Abstract
Mitochondrial dysfunction in obesity and diabetes can be caused by excessive production of free radicals, which can damage mitochondrial DNA. Because mitochondrial DNA plays a key role in the production of ATP necessary for cardiac work, we hypothesized that mitochondrial dysfunction, induced by mitochondrial DNA damage, uncouples coronary blood flow from cardiac work. Myocardial blood flow (contrast echocardiography) was measured in Zucker lean (ZLN) and obese fatty (ZOF) rats during increased cardiac metabolism (product of heart rate and arterial pressure, i.v. norepinephrine). In ZLN increased metabolism augmented coronary blood flow, but in ZOF metabolic hyperemia was attenuated. Mitochondrial respiration was impaired and ROS production was greater in ZOF than ZLN. These were associated with mitochondrial DNA (mtDNA) damage in ZOF. To determine if coronary metabolic dilation, the hyperemic response induced by heightened cardiac metabolism, is linked to mitochondrial function we introduced recombinant proteins (intravenously or intraperitoneally) in ZLN and ZOF to fragment or repair mtDNA, respectively. Repair of mtDNA damage restored mitochondrial function and metabolic dilation, and reduced ROS production in ZOF; whereas induction of mtDNA damage in ZLN reduced mitochondrial function, increased ROS production, and attenuated metabolic dilation. Adequate metabolic dilation was also associated with the extracellular release of ADP, ATP, and H2O2 by cardiac myocytes; whereas myocytes from rats with impaired dilation released only H2O2. In conclusion, our results suggest that mitochondrial function plays a seminal role in connecting myocardial blood flow to metabolism, and integrity of mtDNA is central to this process.
Keywords: Coronary microcirculation, Obesity, Diabetes, Coronary circulation, Mitochondria
Introduction
Mitochondrial dysfunction and excessive generation of reactive oxygen species are common pathophysiological observations for several cardiovascular diseases [1, 12, 25, 26, 35, 36], but whether the dysfunction is a cause or effect of the pathology is not resolved. Zucker obese fatty (ZOF) rats, a model for the metabolic syndrome, demonstrate a phenotype of mitochondrial dysfunction, e.g., impaired oxygen consumption, deceased oxidative substrate utilization, increased generation of mitochondrial reactive oxygen species (ROS) [2, 4, 29, 38], which could also damage mitochondrial DNA (mtDNA) [7, 9]. If proper mitochondrial function is critical to metabolic dilation, the coupling between metabolism and blood flow, then it would follow that an animal model such as the ZOF could show an impairment in this fundamental mechanism of blood flow regulation. This situation could become a “vicious cycle” in that inadequate myocardial perfusion begets further ischemia and more mitochondrial damage, impairing flow regulation even more.
Accordingly, we hypothesized that coronary metabolic dilation is dependent on normal mitochondrial function. To test this hypothesis, we studied coronary metabolic dilation in Zucker Lean (ZLN) rats and ZOF rats. To demonstrate that the findings are “cause and effect” between mitochondrial function and coronary metabolic dilation, we manipulated mitochondrial function by either repairing fragmented mtDNA in ZOF rats or fragmenting mtDNA in ZLN rats, and then assessed the connection between flow and metabolism in the heart. We also evaluated the effects of these treatments on mitochondrial function and superoxide ( ) production. Our results support the conclusion, in which mitochondrial function is intimately connected to coronary metabolic dilation and that the integrity of mtDNA plays a key role in maintaining this connection through effects on mitochondrial function.
Methods
Animals and anesthesia
Male Zucker lean rats (ZLN, N = 32) and Zucker Obese Fatty (ZOF, N = 32) were purchased from Harlan Laboratories (USA). Rats were housed in the Comparative Medicine Facility at Northeast Ohio Medical University and maintained with a light–dark cycle of 12 h, with free access to water and food ad libitum. Rats were studied when they were 8–12 months in age. The experimental use and care of the animals was approved by the Northeast Ohio Medical University Institutional Animal Use and Care Committee, in accordance with the Guidelines for the care and the use of laboratory animals by the National Institutes of Health (NIH publication no. 85-23, revised 1996). During surgical and echocardiographic procedures, anesthesia was induced with isoflurane 4 %, and once anesthetized, the rats were maintained with 2–3 % isoflurane through a nose cone with supplemental O2 (0.6–0.8 L/min), via spontaneous breathing. Rats were divided into four groups: ZLN controls; ZOF controls; ZLN treated with a recombinant protein containing a mitochondrial localization sequence, the tat protein from HIV to enable cell penetration, and exonuclease III to fragment mtDNA (ZLN + mt-tat-ExoIII) [32]; and ZOF treated with a recombinant protein containing a mitochondrial localization sequence, the tat protein from HIV to enable cell penetration, and endonuclease III to facilitate the repair of fragmented mtDNA (ZOF + mt-tat-EndoIII) [32].
Blood glucose, triglycerides, and total cholesterol
Plasma levels of triglycerides, cholesterol, and glucose were measured in ZLN and ZOF rats before (control) and 3 days after mt-tat-ExoIII (ZLN) or mt-tat-EndoIII treatment (ZOF). Triglyceride Liquid Stable Reagent, Cholesterol Liquid Stable Reagent and Glucose Oxidase Liquid Stable Reagent from Infinity (Thermo Fisher Scientific, Waltham, MA, USA) were used according to the manufacturer’s instructions.
Induction and repair of mtDNA fragmentation
Fragmentation of mtDNA was induced in lean rats (ZLN, N = 16), by intravenous (i.v.) or intraperitoneal (i.p.) administration of mt-tat-ExoIII. In preliminary studies, we found that either route of delivery produced an equivalent effect. For i.v. infusion, rats were anesthetized and a catheter was placed in the right jugular vein and exteriorized through the nape of the neck. Rats were allowed to recover (3 days) and then were treated for 3 days with either 80 µg/day of mt-tat-ExoIII or heparinized saline in the control group (ZLN, N = 16). The same dose was given as an i.p injection, also for 3 days. Repair of fragmented mtDNA was achieved by i.v. or i.p. administration (as described above) of mt-tat-EndoIII in ZOF (N = 16). Animals were treated either 1 µg/g of body weight per day (i.v. or i.p) of mt-tat-EndoIII (ZOF + EndoIII) or heparinized saline in the sham group (ZOF, N = 16).
Evaluation of cardiac function
All animals in the four study groups (ZLN, ZLN + mt-tat-ExoIII, ZOF, ZOF + mt-tat-EndoIII) underwent echocardiographic assessment of systolic and diastolic left ventricular function after 3-day treatment with vehicle or recombinant protein. The rats were anesthetized with isoflurane as described above and cardiac function was measured using a Vevo 770 (VisualSonics, Canada) using a 12–38 MHz linear transducer (RMV716). Systolic and diastolic function of left ventricle was assessed according to the guidelines of the American Society of Echocardiography [17].
Systolic and diastolic thickness of the anterior and the posterior walls of the left ventricle was recorded by M-mode images, using the average of 5–10 consecutive heart cycles measurements, obtained in parasternal short axis view at the level of papillary muscles. For the evaluation of left ventricular systolic function, the following parameters were calculated: ejection fraction (EF %), fractional shortening (FS %), indexed cardiac output (CI, ml/min/g). The left ventricular diastolic function was assessed by pulsed Doppler analysis. Early (E wave) and late (A wave) peak velocities, E wave deceleration time (ED) and E/A ratio were obtained from apical four chamber view. In addition to these parameters, left ventricular mass was calculated using the Troy formula [34].
Measurement of myocardial blood flow (MBF)
Myocardial contrast echocardiography (MCE) was performed with a linear transducer probe (7 MHz; Acuson Sequoia C512 system; Siemens, Mountain View, CA, USA) at a low mechanical index (MI <0.2) imaging, using contrast pulse sequencing [19–21] and as we recently described for measurements of myocardial perfusion in mice [27]. MBF and systemic hemodynamics (arterial pressures, heart rate) were measured in the under basal conditions (isoflurane anesthesia), after receiving hexamethonium (5 µg/kg body weight) to prevent cardiovascular reflexes during norepinephrine administration and during different i.v. doses of norepinephrine to increase cardiac work. Mean arterial pressure (MAP) was calculated as diastolic pressure + 1/3 pulse pressure. The double product of MAP and HR was used to estimate the cardiac work (CW) as surrogate for myocardial oxygen consumption during contrast stress echocardiography. All measured variables were continuously recorded, stored and analyzed off-line using the PowerLab system (ADInstruments).
Mitochondrial DNA fragmentation, mitochondrial function and mitochondrial ROS production
Mitochondrial DNA fragmentation was measured using a Southern analysis, in which fragmentation was analyzed by the reduction in size of a BamHI digest of mtDNA [8].
For functional measurements, mitochondria (containing a mixture of subsarcolemmal and interfibrillar mitochondria) were prepared from rat hearts of ZLN or ZOF by differential centrifugation [18]. Mitochondria were precipitated by centrifugation at 20,000×g for 10 min in the final step and re-suspended in media (M-buffer) containing the following agents (in mM): mannitol (230), sucrose (70), EDTA (1), Trizma (1), pH 7.4. Mitochondrial respiration was measured by the polarographic method using a Clark-type oxygen electrode (Oxytherm, Hansatech Instruments, Norfolk, England) at 30 °C and reported in nmol/min. The NADH-linked respiration buffer containing malate plus glutamate included (in mM): potassium glutamate (140), NaCl (10), MgCl2 (1), EGTA (1), malate (5), Trizma (1), phosphate (2.5), and cytochrome c (0.01), and was adjusted to pH 7.4. Mitochondrial preparations were added to the respiration buffer for a final concentration of 0.5 mg of total protein/ml. OCRs were measured as follows: state 2, OCR of mitochondrial preparations with malate/glutamate; state 3, OCR stimulated by ADP (0.2 mM); state 4, OCR after the addition of oligomycin (2 µg/ml) following ADP addition; uncoupling respiration, OCR after the addition of FCCP (2.5 µM). The oxygen electrode was calibrated at 1 atm by assuming the concentration of O2 in the respiration buffer at 30 °C to be 250 µM.
Electron paramagnetic resonance (EPR) measurements of generation by isolated mitochondria were carried out on a Bruker EMX Micro spectrometer operating at 9.43 GHz with 100 kHz modulation frequency at room temperature [14,15].
Tissue hypoxia
To assess the presence of myocardial ischemia in the four treatment groups, some rats were administered intravenously pimonidazole hydrochloride, a substance known commercially as Hypoxyprobe (HPI Inc, Burlington, MA, USA). Hypoxyprobe binds to proteins that have been modified when oxygen tension is less than 10 mmHg (PO2 normal values in non-ischemic myocardial tissue, greater than 15 mmHg). Hypoxyprobe (60 mg/kg of body weight) was administered intravenously during high-dose NE infusion (10 µg/kg per min) for 20 min, after which the rats were killed.
Tissue sections of the left ventricle from the peri-papillary portions were sectioned with a microtome and immediately fixed and mounted. At least 30 sections of about 5 µm were obtained for each animal and 10 sections per animal were randomly selected for immunostaining. Each section was blocked with serum, followed by treatment with anti-pimonidazole antibody (primary) and then stained with secondary antibodies (donkey anti-mouse direct 595 nm). After binding, the sections were washed and fluorescence images were acquired using an epifluorescence microscope. At least ten fields, randomly chosen were acquired, analyzed and compared.
Isolation of cardiac myocytes
Cardiac myocytes were enzymatically isolated from ZLN and ZOF rat hearts (N = 4) as described previously [24, 33]. Isolated cardiomyocytes were electrically stimulated at 200 and 400 bpm, and buffer was collected after 20 min of stimulation or quiescence (no stimulation) to evaluate the production of specific vasoactive substances released by the cardiomyocytes (H2O2 and purines) and the vasoactive effects in coronary arterioles isolated from both ZL and ZOF rats.
Assessment of vasodilation in isolated arterioles
Each rat was used for studies of isolated coronary arterioles and isolated cardiac myocytes as described previously using arterioles studied under isobaric conditions (60 cmH2O) [24, 33]. Aliquots of conditioned buffer were removed from the suspension of myocytes and administered to isolated coronary arterioles. The ZLN and ZOF conditioned buffer (ZLN-CB and ZOF-CB, respectively) were added to the bath of isolated coronary arterioles from both strains (ZOF and ZLN), to test the vasodilator response (increase in diameter) and to clarify whether a different response was possibly due to the production of different vasoactive compounds from ZLN-CB or ZOF-CB or to impaired arteriolar response (ZOF vs. ZLN). To evaluate the effects of treatment of ZLN with mt-tat-ExoIII and ZOF with mt-tat-EndoIII, we assessed dilation of CB from these myocytes when administered to arterioles from the ZLN. When applied to the isolated arterioles the conditioned buffer was incubated with catalase (500–1000 U/ml) and the combination of catalase + 8-pSPT ([8-(p-sulfophenyl) theophylline] to determine the roles of H2O2 and purines, respectively.
Measurement of vasoactive metabolites and purines
Concentrations of H2O2 (ZLN and ZOF), and purines (from the four groups) were measured in conditioned buffer. Samples of conditioned buffer were obtained from non-stimulated myocytes and after 20 min of stimulation at 200 or 400/min. Hydrogen peroxide (H2O2) production was measured electrochemically using the APOLLO 4000 system (World Precision Instruments, Sarasota, FL, USA). The concentration of purines (AMP, ADP, ATP and adenosine) in the conditioned buffer was measured using high pressure liquid chromatography with UV spectrophotometric detector at 254 nm [22]. ATP levels were also measured in myocardial tissue [22].
Data analysis
Results are expressed as mean ± SEM, unless otherwise stated. A one-way ANOVA followed by the Bonferroni post hoc test were used for multiple comparisons where appropriate. For analysis of the mitochondrial function data, analyses and samples were analyzed in pairs, e.g., ZOF and ZOF + mt-tat-EndoIII so that t tests were used to measure differences in function due to the intervention. A probability value of P <0.05 was used to establish statistical significance.
Results
Blood chemistry, hemodynamics and cardiac function
ZOF had higher systolic pressures than ZLN, and treatment with the recombinant proteins had small effects on pressures. Heart rate (HR) was comparable among groups. In the obese or lean groups, body weights were not changed by the treatments (Table 1, Supplement). Treatment of ZOF with mt-tat-EndoIII significantly reduced triglycerides (P < 0.05), but levels glucose, triglycerides or cholesterol were not affected by the treatment in the other groups (Table 1). Blood glucose, total cholesterol and triglycerides were significantly higher in the ZOF compared to ZLN groups (P <0.05).
Table 1.
Hemodynamics, blood chemistries, and body weight
| ZLN | ZLN + mt-tat-ExoIII | ZOF | ZOF + mt-tat-EndoIII | P <0.05 | |
|---|---|---|---|---|---|
| Systolic pressure (mmHg) | 121 ± 3 | 125 ± 2 | 154 ± 1 | 135 ± 1 | *,╪,¤ |
| Diastolic pressure (mmHg) | 80 ± 2 | 86 ± 1 | 79 ± 0 | 75 ± 1 | ¥,╪ |
| MAP (mmHg) | 93 ± 2 | 99 ± 1 | 104 ± 1 | 95 ± 2 | *,¤ |
| HR (min−1) | 359 ± 5 | 350 ± 4 | 350 ± 4 | 362 ± 2 | NS |
| Glucose (mg/dl) | 160 ± 18 | 167 ± 30 | 268 ± 35 | 242 ± 26 | *,╪ |
| Cholesterol (mg/dl) | 61 ± 10 | 69 ± 3 | 231 ± 59 | 237 ± 34 | *,╪ |
| Triglycerides (mg/dl) | 300 ± 84 | 244 ± 5 | 936 ± 203 | 410 ± 31 | *,¤ |
| Body weight (g) | 425 ± 21 | 376 ± 11 | 875 ± 46 | 863 ± 31 | *,╪ |
P <0.05 ZLN vs. ZOF;
P <0.05 ZLN vs. mt-tat-EndoIII;
P <0.05 ZLN vs. ZLN + mt-tat-ExoIII;
P <0.05 ZOF vs. ZOF + mt-tat-EndoIII
Although baseline measurements of EF, FS and CI were not different between ZOF and ZLN, CI during stress with NE at 10 µg/kg/min was significantly lower in ZOF compared to ZLN (P <0.05, Table 1). Treatment of ZOF with mt-tat-EndoIII improved stress CI and EF compared to ZOF vehicle controls (P <0.05, Table 1). Treatment of ZLN with mt-tat-ExoIII decreased the E/A ratio, but did not alter basal parameters of function compared to the ZLN (Table 1). The E/A ratio was significantly less in ZOF compared to ZLN suggesting diastolic dysfunction in the obese rats (Table 1). Three days of mt-tat-EndoIII treatment in ZOF increased E/A ratio compared to ZOF vehicle controls (P <0.05). Cardiac work, measured as MAP × HR, was comparable in the four groups in resting conditions (after hexamethonium injection) and during stress with norepinephrine. Although the triple product may provide a more precise estimate of cardiac work than the double product, we recently reported an excellent correlation between these two variables in mice [27], suggesting that the double product provides a reasonable estimate of cardiac work.
Mitochondrial function and mtDNA lesions
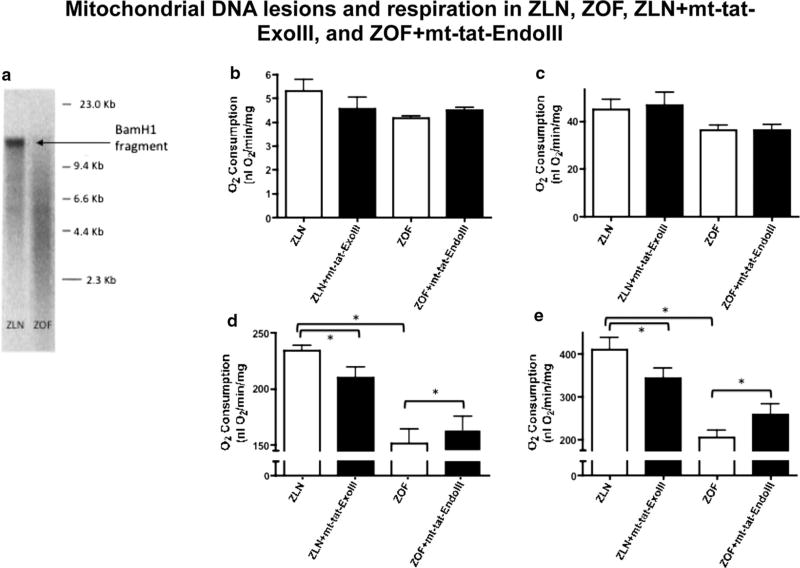
Mitochondrial DNA isolated from cardiac tissue revealed an increased number of base lesions in ZOF compared to ZLN (Fig. 1a). Treatment of ZLN with mt-tat-ExoIII increased the number of base lesions; whereas treatment of ZOF with mt-tat-EndoIII decreased the number of lesions comparable to control. The respiratory control ratio (RCR, Fig. 1b) and state 4 respiration (Fig. 1c) were not different among the four groups. State 3 (ADP-dependent) respiration (Fig. 1d) and maximal O2 consumption (uncoupled oxygen consumption, Fig. 1e) were lower in ZOF than in ZLN (P <0.05). Repair of mtDNA damage in ZOF + mt-tat-EndoIII improved these attributes of mitochondrial function compared to ZOF (an 8 % increase in state 3 respiration and a 26 % increase in uncoupled respiration, both P < 0.05). Treatment with mt-tat-ExoIII reduced the both state 3 and uncoupled mitochondrial respiration in ZLN rats by 11 and 16 %, respectively (ZLN vs. ZLN + mt-tat-ExoIII, both P < 0.05). Although the magnitude of the changes induced by the recombinant proteins appears small, in every animal treatment with mt-tat-En-doIII improved the attributes of respiration in ZOF, whereas treatment with mt-tat-ExoIII decreased respiration in every ZLN rat.
Fig. 1.
Evaluation of mitochondrial DNA damage (a) and respiration (NADH dependent) in isolated cardiac mitochondria (b–e). In ZOF, the size of the BamHI digest band was greatly reduced compared to ZLN (a), indicating mtDNA damage, and about a fourfold higher lesion frequency. The respiratory control ratio (RCR) (b) and state 4 respiration (c) were not different among the four groups. Decreased state 3 respiration (d) was shown in ZOF and ZOF + mt-tat-EndoIII compared to ZLN. (e) Maximal respiration during uncoupling with FCCP revealed decreased respiration in ZLN treated with mt-tat-ExoIII (vs. ZLN). Also, treatment of ZOF with mt-tat-EndoIII improved respiration compared to ZOF (P < 0.05). N = 4–5 for all measurements
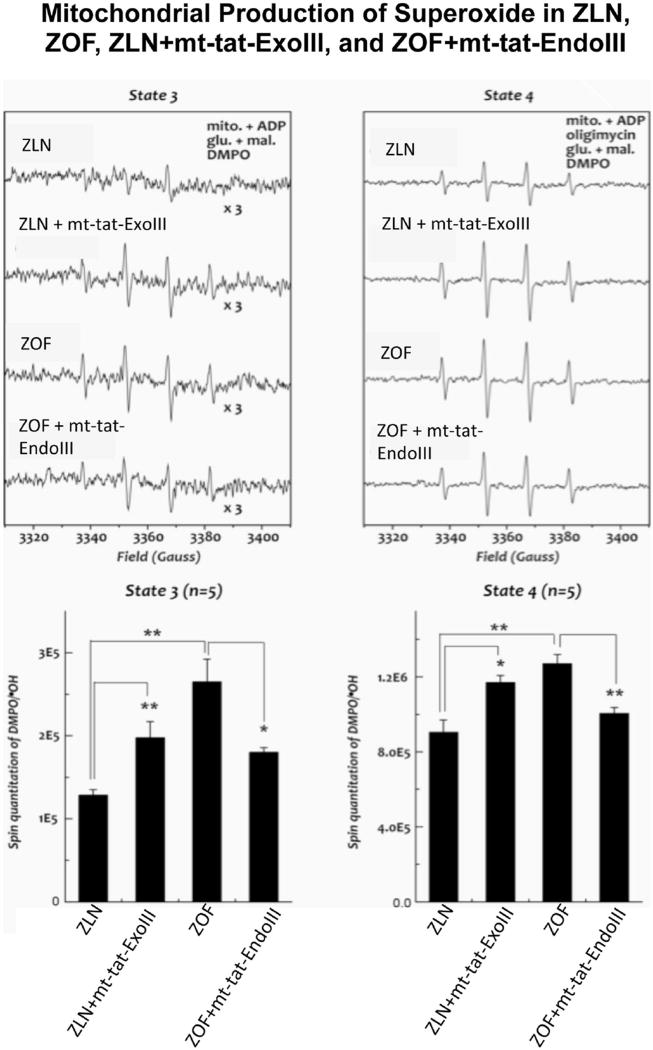
In ZLN rats ROS production was lower than ZOF (P < 0.05, Fig. 2). Treatment with mt-tat-ExoIII increased ROS production in ZLN compared to untreated ZLN (P < 0.05); whereas treatment of ZOF with mt-tat-EndoIII decreased ROS production compared to ZOF (P <0.05).
Fig. 2.
Mitochondrial production of reactive oxygen species (ROS) in Zucker lean (ZLN), Zucker obese fatty (ZOF), ZLN + mt-tat-ExoIII, and ZOF + mt-tat-EndoIII during state 3 and state 4 respiration. Top representative EPR signals from the four groups. Bottom quantified EPR signals in the four groups. ZOF produced greater amount of ROS than ZLN (P <0.05). Treatment of mt-tat-ExoIII in ZLN increased ROS production compared to ZLN; whereas treatment of mt-tat-EndoIII in ZOF decreased ROS production compared to ZOF. Overall ROS production was higher during state 4 vs. state 3. N = 5 for all measurements
In vivo coronary metabolic vasodilation
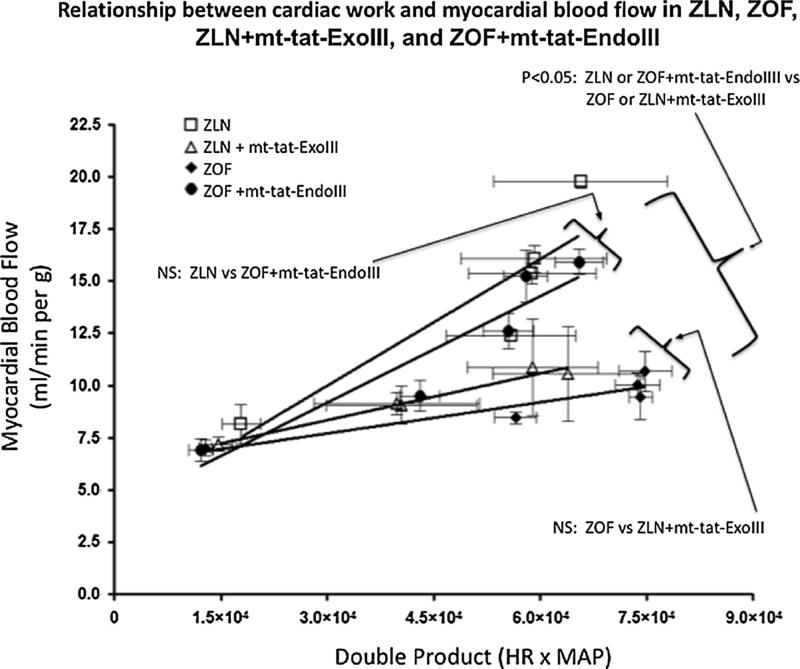
Myocardial blood flow values (MBF) at baseline, after administration of the ganglionic blocker hexamethonium, were comparable among the four groups. During increased metabolic demand by i.v. NE, the increase in blood flow was less in ZOF than in ZLN (Fig. 3). Restoration of mitochondrial function via repair of mtDNA damage in ZOF (ZOF + mt-tat-EndoIII) restored the coupling of myocardial blood flow to metabolism (not different than ZLN group). Conversely, impairing mitochondrial function in ZLN by fragmenting mtDNA (ZLN + mt-tat-ExoIII) impaired the coupling of flow to metabolism to a level comparable to that observed in ZOF. These data (Fig. 3) show that the metabolic regulation of myocardial blood flow is not only predicated on proper mitochondrial function (ZLN and ZOF + mt-tat-EndoIII), but also attenuated during mitochondrial dysfunction (ZOF and ZLN + mt-tat-ExoIII).
Fig. 3.
Relationship between cardiac work and myocardial blood flow during stress with NE (NE 2.5–5.0–7.5–10 µg/kg/min). MBF values at baseline are comparable in the four groups (N = 10 per group). The relation between myocardial blood flow and the double product in ZLN was significantly greater than in the ZOF (P <0.05). The induction of mt-DNA damage in ZLN resulted in a significant impairment of the blood flow-double product relationship (ZLN vs. ZLN + mt-tat-ExoIII, P <0.05) Repair of mtDNA damage in ZOF (ZOF + mt-tat-EndoIII) significantly increased the flow-double product relationship compared to ZOF (P < 0.05) and rendered this relationship not different from untreated ZLN (ZLN + mt-tat-ExoIII vs. ZOF, P = NS). N = 7–10 per group
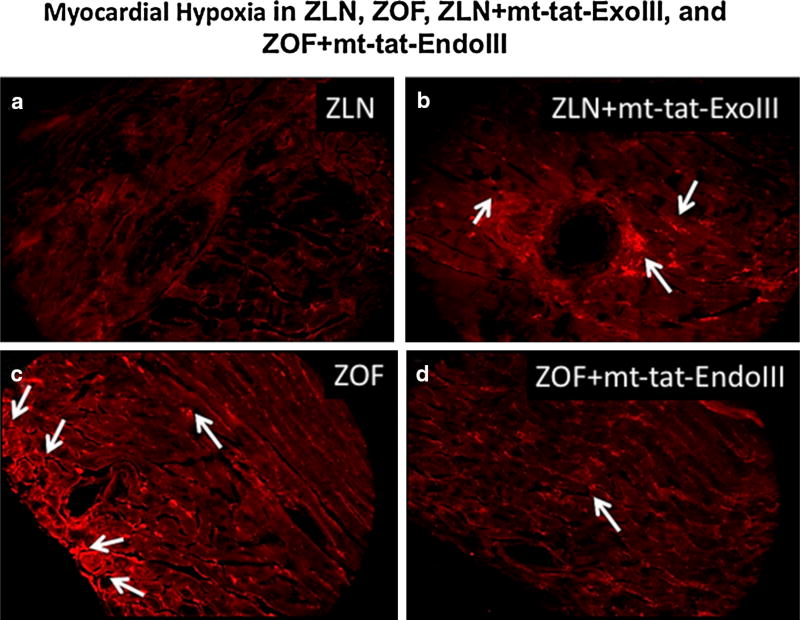
Regional tissue hypoxia
Figure 4 illustrates the consequences of impaired metabolic dilation in the four groups. In Fig. 4a, during increased metabolism via administration of NE (highest dose), the fluorescent signals indicating tissue hypoxia were barely above background indicating adequate dilation to maintain tissue oxygenation in ZLN. However, after mt-DNA damage in ZLN + mt-tat-ExoIII, the fluorescent signals were elevated in certain areas indicating hypoxia during the metabolic stress (Fig. 4b). During increased metabolic demands in ZOF (Fig. 4c) numerous hypoxic regions were evident. In contrast, repair of fragmented mtDNA in ZOF + mt-tat-EndoIII attenuated tissue hypoxia as shown by a decrease in the fluorescent signal (Fig. 4d).
Fig. 4.
Immunostaining of myocardial sections for Hypoxyprobe after cardiac stress with norepinephrine. Note the presence of areas of hypoxia (white arrows) in ZOF, ZLN + mt-tat-ExoIII groups (b, c). Repair of mitochondrial DNA damage attenuated the development of hypoxic regions in the group ZOF + mt-tat-EndoIII (d). ZLN (a) did not exhibit a clear hypoxic signal, as evidenced by the absence of fluorescence. N = 1 per group
Arteriolar vasoactivity to metabolites
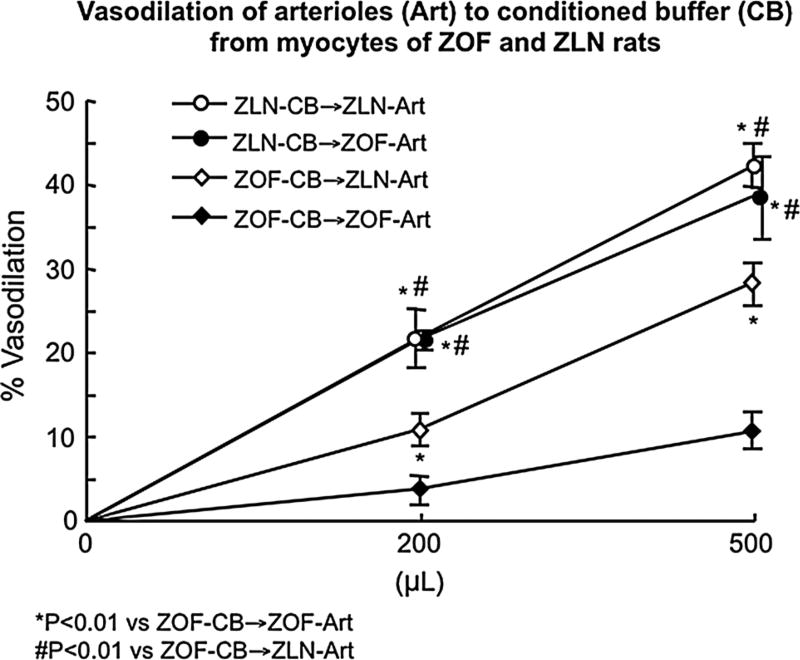
Figure 5 shows the vasodilatory responses to conditioned buffer from cardiac myocytes of the four groups stimulated at 400 bpm. Conditioned buffer (CB) from ZLN myocytes (ZLN/CB) produced comparable dilation of arterioles isolated from hearts of ZLN and ZOF. However, vasodilation was significantly less if ZOF/CB was added to arterioles from either ZLN or ZOF rats. Dilation to CB from ZLN cardiac myocytes was inhibited primarily by catalase and A1 receptor blockade blocked about 30 % of the total dilation (Fig. 6). In contrast, in CB from ZOF rats, catalase blocked the entire dilation and the A1 component was absent (Fig. 6). Conditioned buffer from myocytes of ZLN rats treated with mt-tat-ExoIII showed a response similar to the ZOF-CB, with vasodilation abrogated by catalase; whereas conditioned buffer from ZOF + mt-tat-EndoIII had responses similar to ZLN controls with components blocked by catalase and by A1 receptor antagonism (Fig. 5).
Fig. 5.
Vasodilation induced by conditioned buffer (CB) obtained from cardiomyocytes isolated from ZLN and ZOF (N = 8, each group). CB was obtained from myocytes stimulated at 400/min for 20 min, and was added to the bath of coronary arterioles (Art) from ZOF and ZLN (N = 8 each group). ZOF-CB was less effective in inducing vasodilation than ZLN-CB. ZOF-Art responded equivalently to ZLN-Art to CB isolated from ZLN. N = 6–9 per group/treatment
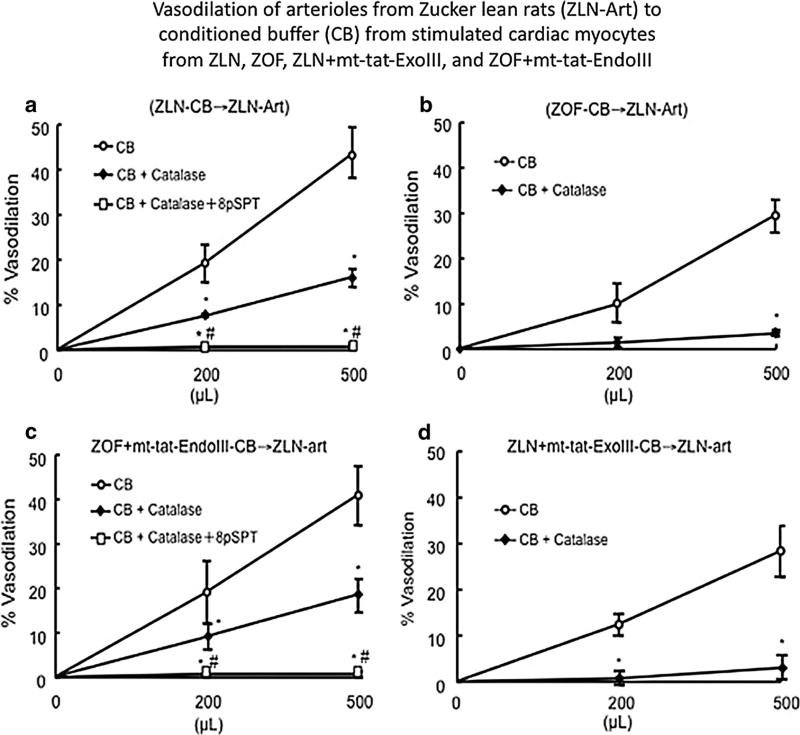
Fig. 6.
Assessment of the vasodilator response of arterioles isolated from Zucker lean rats (N = 8) (ZLN-Art) to conditioned buffer (CB) from the four groups: ZLN, ZOF, ZLN + mt-tat-ExoIII, and ZOF + mt-tat-EndoIII (N = 8, each group). Dilation to ZLN-CB revealed two components: catalase-inhibited and purinergic component blocked by 8-pSPT (a). Catalase inhibited the dilator response of 60–70 % and the use of the A1 purinergic receptor antagonist completely blocked the remaining response. The dilation to ZOF-CB was completely inhibited by catalase and the purinergic component was absent (b). In ZLN + mt-tat-ExoIII-CB, the response was inhibited by catalase (d); whereas in ZOF + mt-tat-EndoIII-CB the purinergic component re-emerged and the dilation was attributed to a catalase-inhibited component and a purinergic component (c). N = 7–9 per group/treatment
Production of vasoactive metabolites
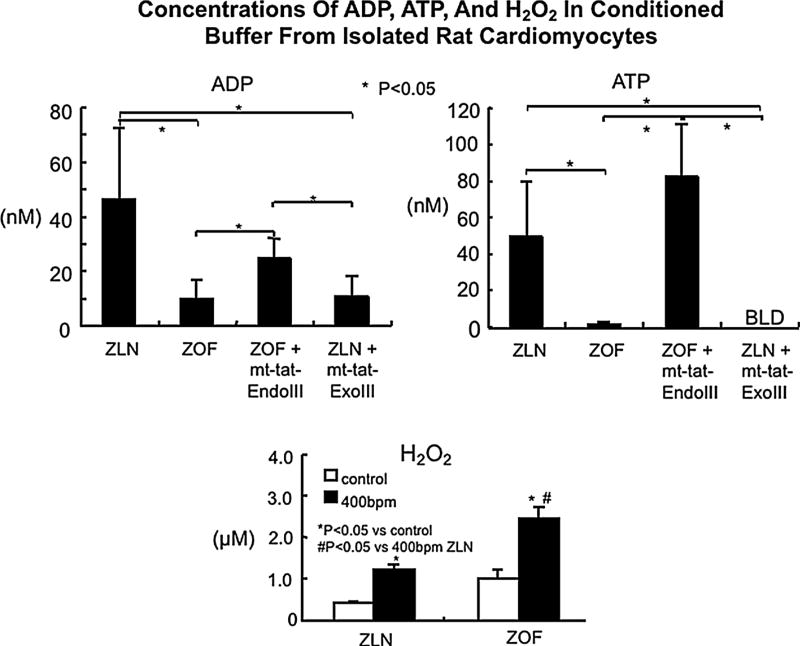
Isolated cardiomyocytes from ZLN and ZOF rats produced H2O2, which increased significantly during stimulation. Myocytes from ZOF produced greater amounts of H2O2 than those from ZLN (Fig. 7). The amount of ADP and ATP was less in conditioned buffer from ZOF and from ZLN + mt-tat-ExoIII vs. the other groups (Fig. 7). Conditioned buffer from cardiac myocytes of ZOF + mt-tat-EndoIII showed levels of ATP and ADP comparable to ZLN and significantly greater than the other two groups. Adenosine and AMP were not detectable in the ZOF and ZLN conditioned buffer.
Fig. 7.
Top panels concentrations of ADP and ATP in conditioned buffer from isolated rat cardiomyocytes. Myocytes were stimulated at 400/min and conditioned buffer (CB) was collected after 20 min of stimulation. Myocytes from ZLN release ADP and ATP into the buffer when stimulated, but the release of these purines was less in ZOF and in ZLN + mt-tat-ExoIII (BLD, below level of detection). Treatment of ZOF with mt-tat-EndoIII increased the release of ATP and ADP, approximating ZLN. AMP and adenosine were below the level of detection. Bottom panel H2O2 production by isolated cardiac myocytes from ZLN and ZOF rat during electrical stimulation at 400/min. The amount of H2O2 generated increased during electrical stimulation and was significantly greater in the ZOF group than ZLN. N = 4–5 for each group
Discussion
In our study, we have observed that the integrity of mitochondrial DNA is not only critically linked to mitochondrial function, and to the connection between myocardial blood flow and cardiac metabolic demands. Disruption of this connection stems from mitochondrial dysfunction and damage to mitochondrial DNA occurring in the metabolic syndrome or during damage to mtDNA with mt-tat-ExoIII. Repair of fragmented mitochondrial DNA in the metabolic syndrome restored the connection between cardiac work and myocardial blood flow. Another important observation was that mitochondrial function is critical for the production of key vasodilator metabolites. Deterioration in mitochondrial function results in a loss of key purine vasodilator metabolites. These observations and conclusions should be considered from the perspective of previous observations we have made as well as cogent observations in the literature.
In the literature, there are many disparate results regarding mitochondrial function in ZOF rats ranging from normal [3, 13] to dysfunction [28, 38]. These discrepant observations can be explained by the study of different ages of animals. Studies reporting normal mitochondrial function have used obese animals about 3 months of age; whereas in the present study the rats were between 8 and 12 months. We have observed that mitochondria from ZOF rats produce elevated amounts of reactive oxygen species [29, 30] and the present study we found evidence for mitochondrial dysfunction in ZOF (reduced state 3 respiration, reduced uncoupled respiration, increased generation). Restoration of mtDNA integrity by mt-tat-EndoIII in ZOF improved mitochondrial function compared to untreated ZOF rats. Also, damage of mtDNA in ZLN by mt-tat-ExoIII, reduced mitochondrial function compared to ZLN. Based on these data, we propose that restoration of mtDNA integrity may be a strategy to restore mitochondrial and vascular functions in the metabolic syndrome. Although we cannot unequivocally exclude an effect of the recombinant proteins on cells other than cardiac myocytes, results from the isolated cardiac myocytes would not be influenced by other cell types. This suggests that cardiac myocytes were the principal target in the responses we measured.
The literature is also mixed about cardiac function in ZOF rats [4, 23, 38]. Our results were somewhat muted in that parameters of systolic function were not reduced in ZOF compared to ZLN, but the measure of diastolic function, E/A ratio, was significantly lower in ZOF and in ZLN following mt-tat-ExoIII vs. ZLN (P <0.05). Although, resting levels of myocardial perfusion were equivalent in all groups of rats, when subjected to a metabolic challenge, hyperemia was impaired in ZOF and ZLN + mt-tat-ExoIII rats. Perhaps this insufficient flow would lead to diffuse fibrosis and diastolic dysfunction. Although, the E/A ratio offers a limited view of diastole, our major goal was not to grade diastolic function, but to assess if mitochondrial dysfunction would impair the relationship between MBF and CW.
Previously, we demonstrated that metabolic dilation in the heart is mediated in part by H2O2 [31]. The concept we proposed is that when the flux of electrons is increased during augmented work and oxygen demands, more would be generated, and thus, more H2O2 would be generated. Similar to the previous study, our present results also supported this concept as isolated cardiac myocytes produced greater amount of H2O2 when electrically stimulated to work. Importantly, the present results are more than confirmatory. Our previous study did not identify some of the other components of metabolic dilation, which appear to be ATP and ADP. Recently several reports have suggested that ATP is a coronary metabolic dilator, although the investigators have made an assumption that the ATP is released by hypoxic red blood cells [5, 6, 10, 11]. Our results would challenge the idea that ATP measured in the venous effluent is exclusively due to release from red blood cells, as we found that cardiac myocytes release ATP during metabolic stimulation.
An unexpected finding was the effects of mitochondrial function on the production of metabolic dilators in the heart; specifically, cardiac myocytes with evidence of mitochondrial dysfunction (ZOF, ZLN + mt-tat-ExoIII) produced only H2O2, but those with normal aspects of mitochondrial function (ZLN, ZOF + mt-tat-EndoIII) produced purines (ADP, ATP) in addition to H2O2. An enigmatic finding is that dilation was attenuated in response to conditioned buffer from ZOF rats (compared to ZLN), despite higher levels of H2O2. Although H2O2 is a critical vasodilator, it would appear that additional factors, e.g., ATP and ADP are needed to adequately couple myocardial blood flow to cardiac work. However, the question of why is the release of purines impaired in the ZOF rats or ZLN rats treated with mt-tat-EndoIII remains unanswered. We speculate that the impairment in mitochondrial function would result into less ATP being produced, and less being released for metabolic dilation. Clearly metabolic dilation and the production of vasodilatory metabolites is dependent on proper mitochondrial function and mtDNA integrity in the heart. We also emphasize that impaired dilation in the ZOF rats is not exclusively related to decreased production of the nucleotides, because responses of isolated arterioles from ZOF rats to metabolites produced from cardiac myocytes isolated from ZLN rats was also blunted. This suggests that the impaired dilation is the net result of impaired vascular responses and altered production of key metabolites. Another detail critical to our study design is that the influences of these metabolites on vasodilation may be a response specific to coronary resistance vessels, because vasoactive responses to many agonists appear to be influenced by the organ system from which they were isolated [16]; thus, it is critical to study these responses in arterioles isolated from the heart.
Another important aspect of our results is that proper mitochondrial function is requisite in maintaining the necessary vasodilation to match metabolic needs of the myocardium to prevent myocardial ischemia. Production of a metabolic stress by intravenous NE induced robust dilation in normal rats (ZLN) or obese rats in which mitochondrial function was restored via reparation of mtDNA (ZOF + mt-tat-EndoIII). Importantly, the tissue levels of hypoxia in these two groups during increased cardiac work were significantly less than in the groups with mitochondrial dysfunction (ZLN + mt-tat-ExoIII, ZOF). Figure 3 could be interpreted as cardiac efficiency is better in ZOF or ZLN + mt-tat-ExoIII than the other two groups, because it appears that for the same amount of work, flow is less. However, the period of time when the measurements of flow and the double product was brief (∼30 s) after which, the preparations decompensated. This suggests that level of flow did not sustain metabolic demands in ZOF or ZLN + mt-tat-ExoIII—a conclusion supported by the elevated level of tissue hypoxia. We also would like to mention that a long-term consequence of impaired metabolic dilation may be diastolic dysfunction. We also were struck by the results suggesting that within as little of 3 days of treatment, systolic and diastolic function was improved in the obese rats by mt-tat-EndoIII suggesting that impairments in mitochondrial function. Interestingly, acute administration of mt-tat-EndoIII was found to reduce infarct size in isolated rat hearts, suggesting that the maintenance of mitochondrial function may also reduce infarct size [37].
An aspect of these results pertains to the relationship between mitochondrial function, mtDNA fragmentation and blood flow in the context of what is causing the impaired metabolic dilation. The impaired metabolic dilation may be related to the mitochondrial dysfunction and also inflammatory reactions due to fragments of mtDNA. Although this last point is speculation, there is a precedent for mtDNA fragments evoking such a response [37]. As an example, we found that mt-tat-EndoIII treatment of ZOF rats restored metabolic dilation, mtDNA integrity, and cardiac function to levels comparable to ZLN rats; yet, mitochondrial function was still lower in the ZOF mt-tat-EndoIII rats compared to ZLN. A similar comment can be made about the treatment of ZLN with mt-tat-ExoIII. The mt-DNA fragmentation was as severe as that in ZOF, but attributes of mitochondrial function were not as depressed as those in ZOF rats. We speculate that part of these issues relate to the duration of the treatment (3 days) vs. the chronic condition of the rats in which the mitochondrial function is affected for months.
We also would like to make the point that the improvement in metabolic dilation did not seem related to an effect on diabetes as blood glucose in the obese rats was not affected by mt-tat-EndoIII. This treatment did reduce triglyceride levels, which we believe is related to the improvement in mitochondrial function, which would result in greater lipid catabolism and thus lower triglycerides. Induction of mtDNA damage and deterioration of mitochondrial function by mt-tat-ExoIII did not affect glucose, cholesterol or triglycerides in the lean animals. We will admit that this could be related to the relatively small change in mitochondrial function we observed following this treatment.
In conclusion, our results suggest in a compelling manner, that normal mitochondrial function is based on the integrity of mtDNA and is required for the proper coupling between myocardial perfusion and cardiac work, i.e., coronary metabolic dilation. Impairments in mitochondrial function, reflected by lesions and fragmentation in mtDNA, blunt the coupling between flow and metabolism. More studies are needed to further test the hypothesis of mitochondrial dysfunction involved in the development of myocardial ischemia and whether maintenance of mtDNA integrity is a viable therapy for cardiovascular pathologies caused by flow limitations in the heart.
Acknowledgments
The authors wish to acknowledge the following grant support: HL032788, HL083366, HL115114, Fibus Family Foundation (WMC); R15HL115540, HL103227, DK095895, AHA14BGIA18770028 (LY); AHA POST4360030 (VO); HL083237 (Y-RC); AHA POST2290021 (YFP) and ES03456 (GLW).
Footnotes
Compliance with ethical standards
Conflict of interest Dr. Wilson has an interest in the company Exscien, which is manufacturing and licensing the recombinant proteins. All other authors have nothing to disclose.
Ethical standards All animal studies were performed using protocols approved by the Northeast Ohio Medical University Institutional Animal Care and Use Committee and comply with the ethical standards laid down in the 1964 Declaration of Helsinki and all later amendments. This manuscript does not contain clinical studies or patient data.
References
- 1.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 3.Brady LJ, Hoppel CL. Hepatic mitochondrial function in lean and obese Zucker rats. Am J Physiol. 1983;245:E239–E245. doi: 10.1152/ajpendo.1983.245.3.E239. [DOI] [PubMed] [Google Scholar]
- 4.Burgmaier M, Sen S, Philip F, Wilson CR, Miller CC, 3rd, Young ME, Taegtmeyer H. Metabolic adaptation follows contractile dysfunction in the heart of obese Zucker rats fed a high-fat “Western” diet. Obesity (Silver Spring) 2010;18:1895–1901. doi: 10.1038/oby.2009.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 6.Dinenno FA, Kirby BS. The age-old tale of skeletal muscle vasodilation: new ideas regarding erythrocyte dysfunction and intravascular ATP in human physiology. Circ Res. 2012;111:e203–e204. doi: 10.1161/CIRCRESAHA.112.279356. [DOI] [PubMed] [Google Scholar]
- 7.Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL. Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J Biol Chem. 2000;275:37518–37523. doi: 10.1074/jbc.M000831200. [DOI] [PubMed] [Google Scholar]
- 8.Druzhyna NM, Musiyenko SI, Wilson GL, LeDoux SP. Cytokines induce nitric oxide-mediated mtDNA damage and apoptosis in oligodendrocytes. Protective role of targeting 8-oxoguanine glycosylase to mitochondria. J Biol Chem. 2005;280:21673–21679. doi: 10.1074/jbc.M411531200. [DOI] [PubMed] [Google Scholar]
- 9.Druzhyna NM, Wilson GL, LeDoux SP. Mitochondrial DNA repair in aging and disease. Mech Ageing Dev. 2008;129:383–390. doi: 10.1016/j.mad.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 11.Farias M, 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288:H1586–H1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- 12.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloway GP, Snook LA, Harris RJ, Glatz JF, Luiken JJ, Bonen A. In obese Zucker rats, lipids accumulate in the heart despite normal mitochondrial content, morphology and long-chain fatty acid oxidation. J Physiol. 2011;589:169–180. doi: 10.1113/jphysiol.2010.198663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang PT, Chen CL, Ren P, Guarini G, Chen YR. BCNU-induced gR2 DEFECT mediates S-glutathionylation of complex I and respiratory uncoupling in myocardium. Biochem Pharmacol. 2014;89:490–502. doi: 10.1016/j.bcp.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang PT, Zhang L, Chen CL, Chen J, Green KB, Chen YR. Protein thiyl radical mediates S-glutathionylation of complex I. Free Radic Biol Med. 2012;53:962–973. doi: 10.1016/j.freeradbiomed.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinbongard P, Schleiger A, Heusch G. Characterization of vasomotor responses in different vascular territories of C57BL/6J mice. Exp Biol Med (Maywood) 2013;238:1180–1191. doi: 10.1177/1535370213502621. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group, American Society of Echocardiography’s Guidelines, Standards Committee European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Lee HL, Chen CL, Yeh ST, Zweier JL, Chen YR. Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2012;302:H1410–H1422. doi: 10.1152/ajpheart.00731.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepper W, Belcik T, Wei K, Lindner JR, Sklenar J, Kaul S. Myocardial contrast echocardiography. Circulation. 2004;109:3132–3135. doi: 10.1161/01.CIR.0000132613.53542.E9. [DOI] [PubMed] [Google Scholar]
- 20.Lindner JR, Sklenar J. Placing faith in numbers: quantification of perfusion with myocardial contrast echocardiography. J Am Coll Cardiol. 2004;43:1814–1816. doi: 10.1016/j.jacc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Lindner JR, Villanueva FS, Dent JM, Wei K, Sklenar J, Kaul S. Assessment of resting perfusion with myocardial contrast echocardiography: theoretical and practical considerations. Am Heart J. 2000;139:231–240. [PubMed] [Google Scholar]
- 22.Lorbar M, Fenton RA, Dobson JG., Jr ATP as a source of interstitial adenosine in the rat heart. Can J Physiol Pharmacol. 1999;77:579–588. [PubMed] [Google Scholar]
- 23.Marsh SA, Powell PC, Agarwal A, Dell’Italia LJ, Chatham JC. Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: role of hydronephrosis. Am J Physiol Heart Circ Physiol. 2007;293:H292–H298. doi: 10.1152/ajpheart.01362.2006. [DOI] [PubMed] [Google Scholar]
- 24.Merkus D, Duncker DJ, Chilian WM. Metabolic regulation of coronary vascular tone: role of endothelin-1. Am J Physiol Heart Circ Physiol. 2002;283:H1915–H1921. doi: 10.1152/ajpheart.00223.2002. [DOI] [PubMed] [Google Scholar]
- 25.Mozaffari, Baban B, Liu JY, Abebe W, Sullivan JC, El-Marakby A. Mitochondrial complex I and NAD(P)H oxidase are major sources of exacerbated oxidative stress in pressure-overloaded ischemic-reperfused hearts. Basic Res Cardiol. 2011;106:287–297. doi: 10.1007/s00395-011-0150-7. [DOI] [PubMed] [Google Scholar]
- 26.Nickel A, Loffler J, Maack C. Myocardial energetics in heart failure. Basic Res Cardiol. 2013;108:358. doi: 10.1007/s00395-013-0358-9. [DOI] [PubMed] [Google Scholar]
- 27.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM. Requisite role of Kv1.5 channels in coronary metabolic dilation. Circ Res. 2015;117:612–621. doi: 10.1161/CIRCRESAHA.115.306642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pajuelo D, Fernandez-Iglesias A, Diaz S, Quesada H, Arola-Arnal A, Blade C, Salvado J, Arola L. Improvement of mitochondrial function in muscle of genetically obese rats after chronic supplementation with proanthocyanidins. J Agric Food Chem. 2011;59:8491–8498. doi: 10.1021/jf201775v. [DOI] [PubMed] [Google Scholar]
- 29.Pung YF, Rocic P, Murphy MP, Smith RA, Hafemeister J, Ohanyan V, Guarini G, Yin L, Chilian WM. Resolution of mitochondrial oxidative stress rescues coronary collateral growth in Zucker obese fatty rats. Arterioscler Thromb Vasc Biol. 2012;32:325–334. doi: 10.1161/ATVBAHA.111.241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pung YF, Sam WJ, Stevanov K, Enrick M, Chen CL, Kolz C, Thakker P, Hardwick JP, Chen YR, Dyck JR, Yin L, Chilian WM. Mitochondrial oxidative stress corrupts coronary collateral growth by activating adenosine monophosphate activated kinase-alpha signaling. Arterioscler Thromb Vasc Biol. 2013;33:1911–1919. doi: 10.1161/ATVBAHA.113.301591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitoh S-I, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick G, Swafford A, Chilian WM. Hydrogen peroxide A feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006 doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 32.Shokolenko IN, Alexeyev MF, LeDoux SP, Wilson GL. TAT-mediated protein transduction and targeted delivery of fusion proteins into mitochondria of breast cancer cells. DNA Repair (Amst) 2005;4:511–518. doi: 10.1016/j.dnarep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Tiefenbacher CP, DeFily DV, Chilian WM. Requisite role of cardiac myocytes in coronary alpha1-adrenergic constriction. Circulation. 1998;98:9–12. doi: 10.1161/01.cir.98.1.9. [DOI] [PubMed] [Google Scholar]
- 34.Troy BL, Pombo J, Rackley CE. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation. 1972;45:602–611. doi: 10.1161/01.cir.45.3.602. [DOI] [PubMed] [Google Scholar]
- 35.Tullio F, Angotti C, Perrelli MG, Penna C, Pagliaro P. Redox balance and cardioprotection. Basic Res Cardiol. 2013;108:392. doi: 10.1007/s00395-013-0392-7. [DOI] [PubMed] [Google Scholar]
- 36.Vogt AM, Kubler W. Heart failure: is there an energy deficit contributing to contractile dysfunction? Basic Res Cardiol. 1998;93:1–10. doi: 10.1007/s003950050055. [DOI] [PubMed] [Google Scholar]
- 37.Yang XM, Cui L, White J, Kuck J, Ruchko MV, Wilson GL, Alexeyev M, Gillespie MN, Downey JM, Cohen MV. Mitochondrially targeted Endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2015;110:3. doi: 10.1007/s00395-014-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–2595. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]