Abstract
Discovered in the brains of multiple animal species, piRNAs may contribute to the pathogenesis of neuropsychiatric illnesses. The present study aimed to identify brain piRNAs across transcriptome that are associated with Alzheimer’s disease (AD). Prefrontal cortical tissues of six AD cases and six controls were examined for piRNA expression levels using an Arraystar HG19 piRNA array (containing 23,677 piRNAs) and genotyped for 17 genome-wide significant and replicated risk SNPs. We examined whether piRNAs are expressed differently between AD cases and controls and explored the potential regulatory effects of risk SNPs on piRNA expression levels. We identified a total of 9453 piRNAs in human brains, with 103 nominally (p<0.05) differentially (> 1.5 fold) expressed in AD cases vs. controls and most of the 103 piRNAs nominally correlated with genome-wide significant risk SNPs. We conclude that piRNAs are abundant in human brains and may represent risk biomarkers of AD.
Keywords: piRNA, brain, Alzheimer’s disease, microarray, transcriptome, differential expression
Introduction
Alzheimer’s disease (AD) is a degenerative brain disorder, affecting millions of people worldwide. Genetic mechanisms underlying the development of AD have been widely explored, including the direct effects of protein-coding genes, e.g., APOE, and the indirect effects of the non-coding RNAs (ncRNAs), e.g., BACEAS (Zuo, et al., 2016a). The ncRNAs include long non-coding RNAs (LncRNAs) and small non-coding RNAs such as miRNAs, piwi-interacting RNAs (piRNAs), siRNAs, snoRNAs and rasiRNAs. In this study, we examined the potential association of piRNAs with AD.
piRNAs are the ncRNAs with 24–32 nucleotides (nt). They exhibit stark differences in length, expression pattern, abundance, and genomic organization from miRNAs (Mani and Juliano, 2013, Zuo, et al., 2016b). They interact with piwi proteins and function as a complex to regulate cellular activities by RNA silencing (Lau, et al., 2006). Most piRNAs are distributed in the mammalian germline cells. In recent studies piRNAs have also been discovered in the brains of multiple species (Iyengar, et al., 2014, Lee, et al., 2011, Perrat, et al., 2013, Rajasethupathy, et al., 2012, Ross, et al., 2014, Weick and Miska, 2014). The amount of piRNAs in the brain is about one-tenth of that in the germline (Dharap, et al., 2011, Lee, et al., 2011, Peng and Lin, 2013, Yan, et al., 2011). There are hundreds of thousands piRNA sequences in each species; however, because piRNAs are poorly conserved even between closely related species (Mani and Juliano, 2013) and are tissue-specific, their distributions in the human brains cannot be predicted from other species or other human tissues. The current study would be the first to investigate the presence of piRNAs in human brains and their potential roles in neurodegenerative diseases.
Numerous lines of evidence indicate that piRNAs carry important functional roles, including suppressing transposon (Mani and Juliano, 2013), preserving genomic integrity (Czech and Hannon, 2016, Stefani and Slack, 2008), remodeling euchromatin and epigenetic programming (Akkouche, et al., 2013, Ross, et al., 2014), regulating translation (Grivna, et al., 2006), regulating target mRNAs (Lee, et al., 2011), modulating mRNA stability (Grivna, et al., 2006), and developmental regulation. The most widely-recognized and well-characterized function of piRNAs is to suppress the activities of transposable elements at genomic and epigenetic levels, suggesting that piRNAs may be involved in the etiological processes of human diseases. The present study aimed to identify the piRNAs associated with AD across transcriptome. Furthermore, we explored whether these AD-associated piRNAs were brain-specific, whether their nearest protein-coding genes were expressed in brains, and whether these genes were related to the APOE expression in brains.
For a decade scientists have scanned the whole genome to search for the risk variants of AD. We reviewed all published genome-wide association studies (GWASs) and whole genome/exome sequencing studies of AD. The results showed associations of 17 variants that were genome-wide significant (1.0×10−295≤p≤9.0×10−9) and replicated across at least two independent studies at single-point level. These 17 variants are located in 11 genes/snRNAs/LncRNAs in eight loci. They are rs6859, rs157580, rs2075650, rs429358+rs7412 (ε2/ε3/ε4) and rs4420638 within APOE cluster (NECTIN2-TOMM40-APOE-APOC1) (Abraham, et al., 2008, Antunez, et al., 2011, Coon, et al., 2007, Feulner, et al., 2010, Harold, et al., 2009, Heinzen, et al., 2010, Kamboh, et al., 2012a, Kamboh, et al., 2012b, Kim, et al., 2011, Lambert, et al., 2009, Li, et al., 2008, Logue, et al., 2011, Meda, et al., 2012, Melville, et al., 2012, Naj, et al., 2010, Nelson, et al., 2014, Perez-Palma, et al., 2014, Ramanan, et al., 2014, Ramirez, et al., 2014, Seshadri, et al., 2010, Shen, et al., 2010, Webster, et al., 2008), rs2279590 and rs9331896 at APOJ (Jun, et al., 2016, Lambert, et al., 2009, Lambert, et al., 2013), rs11218343 at SORL1 (Jun, et al., 2016, Lambert, et al., 2013, Miyashita, et al., 2013), rs10498633 at SLC24A4 (Jun, et al., 2016, Lambert, et al., 2013), rs6656401 at CR1 (Lambert, et al., 2009, Lambert, et al., 2013), rs3865444 at CD33 (Lambert, et al., 2013, Naj, et al., 2011), rs7561528, rs6733839 and rs744373 at LOC105373605 (Antunez, et al., 2011, Hollingworth, et al., 2011, Hu, et al., 2011, Jun, et al., 2016, Kamboh, et al., 2012b, Lambert, et al., 2013, Naj, et al., 2011) and rs10792832 and rs3851179 at RNU6-560P (Harold, et al., 2009, Jun, et al., 2016, Lambert, et al., 2013). Numerous candidate gene studies including ours (Zuo, et al., 2006) supported these GWAS findings. However, the mechanisms underlying SNP-AD associations remain unclear. Here, we examined whether the AD-related piRNAs might mediate these associations, in support of the potential roles of piRNAs in the pathogenesis of AD.
Summary of Materials and Methods
In this pilot study we used prefrontal cortex tissues from the primary brain cohort of 6 AD cases and 6 controls. As a contrast, eight stomach tissue samples were also examined. The samples were examined using the Arraystar HG19 piRNA array (Arraystar, Inc.) that included 23,677 piRNAs. Raw signal intensities were normalized, quality checked, filtered and then log2-transformed. Three piRNAs from the array were examined by qPCR for technical validation. The transformed intensity values were compared between AD cases and controls to identify the piRNAs associated with AD; these values were also compared between control brain tissues and stomach tissues to identify piRNAs “specific” to the brain. The mRNA expression of the nearest genes, within or close to which the AD-associated piRNAs are located, and the density of the proteins encoded by these genes was examined in brain tissues of four other independent auxiliary cohorts, to explore the expression of these genes in the brain. The correlation of expression between APOE and all risk genes in the brain was tested, to examine if the risk genes were related to this most robust and well-recognized AD-associated gene. The 17 genome-wide significant and replicated risk variants for AD were genotyped in our primary cohort (6 AD cases and 6 controls) too. Associations between the genotypes and the expression level of each AD-associated piRNA were analyzed in this primary cohort, to investigate whether these robust risk DNA variants controlled piRNA expression. The details were described in the Supplementary Materials, Methods, Table S1, and Figures S1 and S2. The design of whole study was based on a regulation pathway illustrated in Figure 1.
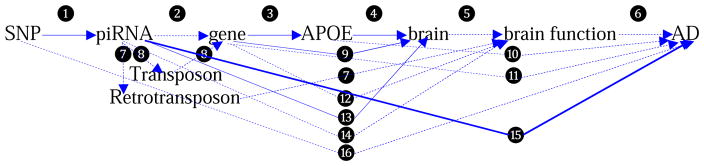
Figure 1. Illustration for the pathways underlying piRNA-AD association.
[Solid lines: Directly evidenced by our study; Dash lines: Indirectly evidenced by literatures. ➊ piRNA expression is correlated with the risk SNPs (by eQTL analysis); ➋ piRNAs are hypothesized to be most likely to regulate the expression of the nearest protein-coding genes by sequence complementarity; ➌ mRNA expression of the risk genes is correlated to APOE mRNA expression (by correlation analysis); ➍ mRNA/protein of APOE is expressed in brain (by RNA-Seq, RNA microarray and mass spectrometry-based proteomics microarray analyses); ➎ RNAs/proteins expressed in brain are assumed to have potential brain functions; ➏ many brain functions are assumed to be related to the development of AD; ➐ piRNAs are hypothesized to be related to L1 retrotransposons that are involved in brain functions; ➑ piRNAs are hypothesized to use the transposons to regulate gene expression and cellular function; ➒ mRNAs/proteins of the risk protein-coding genes are expressed in brain (by RNA-Seq, RNA microarray and mass spectrometry-based proteomics microarray analyses); ➓ association between APOE and AD is most robust and widely-recognized; ⓫ many genes have been associated with AD in literatures; ⓬ some genes regulated by piRNAs can control brain functions; ⓭ piRNA expression in brain is detected by microarray analysis; ⓮ some piRNAs can target at brain cells that may be implicated in brain functions; ⓯ piRNAs are associated with AD (by differential expression analysis), which is the main goal of the present study; ⓰ associations between SNPs and AD are identified by GWAS
Results
1. Detection of piRNAs in the brain
Among the 23k piRNAs, 9453 (41%) were detected in human brains. Among the 9453 brain piRNAs, 6853 (73%) were significantly differentially expressed between brain and stomach (1.2×10−14≤p<0.05); and 1251 (13%) were “specific” to brain (i.e., absent in stomach). The three selected piRNAs, including DQ597973, DQ576872 and DQ597479 (Table 1) were well-validated by qPCR.
Table 1.
Top piRNAs significantly differentially expressed between AD cases and controls
| piRNA | Alias | length (nt) | Chr | Normalized intensity | AD vs. Control | Brain vs. Stomach | Gene | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AD | Control | FC | p | FC | p | |||||
| With top intensities in AD (intensities>12) | ||||||||||
| DQ571030 | piR-hsa-1281 | 29 | chr19 | 29291.9 | 15703.3 | 1.9 ↑ | 0.024 | 17.1 ↑ | 2.2×10−6 | C19orf18 B |
| DQ571029 | piR-hsa-1280 | 27 | chr19 | 28564.7 | 16838.6 | 1.7 ↑ | 0.031 | 14.6 ↑ | 4.5×10−7 | C19orf18 B |
| DQ571031 | piR-hsa-1282 | 32 | chr19 | 20583.6 | 11557.8 | 1.8 ↑ | 0.049 | 18.8 ↑ | 7.6×10−6 | C19orf18 B |
| DQ597217 | piR-hsa-27492 | 28 | chr11 | 24283.1 | 13388.0 | 1.8 ↑ | 0.042 | 17.6 ↑ | 7.3×10−7 | GALNT18 B |
| DQ597216 | piR-hsa-27491 | 26 | chr11 | 24078.7 | 12986.4 | 1.9 ↑ | 0.037 | 14.9 ↑ | 8.1×10−8 | GALNT18 B |
| DQ597479 | piR-hsa-27725 | 28 | chr3 | 11637.5 | 5522.6 | 2.1 ↑ | 0.020 | 14.0 ↑ | 1.9×10−6 | ANKRD28 B |
| DQ585095 | piR-hsa-15406 | 30 | chr1 | 9853.0 | 6558.0 | 1.5 ↑ | 0.032 | 3.9 ↑ | 5.8×10−4 | ATAD3B B |
| DQ576872 | piR-hsa-7193 | 31 | chr2 | 7703.6 | 3777.0 | 2.0 ↑ | 0.034 | 6.8 ↑ | 2.2×10−5 | DOCK10 B |
| DQ571243 | piR-hsa-1580 | 29 | chr11 | 9655.2 | 5028.9 | 1.9 ↑ | 0.042 | 7.0 ↑ | 8.7×10−6 | to C11orf87 B |
| DQ597973 | piR-hsa-28188 | 27 | chr11 | 9039.6 | 4490.8 | 2.0 ↑ | 0.027 | 7.2 ↑ | 3.5×10−6 | to C11orf87 B |
| DQ597974 | piR-hsa-28189 | 28 | chr11 | 8107.7 | 4113.4 | 2.0 ↑ | 0.031 | 7.6 ↑ | 6.6×10−6 | to C11orf87 B |
| DQ597972 | piR-hsa-28187 | 26 | chr11 | 5615.2 | 2771.6 | 2.0 ↑ | 0.020 | 6.5 ↑ | 6.1×10−6 | to C11orf87 B |
| With top FC↑ between cases and controls (FC≥2) | ||||||||||
| DQ576492 | piR-hsa-6740 | 30 | chr10 | 19.0 | 7.8 | 2.4 ↑ | 0.021 | 1.1 ↑ | 0.809 | LINC00837 |
| DQ590835 | piR-hsa-21131 | 28 | chr9 | 210.9 | 89.0 | 2.4 ↑ | 0.030 | 5.5 ↑ | 3.2×10−5 | PTPRD B |
| DQ574023 | piR-hsa-4300 | 26 | chr13 | 18.7 | 7.9 | 2.4 ↑ | 0.029 | 1.1 ↑ | 0.675 | B3GALTL B |
| DQ599205 | piR-hsa-29476 | 26 | chr1 | 48.0 | 22.1 | 2.2 ↑ | 0.004 | 1.2 ↑ | 0.753 | KIAA0319L B |
| DQ598028 | piR-hsa-28243 | 29 | chr19 | 37.0 | 17.1 | 2.2 ↑ | 0.018 | 1.7 ↓ | 0.468 | FLJ25328 |
| DQ573352 | piR-hsa-3645 | 26 | chr7 | 34.4 | 16.3 | 2.1 ↑ | 0.037 | 1.1 ↓ | 0.884 | ABCA13 B |
| DQ581610 | piR-hsa-11139 | 29 | chr17 | 23.1 | 10.9 | 2.1 ↑ | 0.019 | “Brain” | - | to EVPLL |
| DQ599207 | piR-hsa-29478 | 29 | chr22 | 65.6 | 31.5 | 2.1 ↑ | 0.018 | 1.6 ↑ | 0.215 | to ELFN2 B |
| DQ597479* | piR-hsa-27725 | 28 | chr3 | 11637.5 | 5522.6 | 2.1 ↑ | 0.020 | 14.0 ↑ | 1.9×10−6 | ANKRD28 B |
| DQ597973* | piR-hsa-28188 | 27 | chr11 | 9039.6 | 4490.8 | 2.0 ↑ | 0.027 | 7.2 ↑ | 3.5×10−6 | to C11orf87 B |
| DQ597974* | piR-hsa-28189 | 28 | chr11 | 8107.7 | 4113.4 | 2.0 ↑ | 0.031 | 7.6 ↑ | 6.6×10−6 | to C11orf87 B |
| DQ597972* | piR-hsa-28187 | 26 | chr11 | 5615.2 | 2771.6 | 2.0 ↑ | 0.020 | 6.5 ↑ | 6.1×10−6 | to C11orf87 B |
| DQ576872* | piR-hsa-7193 | 31 | chr2 | 7703.6 | 3777.0 | 2.0 ↑ | 0.034 | 6.8 ↑ | 2.2×10−5 | DOCK10 B |
| With top FC ↓ between cases and controls (FC≥2) | ||||||||||
| DQ579851 | piR-hsa-10106 | 32 | chr15 | 10.8 | 27.0 | 2.5 ↓ | 0.025 | 2.1 ↑ | 0.050 | to PGPEP1L |
| DQ600318 | piR-hsa-30518 | 26 | chr17 | 14.0 | 34.7 | 2.5 ↓ | 0.010 | “Brain” | - | LRRC37A3 B |
| DQ571669 | piR-hsa-1963 | 30 | chr17 | 14.8 | 34.6 | 2.3 ↓ | 0.025 | “Brain” | - | VPS53 B |
| DQ586404 | piR-hsa-16724 | 29 | chr18 | 15.7 | 33.3 | 2.1 ↓ | 0.006 | 1.6 ↑ | 0.234 | to GNAL B |
| With lowest p values between cases and controls and FC↑ (p≤0.010) | ||||||||||
| DQ591371 | piR-hsa-21636 | 28 | chr1 | 42.7 | 27.5 | 1.6 ↑ | 0.002 | 2.0 ↑ | 0.012 | to NBPF4 |
| DQ580484 | piR-hsa-10710 | 30 | chr1 | 17.6 | 9.0 | 1.9 ↑ | 0.002 | 1.2 ↑ | 0.448 | to TDRD5 |
| DQ577835 | piR-hsa-8094 | 30 | chr9 | 40.4 | 23.0 | 1.8 ↑ | 0.003 | 1.5 ↑ | 0.055 | FAM225B |
| DQ586113 | piR-hsa-16363 | 29 | chr17 | 12.9 | 7.5 | 1.7 ↑ | 0.003 | “Brain” | - | to EVPLL |
| DQ599205* | piR-hsa-29476 | 26 | chr1 | 48.0 | 22.1 | 2.2 ↑ | 0.004 | 1.2 ↑ | 0.753 | KIAA0319L B |
| DQ580261 | piR-hsa-10501 | 28 | chr10 | 350.2 | 233.2 | 1.5 ↑ | 0.005 | 3.3 ↑ | 7.6×10−4 | NRG3 B |
| DQ597396 | piR-hsa-27133 | 29 | chr11 | 599.6 | 346.4 | 1.7 ↑ | 0.007 | 1.4 ↑ | 0.045 | to UBASH3B B |
| DQ597397 | piR-hsa-27134 | 31 | chr11 | 863.9 | 545.2 | 1.6 ↑ | 0.010 | 1.6 ↑ | 0.011 | to UBASH3B B |
| With lowest p values between cases and controls and FC ↓ (p≤0.002) | ||||||||||
| DQ575353 | piR-hsa-5645 | 28 | chr17 | 19.0 | 28.6 | 1.5 ↓ | 0.002 | 2.2 ↑ | 1.0×10−3 | LRRC37A B |
| DQ577904 | piR-hsa-8163 | 30 | chr1 | 12.0 | 18.3 | 1.5 ↓ | 0.005 | 2.0 ↑ | 0.101 | to RABGEF1 B |
| DQ595753 | piR-hsa-25985 | 29 | chr1 | 11.1 | 19.9 | 1.8 ↓ | 0.005 | “Brain” | - | SCP2 B |
| DQ586404* | piR-hsa-16724 | 29 | chr18 | 15.7 | 33.3 | 2.1 ↓ | 0.006 | 1.6 ↑ | 0.234 | to GNAL B |
| DQ579582 | piR-hsa-9851 | 31 | chr22 | 60.7 | 91.0 | 1.5 ↓ | 0.006 | 2.2 ↑ | 0.012 | POM121L8P |
| DQ584325 | piR-hsa-14547 | 27 | chr15 | 13.0 | 21.5 | 1.7 ↓ | 0.009 | 1.9 ↑ | 0.011 | to C2CD4B |
| With locations at or close to AD-related genes | ||||||||||
| DQ583613 | piR-hsa-13893 | 29 | chr6 | 3694.9 | 2295.2 | 1.6 ↑ | 0.038 | 4.2 ↑ | 2.3×10−4 | to HIST1H4H B |
| DQ584879 | piR-hsa-14621 | 28 | chr15 | 13.4 | 8.7 | 1.5 ↑ | 0.011 | “Brain” | - | CYP19A1 B |
| DQ597214 | piR-hsa-27489 | 27 | chr3 | 117.0 | 61.7 | 1.9 ↑ | 0.025 | 3.4 ↑ | 9.9×10−3 | to PLCH1 B |
| DQ583911 | piR-hsa-14148 | 30 | chr6 | 16.3 | 9.5 | 1.7 ↑ | 0.040 | “Brain” | - | to CCR6 B |
| DQ599147 | piR-hsa-29114 | 31 | chr17 | 699.9 | 386.3 | 1.8 ↑ | 0.048 | 3.1 ↑ | 1.7×10−3 | CTC1 B |
| DQ600513 | piR-hsa-30713 | 28 | chr10 | 311.9 | 182.1 | 1.7 ↑ | 0.014 | 3.5 ↑ | 5.1×10−4 | DOCK1 B |
| DQ575353 | piR-hsa-5645 | 28 | chr17 | 19.0 | 28.6 | 1.5 ↓ | 0.002 | 2.2 ↑ | 1.0×10−3 | LRRC37A B |
| DQ575681 | piR-hsa-5959 | 28 | chr11 | 31.8 | 47.9 | 1.5 ↓ | 0.029 | “Brain” | - | BACE1 B |
| DQ574452 | piR-hsa-4685 | 30 | chr14 | 9.0 | 13.8 | 1.5 ↓ | 0.040 | “Brain” | - | to KCNK10 B |
| DQ596958 | piR-hsa-27248 | 30 | chr15 | 14.0 | 21.3 | 1.5 ↓ | 0.039 | “Brain” | - | CYP19A1 B |
These genes are expressed in brain; chr, chromosome;
↑, up-regulated; ↓, down-regulated; “Brain”, brain-specific expression in constrast to stomach. FC, fold-changes; p, p values from t-test; AD, Alzheimer’s disease; “to”, proximate to.
appears at least twice in this table.
2. Differential expression of piRNAs between cases and controls (Figures 2 and S3, and Tables S2 and 1)
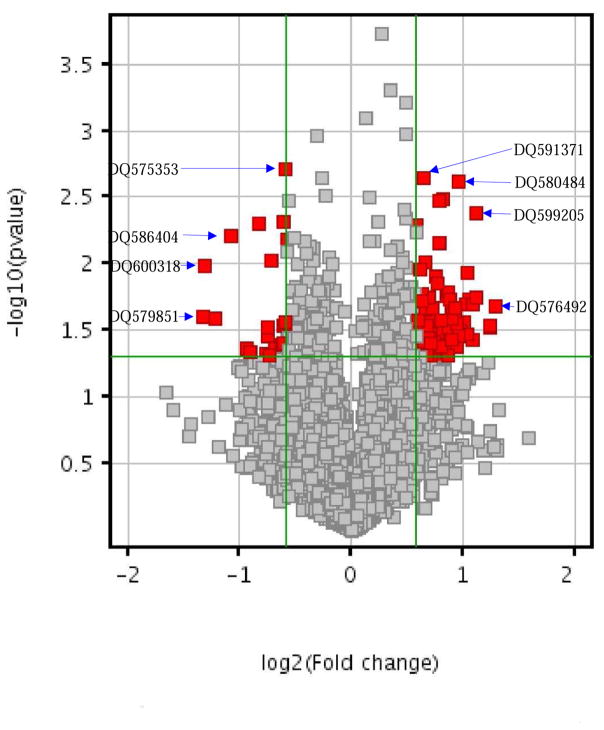
Figure 2. The differential expression between cases and controls.
[X-axis: Fold-change; Y-axis: −log10(p); Red points: the differentially expressed piRNAs with 1.5-fold change and p< 0.05]
The mean log2-transformed normalized intensity of expression of all 9453 piRNAs was 7.00±2.91 (mean ± SD; range: 3.16–18.4) in AD cases, and 7.02±2.90 (2.87–18.4) in controls. 103 piRNAs with length of 26–32nt were nominally differentially expressed between cases and controls (FC>1.5; p<0.05; without correction) (Figure 2; Table S2). The mean transformed normalized intensity of these 103 piRNAs was 6.77±3.57 (3.16–14.8) in AD cases, and 6.30±3.43 (2.88–14.0) in controls. Among the 103 risk/protective piRNAs, 81 were up-regulated and 22 were down-regulated in cases in contrast to controls. Among the 103 piRNAs, 24 were “specific” to brain (i.e., no significant expression in stomach), 69 were expressed in brain higher than in stomach (1.0<FC<18.8), and 10 were expressed in brain lower than in stomach (1.1<FC<2.4). Among the 103 piRNAs, 100 piRNAs map to genomic locations that are located within or close to 66 protein-coding genes, and three piRNAs map to unknown locations. 42 piRNAs map to 37 protein-coding genes, and two map to ncRNA genes. Among these 103 piRNAs, 56 piRNAs are intergenic, proximate to 32 protein-coding or ncRNA genes; 50 of these protein-coding genes that 100 piRNAs map or are proximate to are expressed in brains (data not shown). 45 are located in piRNA clusters. 29 piRNA clusters are located in intergenic regions, consistent with earlier literature (Zuo, et al., 2016b). 66% of these 50 protein-coding brain genes have been related to neurodegenerative or neuropsychiatric disorders (Table S2).
9 piRNAs had log2-transformed normalized intensities > 13 (i.e., > 9000 before transformed). The top five piRNAs with highest intensities in cases were DQ571030, DQ571029 and DQ571031 at C19orf18 (on chr19) (Figure S3), and DQ597217 and DQ597216 at GALNT18 (on chr11). They were also the top five with highest intensities in controls, and the top five with highest FC (14.6≤FC≤18.8) in brain compared to stomach (Table 1).
14 piRNAs were expressed with >2 FCs in cases compared to controls; the top five were DQ590835 at PTPRD (chr9), DQ576492 at LINC00837 (chr10), DQ574023 at B3GALTL (chr13), DQ599205 at KIAA0319L (chr1), and DQ598028 at FLJ25328 (chr19). 4 piRNAs were expressed with >2 FCs in controls compared to cases; they were DQ579851 at chr15, DQ600318 at chr17, DQ571669 at VPS53 (chr17), and DQ586404 at chr18 (Table 1).
14 piRNAs were significantly differentially expressed between cases and controls with p<0.01. The five most significant ones with higher FCs in cases were DQ591371 proximate to NBPF4 on chr 1, DQ580484 proximate to TDRD5 on chr 1, DQ577835 at FAM225B on chr 9, DQ586113 proximate to EVPLL on chr 17, and DQ599205 at KIAA0319L on chr 1 (0.002≤p≤0.004). The five most significant ones with lower FCs in cases were DQ575353 at LRRC37A on chr 17, DQ577904 proximate to RABGEF1 on chr 1, DQ595753 at SCP2 on chr 1, DQ586404 proximate to GNAL on chr 18, and DQ579582 at POM121L8P on chr 22 (0.002≤p≤0.006) (Table 1).
Two piRNAs, including DQ599147 at CTC1 (FC=1.8, p=0.048) and DQ597974 near C11orf87 (FC=2.0, p=0.031), expressed in significantly higher levels in cases than controls (Table S2) replicated previous findings (Roy, et al., 2017).
Some genes that the AD-associated piRNAs are located within or near to have been reported to be associated with AD or its biomarkers, including BACE1, CYP19A1, CTC1, LRRC37A, CCR6, KCNK10, HIST1H4H, C1orf174, DOCK1, and PLCH1. Over 540 studies have reported the associations between BACE1 and AD, 9 studies for CYP19A1, 6 studies for CTC1, and at least one study for each of other genes. (Table S2)
3. Gene expression in brains
Among the 73 risk genes, 52 (71.2%) were expressed in human brains (Table S2). Among the 38 genes of strongest associations with AD risk, as listed in Table 1, 27 (71.1%) were expressed in human brains (Table 1). The expression of all of the 27 genes was significantly correlated with APOE expression in at least one brain region (2.9×10−39≤p<1.8×10−4; Table S3).
4. The piRNA expression correlated with SNPs
eQTL analysis showed that most AD-associated piRNAs were nominally correlated with the genome-wide significant risk SNPs (p<0.05; Table 2). After Bonferroni correction (α=5×10−4), the correlations of rs429358 (p=2.8×10−4) and rs4420638 (p=3.4×10−4) at APOE cluster with DQ581734, rs2075650 (p=3.3×10−4) at APOE cluster with DQ592330, rs4420638 (p=2.3×10−4) at APOE cluster with DQ600318, rs2279590 (p=2.7×10−4) at APOJ with DQ597397, and rs7561528 (p=9.2×10−5) at LOC105373605 with DQ574023 remained significant. In view of the small sample size, we may have missed some potentially significant correlations, so we listed more modest correlations in Table 2 (p<8×10−3). Most eQTL signals occurred in APOE cluster and APOJ loci, and all 5 variants at the LncRNA LOC105373605 or snRNA RNU6-560P presented modest eQTL signals (Table 2). The results presented above are also illustrated in Figure 1.
Table 2.
The expression of piRNAs correlated with the genome-wide significant replicated risk SNPs for AD (p<0.008)
| Gene (chr) | LOC105373605 (chr2) | APOJ (chr8) | SORL1 (chr11) | RNU6-560P (chr11) | SLC24A4 (chr14) | NECTIN2-TOMM40-APOE-APOC1 (chr19) | CD33 (chr19) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNPs | rs6733839 | rs744373 | rs7561528 | rs2279590 | rs9331896 | rs11218343 | rs3851179 | rs10792832 | rs10498633 | rs2075650 | rs429358 | rs4420638 | ε2/ε3/ε4 | rs3865444 | |
| In cases and controls | |||||||||||||||
| DQ571669 | VPS53 | 3.7×10−3 | 4.7×10−3 | ||||||||||||
| DQ573352 | ABCA13 | 7.3×10−3 | |||||||||||||
| DQ573721 | - | 1.1×10−3 | |||||||||||||
| DQ574452 | to_KCNK10 | 6.0×10−3 | |||||||||||||
| DQ577835 | FAM225B | 3.0×10−3 | |||||||||||||
| DQ579851 | to_PGPEP1L | 8.8×10−4 | 2.9×10−3 | ||||||||||||
| DQ581441 | to_CHST1 | 3.5×10−3 | |||||||||||||
| DQ581734 | TYSND1 | 2.8×10−4 | 3.4×10−4 | ||||||||||||
| DQ583613 | to_HIST1H4H | 6.7×10−3 | |||||||||||||
| DQ584325 | to_C2CD4B | 2.2×10−3 | |||||||||||||
| DQ584637 | AGAP1 | 1.2×10−3 | |||||||||||||
| DQ584879 | CYP19A1 | 7.5×10−3 | |||||||||||||
| DQ584936 | to_METTL14 | 3.5×10−3 | |||||||||||||
| DQ592330 | to_ELFN2 | 3.3×10−4 | |||||||||||||
| DQ594768 | to_CHEK2P2 | 3.5×10−3 | |||||||||||||
| DQ597396 | to_UBASH3B | 6.1×10−3 | |||||||||||||
| DQ597397 | to_UBASH3B | 2.7×10−4 | 6.0×10−4 | ||||||||||||
| DQ597401 | to_VN1R10P | 3.1×10−3 | 5.4×10−3 | ||||||||||||
| DQ597402 | to_VN1R10P | 4.1×10−3 | 5.9×10−3 | ||||||||||||
| DQ597403 | to_VN1R10P | 3.4×10−3 | 4.6×10−3 | ||||||||||||
| DQ597886 | to_ILF2 | 7.5×10−3 | |||||||||||||
| DQ598571 | to_CHST1 | 4.8×10−3 | |||||||||||||
| DQ600318 | LRRC37A3 | 7.0×10−3 | 2.3×10−4 | ||||||||||||
| In controls | |||||||||||||||
| DQ574023 | to_B3GALTL | 9.2×10−5 | |||||||||||||
| DQ576492 | LINC00837 | 3.6×10−3 | |||||||||||||
| DQ577835* | FAM225B | 5.1×10−3 | |||||||||||||
| DQ584879* | CYP19A1 | 6.4×10−3 | |||||||||||||
| DQ586113 | to_EVPLL | 3.0×10−3 | |||||||||||||
| DQ590261 | ANKRD20A19P | 1.2×10−3 | 1.2×10−3 | ||||||||||||
| DQ597109 | to_HIST1H4H | 5.7×10−3 | |||||||||||||
| DQ597397* | to_UBASH3B | 3.8×10−3 | 4.6×10−3 | 4.6×10−3 | |||||||||||
| DQ597402* | to_VN1R10P | 5.4×10−3 | 5.4×10−3 | ||||||||||||
| DQ598028 | FLJ25328 | 2.9×10−3 | |||||||||||||
| DQ599147 | CTC1 | 6.0×10−3 | |||||||||||||
| DQ599205 | KIAA0319L | 5.9×10−3 | |||||||||||||
| In cases | |||||||||||||||
| DQ597973 | to C11orf87 | 7.3×10−3 | |||||||||||||
| DQ597402* | to_VN1R10P | 7.2×10−3 | |||||||||||||
| DQ598571* | to_CHST1 | 3.8×10−3 | |||||||||||||
| DQ581441* | to_CHST1 | 4.9×10−3 | |||||||||||||
| DQ600513 | to C11orf87 | 6.4×10−3 | |||||||||||||
The correlations with p<α=5×10−4 (=0.05/103 piRNAs) were bold; “to”, proximate to;
, appears at least twice in this table.
Discussion
The present study showed that piRNAs are abundant in human brains and may contribute to the risk for AD. Although most differential expressions did not survive the conservative Bonferroni correction for multiple comparisons, the potential roles of piRNAs in AD cannot be ignored considering that this was a pilot screening study with small sample sizes (Hebert, et al., 2013). Many piRNAs were brain-“specific”, and their nearest protein-coding genes were expressed in brains and related to the APOE expression in brains. Further, the expression of these piRNAs were controlled by the most robust risk DNA variants. Together, these findings support a functional role of piRNAs in the pathogenesis of AD. We illustrate possible mechanisms underlying these findings in Figure 1.
The piRNAs in the brain usually demonstrate unique biogenesis patterns with a predominantly nuclear localization (Rajasethupathy, et al., 2012). piRNAs located within genes or from the intergenic regions may modulate the stability and translation of the mRNAs of the proximate genes (Grivna, et al., 2006, Lee, et al., 2011, Mani and Juliano, 2013). However, unlike miRNAs and siRNAs, piRNAs are not derived from the dsRNA precursors, which makes it difficult to derive the unique location of each piRNA on the genome. Because piRNAs are short, they might correspond to multiple positions on the genome. Across transcriptome, only 5 percent of piRNAs can be mapped to protein-coding genes (Brennecke, et al., 2007); however, among the AD-associated piRNAs identified in this study, 41% are enriched in the protein-coding genes, suggesting a strong correlation among these genes, piRNAs and AD. Sequences of the AD-associated piRNAs are complement to or close to these protein-coding genes, and thus, the piRNAs are most likely to target and regulate these nearest genes by sequence complementarity (Roy, et al., 2017).
Furthermore, we found that 71.2% of these protein-coding genes were expressed in human brains, and their expression levels were all significantly correlated with APOE, the most robustly and well-recognized AD risk gene. 66% of these protein-coding brain genes have already been associated with neurodegenerative or neuropsychiatric disorders including AD, e.g., BACE1, CYP19A1, CTC1 and HIST1H4H, suggesting that they are potentially the direct biological targets for the AD-associated piRNAs to regulate the development of AD. Some of these genes have been implicated in the extensively-studied etiological pathways leading to AD. For example, BACE1 has been implicated in the “Alzheimer’s disease” pathway (www.genome.jp/kegg); CYP19A1 has been implicated in the “Metabolism of lipids and lipoproteins” pathway (www.reactome.org); CTC1 has been implicated in the “Oxidative phosphorylation” pathway (www.genome.jp/kegg); and HIST1H4H has been implicated in the “Telomere maintenance” pathway (www.reactome.org).
We observed that the expression of many nominally AD-related piRNAs was correlated with the AD-risk DNA variants, suggesting that these piRNAs might mediate SNP-AD associations. In particular, all of the five genome-wide and replicated risk variants at LncRNA and snRNA had nominal or even significant regulatory effects on piRNAs, which may in part explain SNP-AD associations at non-coding loci.
Numerous piRNAs are produced from the disruption of transposons in the genome (Halic and Moazed, 2009, Sai Lakshmi and Agrawal, 2008); that is, most piRNAs overlap with the transposons or transposon remnants in sequences (Brennecke, et al., 2007). piRNAs selectively target and silence the RNAs transcribed from transposons (Brennecke, et al., 2007, Gunawardane, et al., 2007), perhaps to balance or to maintain the fitness of the genome. Experimental data supported this proposition. Mili- and Miwi-2 null mice have been found to have increased activity of retrotransposons, which suggests that piRNAs could protect the genome from deleterious transposon insertions to preserve genomic integrity (Stefani and Slack, 2008). Disruption in the piRNAome may lead to uncontrolled transposition with destabilizing genomic and cellular effects (Dharap, et al., 2011, Mani and Juliano, 2013). It has been posited that the Piwi/piRNA complex uses the transposons to regulate a large group of gene expression and cellular functions (Mani and Juliano, 2013), a plausible mechanism to underscore the associations between piRNAs and AD identified in this study. Experimental data suggest that piRNAs can inhibit transposons at either genomic or epigenetic levels. The restriction of transposons by piRNAs has been demonstrated by the up-regulation of transposons as a result of mutations of the Piwi/piRNA complex.
Evidence suggests that Piwi/piRNA complex may be involved in modulating the development of dendritic spines (Lee, et al., 2011). Some Piwi/piRNA complex target Astrotactin, a protein critical to neuronal migration (Adams, et al., 2002). Some Piwi/piRNA complex potentially regulate genes to control other nervous system functions (Lee, et al., 2011). These mechanisms may also underlie piRNA-AD associations.
Another clue regarding the functions of piRNA relates to the discovery of the L1 retrotransposons in the human, mouse and rat brains. In the brains, the L1 retrotransposons are involved in neuronal differentiation, heterogeneity, and somatic mosaicism (Coufal, et al., 2009, Muotri, et al., 2005). Some piRNAs and retrotransposons co-exist in the brains. These piRNAs regulate L1 retrotransposons and their mutants elevate retrotransposon expression in the brains. The co-existence of piRNA and retrotransposons might play important roles during brain development and in maintaining functional integrity of the adult brains, and in the development of AD.
piRNAs are unevenly distributed across the genome. We found that many AD-associated piRNAs were clustered. Although individual piRNA sequences are rarely conserved, the genomic locations of the piRNA clusters are usually conserved across species (Aravin, et al., 2006, Girard, et al., 2006, Lau, et al., 2006). More studies are clearly warranted to investigate the roles of these clusters in the development of AD.
Supplementary Material
Highlights.
This study first profiled piRNA expression in human brains with Alzheimer’s disease
9453 piRNAs were detected in human brains
103 piRNAs were nominally differentially expressed between cases and controls
Among the genes that the AD-associated piRNAs were located within or close to, 71.2% were expressed in human brains
Most AD-associated piRNAs were nominally correlated with the genome-wide significant risk SNPs
Acknowledgments
This work was supported by the China Human Brain Bank Consortium and in part by National Natural Science Foundation of China (81373150; 81271239; 81202371; 81201057; 81171091 and 91632113), the IBMS/CAMS Dean’s Fund (2011RC01), the CAMS Neuroscience Center Special Fund (#2014C01), the CAMS Innovation Fund for Medical Sciences (CIFMS), the Natural Science Foundation and Major Basic Research Program of Shanghai (16JC1420500, 16JC1420502), the National High Technology Research and Development Program (“863” Program) of China (2013AA020106), Shanghai municipal commission award (#20124109), National Institute on Drug Abuse (NIDA) grant K01 DA029643, and National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R21 AA021380, R21 AA020319 and R21 AA023237. We thank for Prof. Haifan Lin’s helpful comments.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham R, Moskvina V, Sims R, Hollingworth P, Morgan A, Georgieva L, Dowzell K, Cichon S, Hillmer AM, O’Donovan MC, Williams J, Owen MJ, Kirov G. A genome-wide association study for late-onset Alzheimer’s disease using DNA pooling. BMC Med Genomics. 2008;1:44. doi: 10.1186/1755-8794-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129(4):965–72. doi: 10.1242/dev.129.4.965. [DOI] [PubMed] [Google Scholar]
- Akkouche A, Grentzinger T, Fablet M, Armenise C, Burlet N, Braman V, Chambeyron S, Vieira C. Maternally deposited germline piRNAs silence the tirant retrotransposon in somatic cells. EMBO Rep. 2013;14(5):458–64. doi: 10.1038/embor.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunez C, Boada M, Gonzalez-Perez A, Gayan J, Ramirez-Lorca R, Marin J, Hernandez I, Moreno-Rey C, Moron FJ, Lopez-Arrieta J, Mauleon A, Rosende-Roca M, Noguera-Perea F, Legaz-Garcia A, Vivancos-Moreau L, Velasco J, Carrasco JM, Alegret M, Antequera-Torres M, Manzanares S, Romo A, Blanca I, Ruiz S, Espinosa A, Castano S, Garcia B, Martinez-Herrada B, Vinyes G, Lafuente A, Becker JT, Galan JJ, Serrano-Rios M, Vazquez E, Tarraga L, Saez ME, Lopez OL, Real LM, Ruiz A Alzheimer’s Disease Neuroimaging I. The membrane-spanning 4-domains, subfamily A (MS4A) gene cluster contains a common variant associated with Alzheimer’s disease. Genome Med. 2011;3(5):33. doi: 10.1186/gm249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442(7099):203–7. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128(6):1089–103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Coon KD, Myers AJ, Craig DW, Webster JA, Pearson JV, Lince DH, Zismann VL, Beach TG, Leung D, Bryden L, Halperin RF, Marlowe L, Kaleem M, Walker DG, Ravid R, Heward CB, Rogers J, Papassotiropoulos A, Reiman EM, Hardy J, Stephan DA. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry. 2007;68(4):613–8. doi: 10.4088/jcp.v68n0419. [DOI] [PubMed] [Google Scholar]
- Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, Morell M, O’Shea KS, Moran JV, Gage FH. L1 retrotransposition in human neural progenitor cells. Nature. 2009;460(7259):1127–31. doi: 10.1038/nature08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, Hannon GJ. One Loop to Rule Them All: The Ping-Pong Cycle and piRNA-Guided Silencing. Trends in biochemical sciences. 2016 doi: 10.1016/j.tibs.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Nakka VP, Vemuganti R. Altered expression of PIWI RNA in the rat brain after transient focal ischemia. Stroke. 2011;42(4):1105–9. doi: 10.1161/STROKEAHA.110.598391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feulner TM, Laws SM, Friedrich P, Wagenpfeil S, Wurst SH, Riehle C, Kuhn KA, Krawczak M, Schreiber S, Nikolaus S, Forstl H, Kurz A, Riemenschneider M. Examination of the current top candidate genes for AD in a genome-wide association study. Mol Psychiatry. 2010;15(7):756–66. doi: 10.1038/mp.2008.141. [DOI] [PubMed] [Google Scholar]
- Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442(7099):199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- Grivna ST, Beyret E, Wang Z, Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20(13):1709–14. doi: 10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science. 2007;315(5818):1587–90. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- Halic M, Moazed D. Transposon silencing by piRNAs. Cell. 2009;138(6):1058–60. doi: 10.1016/j.cell.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nature genetics. 2009;41(10):1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Wang WX, Zhu Q, Nelson PT. A study of small RNAs from cerebral neocortex of pathology-verified Alzheimer’s disease, dementia with lewy bodies, hippocampal sclerosis, frontotemporal lobar dementia, and non-demented human controls. J Alzheimers Dis. 2013;35(2):335–48. doi: 10.3233/JAD-122350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen EL, Need AC, Hayden KM, Chiba-Falek O, Roses AD, Strittmatter WJ, Burke JR, Hulette CM, Welsh-Bohmer KA, Goldstein DB. Genome-wide scan of copy number variation in late-onset Alzheimer’s disease. J Alzheimers Dis. 2010;19(1):69–77. doi: 10.3233/JAD-2010-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Ruther E, Schurmann B, Heun R, Kolsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Gallacher J, Hull M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, van Duijn CM, Breteler MM, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alperovitch A, Lathrop M, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Bjornsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossu P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J consortium E, consortium C, Alzheimer’s Disease Neuroimaging I. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nature genetics. 2011;43(5):429–35. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Pickering E, Liu YC, Hall S, Fournier H, Katz E, Dechairo B, John S, Van Eerdewegh P, Soares H Alzheimer’s Disease Neuroimaging I. Meta-analysis for genome-wide association study identifies multiple variants at the BIN1 locus associated with late-onset Alzheimer’s disease. PLoS One. 2011;6(2):e16616. doi: 10.1371/journal.pone.0016616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar BR, Choudhary A, Sarangdhar MA, Venkatesh KV, Gadgil CJ, Pillai B. Non-coding RNA interact to regulate neuronal development and function. Frontiers in cellular neuroscience. 2014;8:47. doi: 10.3389/fncel.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun G, Ibrahim-Verbaas CA, Vronskaya M, Lambert JC, Chung J, Naj AC, Kunkle BW, Wang LS, Bis JC, Bellenguez C, Harold D, Lunetta KL, Destefano AL, Grenier-Boley B, Sims R, Beecham GW, Smith AV, Chouraki V, Hamilton-Nelson KL, Ikram MA, Fievet N, Denning N, Martin ER, Schmidt H, Kamatani Y, Dunstan ML, Valladares O, Laza AR, Zelenika D, Ramirez A, Foroud TM, Choi SH, Boland A, Becker T, Kukull WA, van der Lee SJ, Pasquier F, Cruchaga C, Beekly D, Fitzpatrick AL, Hanon O, Gill M, Barber R, Gudnason V, Campion D, Love S, Bennett DA, Amin N, Berr C, Tsolaki M, Buxbaum JD, Lopez OL, Deramecourt V, Fox NC, Cantwell LB, Tarraga L, Dufouil C, Hardy J, Crane PK, Eiriksdottir G, Hannequin D, Clarke R, Evans D, Mosley TH, Jr, Letenneur L, Brayne C, Maier W, De Jager P, Emilsson V, Dartigues JF, Hampel H, Kamboh MI, de Bruijn RF, Tzourio C, Pastor P, Larson EB, Rotter JI, O’Donovan MC, Montine TJ, Nalls MA, Mead S, Reiman EM, Jonsson PV, Holmes C, St George-Hyslop PH, Boada M, Passmore P, Wendland JR, Schmidt R, Morgan K, Winslow AR, Powell JF, Carasquillo M, Younkin SG, Jakobsdottir J, Kauwe JS, Wilhelmsen KC, Rujescu D, Nothen MM, Hofman A, Jones L, Haines JL, Psaty BM, Van Broeckhoven C, Holmans P, Launer LJ, Mayeux R, Lathrop M, Goate AM, Escott-Price V, Seshadri S, Pericak-Vance MA, Amouyel P, Williams J, van Duijn CM, Schellenberg GD, Farrer LA Consortium I. A novel Alzheimer disease locus located near the gene encoding tau protein. Mol Psychiatry. 2016;21(1):108–17. doi: 10.1038/mp.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh MI, Barmada MM, Demirci FY, Minster RL, Carrasquillo MM, Pankratz VS, Younkin SG, Saykin AJ, Sweet RA, Feingold E, DeKosky ST, Lopez OL Alzheimer’s Disease Neuroimaging I. Genome-wide association analysis of age-at-onset in Alzheimer’s disease. Mol Psychiatry. 2012a;17(12):1340–6. doi: 10.1038/mp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh MI, Demirci FY, Wang X, Minster RL, Carrasquillo MM, Pankratz VS, Younkin SG, Saykin AJ, Jun G, Baldwin C, Logue MW, Buros J, Farrer L, Pericak-Vance MA, Haines JL, Sweet RA, Ganguli M, Feingold E, Dekosky ST, Lopez OL, Barmada MM Alzheimer’s Disease Neuroimaging I. Genome-wide association study of Alzheimer’s disease. Transl Psychiatry. 2012b;2:e117. doi: 10.1038/tp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Swaminathan S, Shen L, Risacher SL, Nho K, Foroud T, Shaw LM, Trojanowski JQ, Potkin SG, Huentelman MJ, Craig DW, DeChairo BM, Aisen PS, Petersen RC, Weiner MW, Saykin AJ Alzheimer’s Disease Neuroimaging I. Genome-wide association study of CSF biomarkers Abeta1–42, t-tau, and p-tau181p in the ADNI cohort. Neurology. 2011;76(1):69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P European Alzheimer’s Disease Initiative I. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nature genetics. 2009;41(10):1094–9. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Moron FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fievet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossu P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nothen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P European Alzheimer’s Disease I Genetic Environmental Risk in Alzheimer’s D, Alzheimer’s Disease Genetic C, Cohorts for H. Aging Research in Genomic E. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature genetics. 2013;45(12):1452–8. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE. Characterization of the piRNA complex from rat testes. Science. 2006;313(5785):363–7. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Banerjee S, Zhou H, Jammalamadaka A, Arcila M, Manjunath BS, Kosik KS. Identification of piRNAs in the central nervous system. RNA. 2011;17(6):1090–9. doi: 10.1261/rna.2565011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wetten S, Li L, St Jean PL, Upmanyu R, Surh L, Hosford D, Barnes MR, Briley JD, Borrie M, Coletta N, Delisle R, Dhalla D, Ehm MG, Feldman HH, Fornazzari L, Gauthier S, Goodgame N, Guzman D, Hammond S, Hollingworth P, Hsiung GY, Johnson J, Kelly DD, Keren R, Kertesz A, King KS, Lovestone S, Loy-English I, Matthews PM, Owen MJ, Plumpton M, Pryse-Phillips W, Prinjha RK, Richardson JC, Saunders A, Slater AJ, St George-Hyslop PH, Stinnett SW, Swartz JE, Taylor RL, Wherrett J, Williams J, Yarnall DP, Gibson RA, Irizarry MC, Middleton LT, Roses AD. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65(1):45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- Logue MW, Schu M, Vardarajan BN, Buros J, Green RC, Go RC, Griffith P, Obisesan TO, Shatz R, Borenstein A, Cupples LA, Lunetta KL, Fallin MD, Baldwin CT, Farrer LA Multi-Institutional Research on Alzheimer Genetic Epidemiology Study G. A comprehensive genetic association study of Alzheimer disease in African Americans. Arch Neurol. 2011;68(12):1569–79. doi: 10.1001/archneurol.2011.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SR, Juliano CE. Untangling the web: the diverse functions of the PIWI/piRNA pathway. Mol Reprod Dev. 2013;80(8):632–64. doi: 10.1002/mrd.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Narayanan B, Liu J, Perrone-Bizzozero NI, Stevens MC, Calhoun VD, Glahn DC, Shen L, Risacher SL, Saykin AJ, Pearlson GD. A large scale multivariate parallel ICA method reveals novel imaging-genetic relationships for Alzheimer’s disease in the ADNI cohort. NeuroImage. 2012;60(3):1608–21. doi: 10.1016/j.neuroimage.2011.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville SA, Buros J, Parrado AR, Vardarajan B, Logue MW, Shen L, Risacher SL, Kim S, Jun G, DeCarli C, Lunetta KL, Baldwin CT, Saykin AJ, Farrer LA Alzheimer’s Disease Neuroimaging I. Multiple loci influencing hippocampal degeneration identified by genome scan. Ann Neurol. 2012;72(1):65–75. doi: 10.1002/ana.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita A, Koike A, Jun G, Wang LS, Takahashi S, Matsubara E, Kawarabayashi T, Shoji M, Tomita N, Arai H, Asada T, Harigaya Y, Ikeda M, Amari M, Hanyu H, Higuchi S, Ikeuchi T, Nishizawa M, Suga M, Kawase Y, Akatsu H, Kosaka K, Yamamoto T, Imagawa M, Hamaguchi T, Yamada M, Morihara T, Takeda M, Takao T, Nakata K, Fujisawa Y, Sasaki K, Watanabe K, Nakashima K, Urakami K, Ooya T, Takahashi M, Yuzuriha T, Serikawa K, Yoshimoto S, Nakagawa R, Kim JW, Ki CS, Won HH, Na DL, Seo SW, Mook-Jung I, St George-Hyslop P, Mayeux R, Haines JL, Pericak-Vance MA, Yoshida M, Nishida N, Tokunaga K, Yamamoto K, Tsuji S, Kanazawa I, Ihara Y, Schellenberg GD, Farrer LA, Kuwano R Alzheimer Disease Genetics C. SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese, Koreans and Caucasians. PLoS One. 2013;8(4):e58618. doi: 10.1371/journal.pone.0058618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435(7044):903–10. doi: 10.1038/nature03663. [DOI] [PubMed] [Google Scholar]
- Naj AC, Beecham GW, Martin ER, Gallins PJ, Powell EH, Konidari I, Whitehead PL, Cai G, Haroutunian V, Scott WK, Vance JM, Slifer MA, Gwirtsman HE, Gilbert JR, Haines JL, Buxbaum JD, Pericak-Vance MA. Dementia revealed: novel chromosome 6 locus for late-onset Alzheimer disease provides genetic evidence for folate-pathway abnormalities. PLoS Genet. 2010;6(9):e1001130. doi: 10.1371/journal.pgen.1001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JS, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, St George-Hyslop P, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, DeCarli C, DeKosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nature genetics. 2011;43(5):436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Estus S, Abner EL, Parikh I, Malik M, Neltner JH, Ighodaro E, Wang WX, Wilfred BR, Wang LS, Kukull WA, Nandakumar K, Farman ML, Poon WW, Corrada MM, Kawas CH, Cribbs DH, Bennett DA, Schneider JA, Larson EB, Crane PK, Valladares O, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Scheff SW, Sonnen JA, Haines JL, Pericak-Vance MA, Mayeux R, Farrer LA, Van Eldik LJ, Horbinski C, Green RC, Gearing M, Poon LW, Kramer PL, Woltjer RL, Montine TJ, Partch AB, Rajic AJ, Richmire K, Monsell SE, Schellenberg GD, Fardo DW Alzheimer’ Disease Genetic C. ABCC9 gene polymorphism is associated with hippocampal sclerosis of aging pathology. Acta Neuropathol. 2014;127(6):825–43. doi: 10.1007/s00401-014-1282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JC, Lin H. Beyond transposons: the epigenetic and somatic functions of the Piwi-piRNA mechanism. Curr Opin Cell Biol. 2013;25(2):190–4. doi: 10.1016/j.ceb.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Palma E, Bustos BI, Villaman CF, Alarcon MA, Avila ME, Ugarte GD, Reyes AE, Opazo C, De Ferrari GV Alzheimer’s Disease Neuroimaging I, Group NLNFS. Overrepresentation of glutamate signaling in Alzheimer’s disease: network-based pathway enrichment using meta-analysis of genome-wide association studies. PLoS One. 2014;9(4):e95413. doi: 10.1371/journal.pone.0095413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrat PN, DasGupta S, Wang J, Theurkauf W, Weng Z, Rosbash M, Waddell S. Transposition-driven genomic heterogeneity in the Drosophila brain. Science. 2013;340(6128):91–5. doi: 10.1126/science.1231965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Antonov I, Sheridan R, Frey S, Sander C, Tuschl T, Kandel ER. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell. 2012;149(3):693–707. doi: 10.1016/j.cell.2012.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Risacher SL, Nho K, Kim S, Swaminathan S, Shen L, Foroud TM, Hakonarson H, Huentelman MJ, Aisen PS, Petersen RC, Green RC, Jack CR, Koeppe RA, Jagust WJ, Weiner MW, Saykin AJ Alzheimer’s Disease Neuroimaging I. APOE and BCHE as modulators of cerebral amyloid deposition: a florbetapir PET genome-wide association study. Mol Psychiatry. 2014;19(3):351–7. doi: 10.1038/mp.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A, van der Flier WM, Herold C, Ramonet D, Heilmann S, Lewczuk P, Popp J, Lacour A, Drichel D, Louwersheimer E, Kummer MP, Cruchaga C, Hoffmann P, Teunissen C, Holstege H, Kornhuber J, Peters O, Naj AC, Chouraki V, Bellenguez C, Gerrish A, Heun R, Frolich L, Hull M, Buscemi L, Herms S, Kolsch H, Scheltens P, Breteler MM, Ruther E, Wiltfang J, Goate A, Jessen F, Maier W, Heneka MT, Becker T, Nothen MM International Genomics of Alzheimer’s P, Alzheimer’s Disease Neuroimaging I. SUCLG2 identified as both a determinator of CSF Abeta1–42 levels and an attenuator of cognitive decline in Alzheimer’s disease. Hum Mol Genet. 2014;23(24):6644–58. doi: 10.1093/hmg/ddu372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RJ, Weiner MM, Lin H. PIWI proteins and PIWI-interacting RNAs in the soma. Nature. 2014;505(7483):353–9. doi: 10.1038/nature12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J, Sarkar A, Parida S, Ghosh Z, Mallick B. Small RNA sequencing revealed dysregulated piRNAs in Alzheimer’s disease and their probable role in pathogenesis. Molecular bioSystems. 2017;13(3):565–76. doi: 10.1039/c6mb00699j. [DOI] [PubMed] [Google Scholar]
- Sai Lakshmi S, Agrawal S. piRNABank: a web resource on classified and clustered Piwi-interacting RNAs. Nucleic acids research. 2008;36(Database issue):D173–7. doi: 10.1093/nar/gkm696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, Harold D, Schrijvers EM, Ramirez-Lorca R, Debette S, Longstreth WT, Jr, Janssens AC, Pankratz VS, Dartigues JF, Hollingworth P, Aspelund T, Hernandez I, Beiser A, Kuller LH, Koudstaal PJ, Dickson DW, Tzourio C, Abraham R, Antunez C, Du Y, Rotter JI, Aulchenko YS, Harris TB, Petersen RC, Berr C, Owen MJ, Lopez-Arrieta J, Varadarajan BN, Becker JT, Rivadeneira F, Nalls MA, Graff-Radford NR, Campion D, Auerbach S, Rice K, Hofman A, Jonsson PV, Schmidt H, Lathrop M, Mosley TH, Au R, Psaty BM, Uitterlinden AG, Farrer LA, Lumley T, Ruiz A, Williams J, Amouyel P, Younkin SG, Wolf PA, Launer LJ, Lopez OL, van Duijn CM, Breteler MM Consortium C, Consortium G, Consortium E. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–40. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Kim S, Risacher SL, Nho K, Swaminathan S, West JD, Foroud T, Pankratz N, Moore JH, Sloan CD, Huentelman MJ, Craig DW, Dechairo BM, Potkin SG, Jack CR, Jr, Weiner MW, Saykin AJ Alzheimer’s Disease Neuroimaging I. Whole genome association study of brain-wide imaging phenotypes for identifying quantitative trait loci in MCI and AD: A study of the ADNI cohort. NeuroImage. 2010;53(3):1051–63. doi: 10.1016/j.neuroimage.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nature reviews Molecular cell biology. 2008;9(3):219–30. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- Webster JA, Myers AJ, Pearson JV, Craig DW, Hu-Lince D, Coon KD, Zismann VL, Beach T, Leung D, Bryden L, Halperin RF, Marlowe L, Kaleem M, Huentelman MJ, Joshipura K, Walker D, Heward CB, Ravid R, Rogers J, Papassotiropoulos A, Hardy J, Reiman EM, Stephan DA. Sorl1 as an Alzheimer’s disease predisposition gene? Neurodegener Dis. 2008;5(2):60–4. doi: 10.1159/000110789. [DOI] [PubMed] [Google Scholar]
- Weick EM, Miska EA. piRNAs: from biogenesis to function. Development. 2014;141(18):3458–71. doi: 10.1242/dev.094037. [DOI] [PubMed] [Google Scholar]
- Yan Z, Hu HY, Jiang X, Maierhofer V, Neb E, He L, Hu Y, Hu H, Li N, Chen W, Khaitovich P. Widespread expression of piRNA-like molecules in somatic tissues. Nucleic acids research. 2011;39(15):6596–607. doi: 10.1093/nar/gkr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Tan Y, Wang Z, Wang K, Zhang X, Chen X, Li C, Wang T, Luo X. Long non-coding RNAs in psychiatric disorders. Psychiatric Genet. 2016a;26(3):109–16. doi: 10.1097/YPG.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, van Dyck CH, Luo X, Kranzler HR, Yang BZ, Gelernter J. Variation at APOE and STH loci and Alzheimer’s disease. Behavioral and brain functions : BBF. 2006;2:13. doi: 10.1186/1744-9081-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Wang Z, Tan Y, Chen X, Luo X. piRNAs and their functions in the brain. Int J Hum Genet. 2016b;16(1–2):53–60. doi: 10.1080/09723757.2016.11886278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.