders, 22.9%; and insomnia, 21.3%), Nunberg et al. (2011) found that pain relief (82.6%), sleep improvement (70.6%), and relaxation (55.6%) were the most frequently cited reasons for use. Based on physician evaluation forms of 1,746 patients from MM evaluation clinics in California, Reinarman et al. (2011) found that pain (30.6%), insomnia (15.7%), and anxiety/depression (13%) were the most common conditions for which evaluating physicians approved the use of MM.
3.2.3. Perceived effects
We found six cross-sectional studies assessing perceived effects of MM based on self-reported data (Chong et al., 2006; Clark et al., 2004; Martínez-Rodríguez et al., 2008; Storr et al.. 2014; Waissengrin et al., 2015; Ware et al., 2003). Of six studies, three studies were conducted on Canadian patients with specific medical conditions (Clark et al., 2004; Storr et al., 2014; Ware et al., 2003). Ware et al. (2003) found that among 32 Canadian patients who used marijuana for chronic pain, 78% reported at least moderate pain relief. They also found that MM provided adequate relief of sleep, mood, and muscle stiffness. Storr et al. (2014) showed that among 56 patients who used marijuana for IBD, more than three-quarters of patients reported improvement in abdominal pain (83.9%) and abdominal cramps (76.8%). In a study of Canadian MS patients, MM appeared to have clinical benefits in relieving various MS-related symptoms at least moderately or completely, such as stress, sleep, stiffness, mood, spasms, pain, or weight loss (Clark et al., 2004). Two studies of MS patients, conducted in European countries, reported similar results (Chong et al., 2006; Martínez-Rodríguez et al., 2008). In the U.K., Chong et al. (2006) found that nearly three-quarters reported improvement in pain (75.6%, n=31/41) and limb spasms (81.1%, n=30/37) among MS patients who reported using marijuana for these symptoms. In Spain, nearly one-half of 30 MS patients (46.7%) reported clinical improvement after using MM, which was mainly perceived in sleep disturbance, muscle spasms, pain, tremor, and anxiety (Martínez-Rodríguez et al., 2008). In Israel, pain and general well-being (70% each), appetite (60%), and nausea (50%) were the most frequently reported benefits of MM perceived by 69 cancer patients with a MM license (Waissengrin et al., 2015).
Although none of these studies used a randomized design, evidence obtained from the included studies suggested that MM appeared to relieve various symptoms, particularly chronic pain and other pain conditions, sleep disturbance, and anxiety. However, Leroux et al. (2013) suggested that MM may not provide enough relief for CH. Among 27 French patients who used marijuana for CH, more than one-half of patients (51.8%) indicated variable/uncertain effects and nearly one-fourth of patients (22.3%) indicated negative effects.
3.2.4. Correlates of MMU
3.2.4.1. U.S. Samples from national survey or in health care settings
Two studies from the NSDUH found that those who used prescribed marijuana had increased odds of reporting poor health status and daily/near daily MU, but decreased odds of alcohol use problems (Compton et al., 2017; Lin et al., 2016). Likewise, two studies conducted in health care settings in Colorado (Richmond et al., 2015) and Southern California (Woodruff and Shillington, 2015) found that prescribed MMU was positively associated with frequent MU, but negatively ssociated with drug use problems. In a study of primary care patients in Washington, Roy-Byrne et al. (2015) found that those who used marijuana for medical purposes were more likely than those who used marijuana for recreational purposes have medical and psychological problems, but less likely to have severe drug and alcohol use problems. In summary, frequent MU, mental and psychological problems were the characteristics positively associated with MMU. Those who used MM were less likely than those who used recreational marijuana to engage in drug use problems.
3.2.4.2. Patients with psychiatric disorders
In the U.S., three studies examined correlates of MMU among patients with psychiatric disorders (Ashrafioun et al., 2015; Davis et al., 2016; Nussbaum et al., 2015).
3.2.4.2.1 Demographics
Results on demographics are mixed across studies, except for marital status and race/ethnicity. While Ashrafioun et al. (2015) found that those who used MM for pain had younger age than those who did not (28.7±9.2 vs. 35.9±10.6, p≤.005), Davis et al. (2016) and Nussbaum et al. (2015) reported no significant difference between MM registrants and non-registrants. In terms of sex, Nussbaum et al. (2015) found that MM registrants were more likely than non-registrants to be male (69% vs. 54.5%, p=.04). However, Ashrafioun et al. (2015) and Davis et al. (2016) reported no significant difference by sex. While Davis et al. (2016) found that MM registrants tended to be unemployed (87% vs. 75%, p=.036), Ashrafioun et al. (2015) reported no significant difference. Of the three studies, two studies reported no significant differences by marital status and race/ethnicity (Ashrafioun et al., 2015; Davis et al., 2016),
3.2.4.2.2 Psychological/behavioral factor
Results involving psychological factors are also mixed. Ashrafioun et al. (2015) found that neither pain nor depression was associated with MMU. However, Davis et al. (2016) found that MM registrants had higher levels of pain, sleep problems, and PTSD symptoms than non-registrants. In terms of substance use, Ashrafioun et al. (2015) found that MMU was positively associated with history of recent substance use, such as alcohol, marijuana, cocaine, other opioids, or sedatives. In particular, recent MU was strongly associated with MMU (AOR=4.71, 95% CI: 2.31–9.62). Nussbaum et al. (2015) and Davis et al. (2016) also found that MM registrants were more likely than non-registrants to report MU. Differences in sample demographics might contribute to mixed findings on demographic or psychological factors.
3.2.4.3. Patients with MS
Three studies examined correlates of self-medication with marijuana among patients with MS in different countries, including the U.K. (Chong et al., 2006), Canada (Clark et al., 2004), and Spain (Martínez-Rodríguez et al., 2008),
3.2.4.3.1 Demographic
Results on age and sex differences are mixed. In Spain, age was negatively associated with MMU (AOR=0.93, 95% CI: 0.86–0.99) (Martínez-Rodríguez et al., 2008). In contrast, Chong et al. (2006) and Clark et al. (2004) reported no significant difference by age. While Clark et al. (2004) found that those who used MM tended to be males, Chong et al. (2006) reported no significant difference by sex. Additionally, Chong et al. (2006) found that single adults were less likely than married adults to use MM (AOR=0.26, 95% CI: 0.08–0.81). Mixed results on age and sex differences may be related to differences in sample demographics or countries where the samples were recruited.
3.2.4.3.2 Clinical/behavioral factor
In Spain, pain was positively associated with MMU (AOR=5.20, 95% CI: 1.29–20.92) (Martínez-Rodríguez et al., 2008). In addition, Chong et al. (2006) and Martínez-Rodríguez et al. (2008) found that disability was positively associated with MMU. All three studies showed that tobacco smoking was positively associated with MMU (Chong et al., 2006; Clark et al., 2004; Martínez-Rodríguez et al., 2008). Recreational MU was also related to MMU (Clark et al., 2004). Taken together, pain, disability, tobacco smoking, and recreational MU were found to be characteristics positively associated with MMU among patients with MS.
3.2.4.4. Patients with CNCP
Two studies, conducted in Australia (Degenhardt et al., 2015) and Canada (Ware et al., 2003), examined correlates of MMU among patients with CNCP.
3.2.4.4.1 Demographic
These two studies revealed that those who used marijuana for pain were more likely to be younger than those who did not (Degenhardt et al., 2015; Ware et al., 2003).
3.2.4.4.2 Clinical/behavioral factor
Results on pain are mixed across studies. While Degenhardt et al. (2015) found that those who used marijuana for pain had significantly longer medical months living with pain than those who did not (156 vs. 120), Ware et al. (2003) reported no significant difference in the duration of pain. Degenhardt et al. (2015) also found that greater pain interference was positively associated with MU for pain, after controlling for pain severity. These two studies found that those who used marijuana for pain tended to be tobacco smokers (Ware et al., 2003; Degenhardt et al., 2015). Degenhardt et al. (2015) also found that a history of SUD (alcohol, amphetamine, or heroin) was positively related to MU for pain. Taken together, differences in the measurement scale or study designs may be a potential source of mixed results on the duration of pain. There is a need for in-depth research to determine relationship between clinical/behavior factors and MMU.
3.2.4.5. Patients with HIV/AIDS
Two studies, conducted in Australia (Fogarty et al., 2007) and Canada (Furler et al., 2004), examined correlates of MMU among HIV patients.
3.2.4.5.1 Demographic
Results on age are mixed. In Australia, Fogarty et al. (2007) found that age was negatively associated with MMU (AOR=0.95, 95% CI: 0.91–0.99). In Contrast, Furler et al. (2004) showed that those who used MM and those who used recreational marijuana were similar in terms of average age. These two studies revealed that low-income was positively associated with MMU (Fogarty et al., 2007; Furler et al., 2004).
3.2.4.5.2 Clinical/behavioral factor
Fogarty et al. (2007) found that most recent CD4/T-cell count (AOR=1.36, 95% CI: 1.06–1.75) was positively associated with MMU. Additionally, they found that those who used marijuana for both therapeutic and recreational purposes were less likely to use other recreational drugs than those who used marijuana for recreational purposes. Conversely, Furler et al. (2004) found that they did not differ significantly in terms of the most recent CD4/T-cell count, nor did they differ in past-year substance use (alcohol, cocaine, or other illicit drugs). In terms of patterns of MU, those who used MM were more frequently used marijuana than those who used recreational marijuana. Differences in samples’ inclusion criteria, study designs, and measuring tools might lead to mixed findings regarding age, CD4/T-cell count, and substance use variables. More research is needed to verify the extent to such demographic, clinical, and behavioral factors are associated with MMU.
3.2.4.6. Those renewing their MM card
In the U.S., Ilgen et al. (2013) described how those seeking MM card for the first time differ from those renewing their MM card. Those renewing their MM card were more likely than first-time MM patients to report lifetime illicit drug use (cocaine, hallucinogens, amphetamines, inhalants, or street opioids) and lifetime and daily/almost daily MU. There were no notable differences in recent non-marijuana substance use, except for prescription sedatives (10% vs. 6%, p=.02). With regard to physical and mental component scores (PCS and MCS) of the Short Form-12 Health Survey that measured mental and physical health problems and perceived interference with daily life due to these problems, those renewing their MM card had better PSC (35.1±8.2 vs. 37.9±9.1 vs. p<.01) and MSC (47.3±11.6 vs. 50±11.1, p=.03) than first-time MM patients. Those renewing their MM card rated their current pain level less severely than first-time MM patients did (6.0±2.2 vs. 5.5±2.4, p=.04). In Israel, a study of cancer patients found that younger patients (median age: 52.5 vs. 61, p<.001) and those with metastatic disease (p=.02) were more likely to be those renewing their MM permit (Waissengrin et al., 2015).
3.2.5. Adherence to MMU
In Israel, one cross-sectional study examined factors associated with the extent to which a patient takes MM as prescribed among those with a MM license (Zolotov et al., 2016). Patient’s level of participation in their healthcare (AOR=1.13, 95% CI: 1.03–1.24) and patients’ perceived relationship between them and their physician (AOR=1.40, 95% CI: 1.11–1.77) were positively associated with adherence to MMU. Those diagnosed with cancer were less adherent to MMU than those diagnosed with other types of illness (AOR=0.10, 95% CI: 0.01–0.82).
4. DISCUSSION
This is the first study that reviewed prevalence, reasons for use, perceived effects, and correlates of MMU across age groups or medical conditions. The prevalence of self-reported MMU was 1.1% among a national sample of 12th graders in the U.S. The prevalence of MMU among adults aged ≥18 years who reported past-year marijuana use was 17%, according to the national survey data in the U.S. In the absence of national estimates in other countries, further research is needed to obtain global data on age-specific prevalence of MMU. Although we could not make direct comparisons between the prevalence of prescribed MMU and the prevalence of self-medication with marijuana, the range of prevalence of MMU differed according to how each study defined MMU. While the prevalence of prescribed MMU ranged from <1.7% to 17.4%, the prevalence of self-medication with marijuana ranged from 15% to 30%. This is possibly due to the difficulty in acquiring legal access to MM or social stigma associated with using marijuana as a medicine. The patterns of these results suggest that a substantial proportion of people may use marijuana as a form of self-medicate without a valid doctor’s recommendation.
The most frequently cited reason for either prescribed MMU or self-medication with marijuana by adult patients was pain management. In most studies included in this review, study participants reported using MM for multiple conditions, which might contribute to the high proportions of patients seeking MM for pain relief. Nevertheless, our review found that chronic pain was the leading indication for MMU among adult patients. However, there is a lack of study on why adolescent patients seek MM. Adolescent patients may differ from adult patient in terms of medical conditions and reasons for use. According to data from the Colorado Department of Public Health and Environment, the most commonly reported reason for MMU among adolescent patients was seizures, while severe pain was the most frequently cited reason for MMU among adult patients (Colorado Department of Public Health and Environment, 2016). Clearly, there is a need for research on medical conditions and reasons for MMU among adolescent patients so that healthcare providers can better understand their healthcare needs.
Although clinical trials of the efficacy of MM were not available in this review, findings identified from our review suggest that MM could provide relief of symptoms, such as a range of pain, anxiety, and sleep disturbance, but not for CH symptoms. Given that the majority of individuals seeking MM are patients with chronic pain, clinical trials are needed to evaluate the risks and benefits of using MM for managing chronic pain. Martín-Sánchez et al. (2009) suggested that the beneficial effects of MM for pain could be offset by potential harms of marijuana. Thus, focused efforts are needed to assess the comparative efficacy of MM versus other pain medications, particularly opioids, to guide health practitioners in making informed decisions. Opioid is the most commonly prescribed pain medication in the U.S. (Kuehn, 2007). However, the long-term efficacy of opioids for pain is unclear and there has been an alarming increase in opioid-involved overdose and mortality (Chou et al., 2015; Dart et al., 2015; Martell et al., 2007). As the opioid misuse epidemic in the U.S. gets worse, health practitioners may face challenge in determining whether MM is a more appropriate or safer option for managing pain than opioids. Because both MM and opioids are psychoactive drugs that can lead to drug misuse or abuse problems, the important issue is how to minimize the degree of risk associated with MM or opioid use. A few studies have suggested a synergistic interaction between MM and opioids in reducing pain (Abrams et al., 2011; Cichewicz et al., 2004). In addition, Perron et al. (2015) found that using opioids in conjunction with MM was not associated with serious alcohol and other drug use problems when comparing with MMU only. Taken together, a low-dosage combination of MM and opioids may be an option for alleviating pain, while minimizing side effects accompanied by the use of these psychoactive drugs (Ballantyne and Mao, 2003; Robson, 2001). Research is needed to inform the safety and the appropriate dosage of combined use of MM and opioids and to provide guidance for evidence-based approaches for managing pain.
We found that non-medical MU was the only consistent factor positively associated with MMU across studies. One possible explanation is that those who did not use marijuana tended to have higher risk perception related to MU than those who used marijuana (Kilmer et al., 2007). Additionally, those who used marijuana as a means of self-medication might transition to those seeking a MM recommendation after MM became legal. Indeed, more than 95% of patients seeking a MM recommendation were those who had self-medicated their medical complains with marijuana, according to an informal survey of MM specialty physicians in California (Mikuriya et al., 2007). Taken together, health practitioners should check whether a patient has previous experience of self-medication with marijuana, non-medical MU, or symptoms of MU disorder prior to providing a MM recommendation.
Our review also revealed that those who used MM were generally less likely to involve in alcohol and other drug use problems than those who used recreational marijuana. The results may be explained by the observation that substantial proportions of people have used MM as a substitute for alcohol or illicit drugs (Reiman, 2009). Longitudinal study is needed to elucidate the extent to which MM contributes to the course of substance use and SUDs. Evidence from this review was insufficient to draw solid conclusions about the extent to which demographic or clinical characteristics were associated with MMU. This is possibly due to several reasons, such as differences in the study samples’ inclusion and exclusion criteria, medical conditions, study designs, and measures of specific factors. There is a need for more in-depth research in a large sample to better document subgroup differences in demographic/clinical correlates and outcomes of MMU.
Findings in our review have certain limitations. In most studies, MMU was assessed based on self-reports and relied on a small subset of patients, which might lead to less reliable prevalence estimates. Especially, the accuracy of self-reported responses are subject to bias due to the stigma associated with MMU (Satterlund et al., 2015). In some studies, there was a lack of information on patient’s medical conditions, which might lead to inaccurate proportions of people using MM for pain. Additionally, evidence on perceived effects of MM was based on self-reported data, which are not rigorous enough to reach clinically solid conclusions about the efficacy of MM. Lastly, the causal relationship between non-medical MU and MMU could not be determined due to the nature of observational designs of the studies included in our review. Longitudinal research is needed to elucidate temporal and causal associations between non-medical MU and MMU, factors mediating or moderating their associations, and long-term impacts of MMU on MU disorder.
In conclusion, evidence from this review demonstrates that either prescribed MMU or self-medication with marijuana was common among adults in the U.S., as well as in many other countries, mainly due to pain management. Most individuals using MM self-reported that MM improved their pain conditions, sleep disturbance, and anxiety, but not for CH symptoms. Further clinical trials assessing the risks and benefits of MM would guide evidence-based clinical practice. Considering the lack of evidence supporting the safety of MM, health practitioners should take a cautious approach in recommending MM. MM should be recommended only as a last resort when other medications had failed. Non-medical MU was a common factor associated with MMU across studies, and there is a need for in-depth research to determine whether the association is causal. Especially, it is important to elucidate temporal associations and their underlying mechanisms between non-medical MU and MMU in order to inform preventive and clinical strategies for minimizing marijuana-related harms.
Highlights.
Marijuana use for medical purposes was found among diverse patient groups.
Pain was the most commonly endorsed reason for medical marijuana use.
Medical marijuana appeared to relieve self-reported pain/anxiety for some patients.
Studies are needed to understand long-term outcomes of medical marijuana use.
Non-medical marijuana use was positively associated with medical marijuana use.
Acknowledgments
Role of funding source
This work was made possible by research support from the U.S. National Institutes of Health (UG1DA040317, R01MD007658, R01DA019623, PI, Li-Tzy Wu). The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The opinions expressed in this paper are solely those of the authors.
Footnotes
Contributors
Ji-Yeun Park contributed to the design and concept for this manuscript, conducted literature searches and review, and drafted the manuscripts. Li-Tzy Wu contributed to the design and concept for this manuscript, drafted the manuscripts, and supervised the work. All authors approved of the final manuscript before submission.
Conflict of interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844–851. doi: 10.1038/clpt.2011.188. [DOI] [PubMed] [Google Scholar]
- Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD. Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington State. J Opioid Manag. 2009;5:257–286. doi: 10.5055/jom.2009.0028. [DOI] [PubMed] [Google Scholar]
- Ashrafioun L, Bohnert KM, Jannausch M, Ilgen MA. Characteristics of substance use disorder treatment patients using medical cannabis for pain. Addict Behav. 2015;42:185–188. doi: 10.1016/j.addbeh.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Bachman JG, Johnson LD, O’Malley PM. Explaining recent increases in students’ marijuana use: Impacts of perceived risks and disapproval, 1976 through 1996. Am J Public Health. 1998;88:887–892. doi: 10.2105/ajph.88.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- Blanco C, Hasin DS, Wall MM, Flórez-Salamanca L, Hoertel N, Wang S, Kerridge BT, Olfson M. Cannabis use and risk of psychiatric disorders: Prospective evidence from a US national longitudinal study. JAMA Psychiatry. 2016;73:388–395. doi: 10.1001/jamapsychiatry.2015.3229. [DOI] [PubMed] [Google Scholar]
- Bowles DW. Persons registered for medical marijuana in the United States. J Palliat Med. 2012;15:9–11. doi: 10.1089/jpm.2011.0356. [DOI] [PubMed] [Google Scholar]
- Boyd CJ, Veliz PT, McCabe SE. Adolescents’ use of medical marijuana: A secondary analysis of monitoring the future data. J Adolesc Health. 2015;57:241–244. doi: 10.1016/j.jadohealth.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (CBHSQ) Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2016. [accessed 07.11.16]. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. [Google Scholar]
- Cerda M, Wall M, Keyes KM, Galea S, Hasin D. Medical marijuana laws in 50 states: Investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 2012;120:22–27. doi: 10.1016/j.drugalcdep.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E. Cannabis use in patients with multiple sclerosis. Mult Scler. 2006;12:646–651. doi: 10.1177/1352458506070947. [DOI] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74:1317–1324. doi: 10.1016/j.lfs.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology. 2004;62:2098–2100. doi: 10.1212/01.wnl.0000127707.07621.72. [DOI] [PubMed] [Google Scholar]
- Colorado Department of Public Health and Environment. [accessed 28.11.16];Medical Marijuana Registry Program Statistics: October 31, 2016. 2016 https://www.colorado.gov/pacific/cdphe/medical-marijuana-statistics-and-data.
- Compton WM, Han B, Hughes A, Jones CM, Blanco C. Use of marijuana for medical purposes among adults in the United States. JAMA. 2017;317:209–211. doi: 10.1001/jama.2016.18900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- Davis AK, Bonar EE, Ilgen MA, Walton MA, Perron BE, Chermack ST. Factors associated with having a medical marijuana card among Veterans with recent substance use in VA outpatient treatment. Addict Behav. 2016;63:132–136. doi: 10.1016/j.addbeh.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Lintzeris N, Campbell G, Bruno R, Cohen M, Farrell M, Hall WD. Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend. 2015;147:144–150. doi: 10.1016/j.drugalcdep.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Fairfield KM, Eisenberg DM, Davis RB, Libman H, Phillips RS. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Arch Intern Med. 1998;158:2257–2264. doi: 10.1001/archinte.158.20.2257. [DOI] [PubMed] [Google Scholar]
- Fitzcharles MA, Jamal S. Expanding medical marijuana access in Canada: Considerations for the rheumatologist. J Rheumatol. 2015;42:143–145. doi: 10.3899/jrheum.131514. [DOI] [PubMed] [Google Scholar]
- Fogarty A, Rawstorne P, Prestage G, Crawford J, Grierson J, Kippax S. Marijuana as therapy for people living with HIV/AIDS: Social and health aspects. AIDS Care. 2007;19:295–301. doi: 10.1080/09540120600841930. [DOI] [PubMed] [Google Scholar]
- Furler MD, Einarson TR, Millson M, Walmsley S, Bendayan R. Medicinal and recreational marijuana use by patients infected with HIV. AIDS Patient Care STDS. 2004;18:215–228. doi: 10.1089/108729104323038892. [DOI] [PubMed] [Google Scholar]
- Grella CE, Rodriguez L, Kim T. Patterns of medical marijuana use among individuals sampled from medical marijuana dispensaries in Los Angeles. J Psychoactive Drugs. 2014;46:267–275. doi: 10.1080/02791072.2014.944960. [DOI] [PubMed] [Google Scholar]
- Hall W. The adverse health effects of cannabis use: What are they, and what are their implications for policy? Int J Drug Policy. 2009;20:458–466. doi: 10.1016/j.drugpo.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362:1453–1457. doi: 10.1056/NEJMp1000695. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Bohnert K, Kleinberg F, Jannausch M, Bohnert AS, Walton M, Blow FC. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend. 2013;132:654–659. doi: 10.1016/j.drugalcdep.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Joffe A, Yancy WS American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescence. Legalization of marijuana: Potential impact on youth. Pediatrics. 2004;113:e632–638. [PubMed] [Google Scholar]
- Kilmer JR, Hunt SB, Lee CM, Neighbors C. Marijuana use, risk perception, and consequences: Is perceived risk congruent with reality? Addict Behav. 2007;32:3026–3033. doi: 10.1016/j.addbeh.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kmietowicz Z. Cannabis based drug is licensed for spasticity in patients with MS. BMJ. 2010;340:c3363. doi: 10.1136/bmj.c3363. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Opioid prescriptions soar: Increase in legitimate use as well as abuse. JAMA. 2007;297:249–251. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- Leroux E, Taifas I, Valade D, Donnet A, Chagnon M, Ducros A. Use of cannabis among 139 cluster headache sufferers. Cephalalgia. 2013;33:208–213. doi: 10.1177/0333102412468669. [DOI] [PubMed] [Google Scholar]
- Lin LA, Ilgen MA, Jannausch M, Bohnert KM. Comparing adults who use cannabis medically with those who use recreationally: Results from a national sample. Addict Behav. 2016;61:99–103. doi: 10.1016/j.addbeh.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic review: Opioid treatment for chronic back pain: Prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- Martin JH, Bonomo YA. Medicinal cannabis in Australia: The missing links. Med J Aust. 2016;204:371–373. doi: 10.5694/mja16.00234. [DOI] [PubMed] [Google Scholar]
- Martinez-Rodriguez JE, Munteis E, Carreno M, Blanco Y, Roquer J, Abanades S, Graus F, Saiz A. Cannabis use in Spanish patients with multiple sclerosis: Fulfilment of patients’ expectations? J Neurol Sci. 2008;273:103–107. doi: 10.1016/j.jns.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Martín-Sánchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10:1353–1368. doi: 10.1111/j.1526-4637.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- Mikuriya T, Hergenrather J, Denney P, Lucido F, Bearman D, Nunberg H. Medical marijuana in California, 1996–2006. J Cannabis Clin Pract. 2007 Winter-Spring;:1–4.
- Nunberg H, Kilmer B, Pacula RL, Burgdorf J. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011:4. doi: 10.2202/1941-2851.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum AM, Thurstone C, McGarry L, Walker B, Sabel AL. Use and diversion of medical marijuana among adults admitted to inpatient psychiatry. Am J Drug Alcohol Abuse. 2015;41:166–172. doi: 10.3109/00952990.2014.949727. [DOI] [PubMed] [Google Scholar]
- Parliament of Australia. [accessed 06.02.17];Narcotic Drugs Amendment Bill 2016. 2016 http://www.aph.gov.au/Parliamentary_Business/Bills_Legislation/Bills_Search_Results/Result?bId=r5609.
- Perron BE, Bohnert K, Perone AK, Bonn-Miller MO, Ilgen M. Use of prescription pain medications among medical cannabis patients: comparisons of pain levels, functioning, and patterns of alcohol and other drug use. J Stud Alcohol Drugs. 2015;76:406–413. doi: 10.15288/jsad.2015.76.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman A. Cannabis as a substitute for alcohol and other drugs. Harm Reduct J. 2009;6:35. doi: 10.1186/1477-7517-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinarman C, Nunberg H, Lanthier F, Heddleston T. Who are medical marijuana patients? Population characteristics from nine California assessment clinics. J Psychoactive Drugs. 2011;43:128–135. doi: 10.1080/02791072.2011.587700. [DOI] [PubMed] [Google Scholar]
- Richmond MK, Pampel FC, Rivera LS, Broderick KB, Reimann B, Fischer L. Frequency and risk of marijuana use among substance-using health care patients in Colorado with and without access to state legalized medical marijuana. J Psychoactive Drugs. 2015;47:1–9. doi: 10.1080/02791072.2014.991008. [DOI] [PubMed] [Google Scholar]
- Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Maynard C, Bumgardner K, Krupski A, Dunn C, West II, Donovan D, Atkins DC, Ries R. Are medical marijuana users different from recreational users? The view from primary care. Am J Addict. 2015;24:599–606. doi: 10.1111/ajad.12270. [DOI] [PubMed] [Google Scholar]
- Ryan-Ibarra S, Induni M, Ewing D. Prevalence of medical marijuana use in California, 2012. Drug Alcohol Rev. 2015;34:141–146. doi: 10.1111/dar.12207. [DOI] [PubMed] [Google Scholar]
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- Satterlund TD, Lee JP, Moore RS. Stigma among California’s medical marijuana patients. J Psychoactive Drugs. 2015;47:10–17. doi: 10.1080/02791072.2014.991858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermeyer J, Salomonsen-Sautel S, Price RK, Balan S, Thurstone C, Min SJ, Sakai JT. Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003–11. Drug Alcohol Depend. 2014;140:145–155. doi: 10.1016/j.drugalcdep.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney S. Marijuana use in HIV-positive and AIDS patients: Results of an anonymous mail survey. J Cannabis Ther. 2001;1:35–41. [Google Scholar]
- Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20:472–480. doi: 10.1097/01.MIB.0000440982.79036.d6. [DOI] [PubMed] [Google Scholar]
- Swaim RC. Individual and school level effects of perceived harm, perceived availability, and community size on marijuana use among 12th-grade students: A random effects model. Prev Sci. 2003;4:89–98. doi: 10.1023/a:1022922231605. [DOI] [PubMed] [Google Scholar]
- Sznitman SR, Lewis N. Is cannabis an illicit drug or a medicine? A quantitative framing analysis of Israeli newspaper coverage. Int J Drug Policy. 2015;26:446–452. doi: 10.1016/j.drugpo.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waissengrin B, Urban D, Leshem Y, Garty M, Wolf I. Patterns of use of medical cannabis among Israeli cancer patients: A single institution experience. J Pain Symptom Manage. 2015;49:223–230. doi: 10.1016/j.jpainsymman.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain. 2003;102:211–216. doi: 10.1016/s0304-3959(02)00400-1. [DOI] [PubMed] [Google Scholar]
- Woodruff SI, Shillington AM. Sociodemographic and drug use severity differences between medical marijuana users and non-medical users visiting the emergency department. Am J Addict. 2016;25:385–391. doi: 10.1111/ajad.12401. [DOI] [PubMed] [Google Scholar]
- Wu LT, Zhu H, Swartz MS. Trends in cannabis use disorders among racial/ethnic population groups in the United States. Drug Alcohol Depend. 2016;165:181–190. doi: 10.1016/j.drugalcdep.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotov Y, Baruch Y, Reuveni H, Magnezi R. Adherence to medical cannabis among licensed patients in Israel. Cannabis Cannabinoid Res. 2016;1:16–21. doi: 10.1089/can.2015.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]