Abstract
Objectives:
Lemierre’s syndrome cause by methicillin-sensitive Staphylococcus aureus is rare, but can lead to necrotizing pneumonia and septicaemia. When treating such patient with extracorporeal life support source control can be both challenging and controversial.
Methods:
In this report we present a 12 year old male who presented with Lemierre’s syndrome from which he developed septic shock and severe necrotizing pneumonia. He also showed multiple pulmonary embolisms from the internal jugular vein thrombi, resulting in acute respiratory distress syndrome.
Results:
The patient was treated with extracorporeal life support. Subsequent computed tomography revealed multiple abscesses throughout his lungs and around vertebral bodies C1 and C2, for which source control with drainage of the cervical abscesses was achieved while on extracorporeal life support. The necrotizing pneumonia gradually improved, and partial pneumectomy was avoided. He was successfully separated from extracorporeal life support and respiratory support and recovered from his illness. Follow-up imaging showed almost complete resolution of the pulmonary abscesses. Osteomyelitis of C1/C2 and severe muscle wasting required a prolonged hospital stay.
Conclusion:
This case highlights the challenges of supporting patients suffering from disseminated staphylococcal sepsis with extracorporeal life support and the key role of source control and demonstrates the value of using extracorporeal life support in necrotizing pneumonia.
Keywords: Lemierre’s syndrome, extracorporeal life support, acute respiratory distress syndrome, sepsis
Introduction
Staphylococcus aureus has emerged as the most common pathogen causing community-acquired sepsis in children.1 Invasive staphylococcal infections represent particular challenges in the intensive care unit (ICU), including necrotizing pneumonia, toxin-mediated disease and coagulopathy. Mortality is high, and extracorporeal life support (ECLS) – though associated with great treatment challenges – may lead to successful treatment outcomes.2–4
Lemierre’s syndrome is a clinical constellation of suppurative internal jugular vein thrombophlebitis, oropharyngeal sepsis and septic emboli. We report a child presenting with Lemierre’s syndrome and septic shock caused by methicillin-sensitive Staphylococcus aureus (MSSA) who developed severe cardiorespiratory failure with extensive necrotizing pneumonia. Venoarterial ECLS was successfully employed and source control achieved while on ECLS with good outcome.
Case report
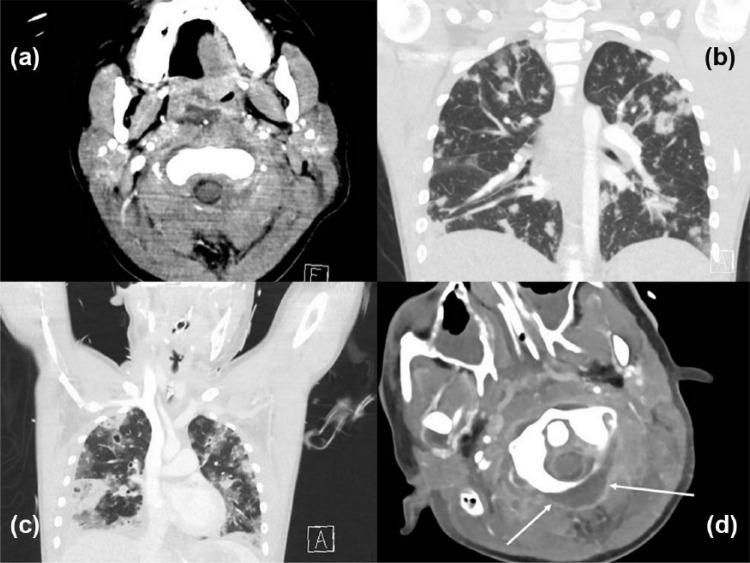
A previously well 12-year-old Maori boy presented to hospital with acute otitis media with perforation, neck swelling and neck pain which had developed over 24 h. He showed features of septic shock requiring urgent ICU admission. After endotracheal intubation, a computed tomography (CT) scan showed a retropharyngeal phlegmon as well as obstructive thrombi both in the entire left internal jugular vein and in parts of the right internal jugular vein (Figure 1). Pulmonary septic emboli as well as multiple pulmonary lesions (evolving lung assesses) were seen. With a differential diagnosis of Lemierre’s syndrome, antibiotics were started, empirically cefotaxime and metronidazole, then flucloxacillin and lincomycin after cultures from both the external ear canal and from blood showed growth of MSSA. The initial echocardiogram was normal and infective endocarditis was excluded. Blood cultures remained positive for the first 3 days, but remained clear of MSSA thereafter.
Figure 1.
Computed tomography images of (a) initial presentation showing an occluded left internal jugular vein, (b) lung on presentation, (c) CT lung on day 3 and (d) abscess (see arrows) posterior of C1 on day 14.
High-frequency ventilation was started within 24 h of admission and – due to ongoing hypoxia – an increase in mean airway pressure to a maximum of 28 cm H2O was required subsequently, with an inspired oxygen fraction of 0.5 (worst oxygenation index 40). A repeat CT scan on day three showed progression of jugular clots, pulmonary emboli as well as pulmonary cavitating lesions. Increasing cardiovascular support was necessary with noradrenaline (0.3 µg/kg/min), adrenaline (0.05 µg/kg/min) and vasopressin (1 U/h) to maintain an adequate blood pressure. In view of the deterioration requiring escalation of cardiorespiratory support and in an attempt to minimize ventilation pressures to prevent both further lung injury and pulmonary air leaks, the patient was commenced on ECLS via femoral veno-arterial cannulation on day three of admission. After ECLS cannulation, ‘rest ventilation’ was instituted with pressure control ventilation (peak inspiratory pressures of 15 cm H2O and a positive end-expiratory pressure of 5 cm H2O). Despite dropping ventilation pressures shortly after ECLS cannulation, a right-sided pneumothorax developed and was drained, followed by a left-sided pneumothorax on day six. Hemoserous drain losses over both drains totalled around 400 mL over the first 7 days of the extracorporeal membrane oxygenation (ECMO) run, without any evidence for frank haemorrhage at any stage.
While on ECLS inflammatory markers remained high, and the patient intermittently required inotropic support, suggesting ongoing systemic inflammation. On day eight, further CT imaging (on ECLS) was performed to identify presumed infectious collections. CT findings showed an abscess formation posterior to cervical vertebra one (Figure 1), signs of osteomyelitis involving the body of C1 and the dens of C2, and an opacified left mastoid. A decision was made to drain the abscess posterior to C1 by open incision and perform a cortical mastoidectomy. The procedure was performed in the paediatric intensive care unit (PICU) in prone position, and the mastoid was drilled out at the same time. The retropharyngeal space was not opened. Heparin was stopped just prior to and for 6 h following the procedure, and tranexamic acid was given. Bleeding around the abscess was well controlled, but periosteal bleeding proved challenging to control. Estimated total blood loss was 600–700 mL of blood. Peri-procedure (procedure day plus 2 days) 1500 mL of red blood cells and 1100 mL of pooled platelet were given. During this period, the fibrinogen remained high, and no cryoprecipitate or concentrated fibrinogen was required. In total, 1800 mg of tranexamic acid was given during this period as well due to the thrombelastogram showing hyperfibrinolysis. During the entire ECMO run, a total of 5700 mL of blood, 3200 mL of platelets and 4600 mg of tranexamic acid was given (patient weight 40 kg). Cultures from the drained paravertebral abscess yielded significant growth of MSSA. The cortical mastoidectomy demonstrated destruction of the malleus and incus with granulation tissue in the attic and a subtotal perforation, suggestive of a chronic middle ear process. The posterior cervical muscle wound was not primarily closed, but rather use of a negative pressure vacuum dressing was employed for approximately 10 days. Delayed primary closure then followed without requirement for further repeat surgical drainage. On day 13, further CT imaging showed progression of lung abscesses, with a particularly big abscess in the right middle lobe. Due to the high risk of inducing a bronchopleural fistula, drainage of this abscess was deferred. With improving pulmonary gas exchange, weaning from ECLS and successful decannulation were achieved on day 16. Magnetic resonance imaging (MRI) post decannulation revealed no further abscesses.
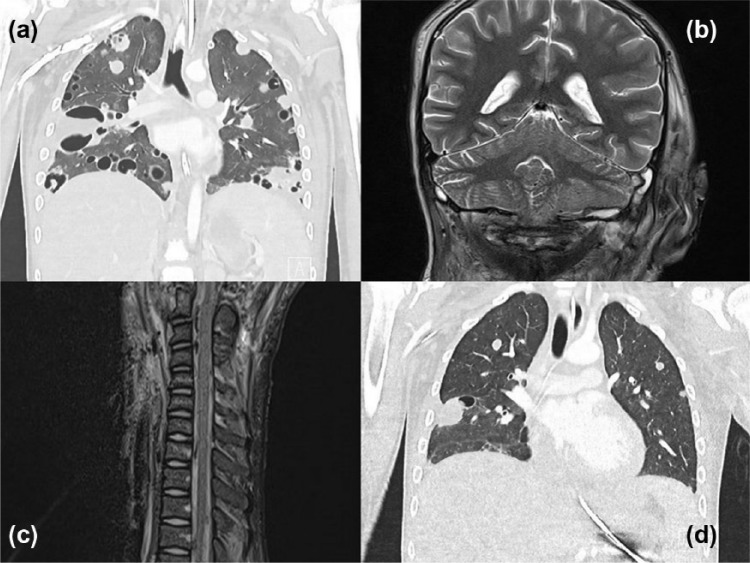
Invasive mechanical ventilation was required until day 23 of the illness. Post extubation respiratory support with non-invasive ventilation and high-flow nasal cannula oxygen was continued for 19 days. Further recovery was complicated by secondary pseudomonal sepsis and profound critical illness myopathy. CT imaging after extubation revealed pathological fractures of the lateral masses of C1. The patient was discharged from ICU on day 43. Follow-up imaging after ICU discharge showed markedly improved lung fields, improvement of cavitations, resolution of fluid collections around C1 and C2, but persistence of thrombi in the jugular veins and part of the venous sinuses (see Figure 2). Intravenous flucloxacillin and intravenous followed by oral Bactrim were continued for eight weeks with oral flucloxacillin planned to continue for a further four months.
Figure 2.
(a) Computed tomography lung post ECLS decannulation, (b) abscess on the right base of the cerebellar hemisphere on day 20, (c) epidural lesions (most likely blood) on day 20 and (d) follow-up CT at day 45.
Central nervous system (CNS) imaging was done repeatedly, both for delineation of the mastoid and C1 collections as well as for screening for intracranial abscesses when the inflammatory response seemed not to abate. While initially small intracranial extradural collections and small scattered foci of haemorrhage throughout both cerebellar hemispheres was found, an MRI six months after discharge showed resolution of all abnormal findings, albeit with a small degree of gliosis in a discrete area of the cerebellum.
Due to osteomyelitis of C1 and C2 and fractures of the lateral masses of C1, a halo-thoracic jacket was applied to prevent spinal injury. Neurocognitive assessment including motor, cognitive and behavioural performance showed normal outcomes six months post the onset of his illness. The Pediatric Overall Performance Category Assessment showed good overall performance (PCPC = 1).
Audiological assessment confirmed a mild conductive loss on the left and bilateral sensorineural mid to high frequency loss. The patient was discharged from hospital four months after initial presentation, with the halo-thoracic jacket still to be removed at a later date.
Consent for this case report was obtained from the parents, and the patient assented to it once he recovered from his illness.
Discussion
Our case report illustrates the use of ECLS in staphylococcal septic shock in a child with severe Lemierre’s syndrome and necrotizing pneumonia. To the best of our knowledge, this is the first report of ECLS support in a patient with Lemierre’s syndrome caused by S. aureus. The use of ECLS in Lemierre’s syndrome associated with Fusobacterium necrophorum has been described previously in two adult patients.5,6
Lemierre’s syndrome is an unusual presentation of invasive staphylococcal infections, likely resulting from a pathogen-induced prothrombotic state, local invasion and haematogenic extension from infections around the mastoid, external auditory canal and retropharyngeal spaces. Associations with Panton–Valentine leukocidin expressing S. aureus strains have been reported and mortality is high.7
ECLS represents a well-recognized rescue treatment for septic shock and should be considered in refractory septic shock.2–4,8 However, the outcomes depend on a number of factors, including age (with best outcomes in neonatal septic shock), pathogens and ECLS flow rates. Staphylococcal infections represent particular challenges on ECLS9 for several reasons, including pathogen adherence and persistence in extracorporeal circuits, haematogenous seeding, hypercoagulable state and toxin-mediated effects. Failure to eradicate the primary focus of infection allows for refractory haematogenous spread, which often lead to pulmonary and septic embolisms, resulting in multisite abscesses. While ECLS is effective in allowing ultra-protective ventilation to minimize air leak and ventilator-induced lung injury in staphylococcal acute respiratory distress syndrome (ARDS), necrotizing pneumonia can lead to extensive lung destruction and severe haemorrhage, particularly where anticoagulation for ECLS treatment is necessary. Overall outcomes for severe necrotizing staphylococcal pneumonia in paediatrics has been reported as poor in some series.10 As a result, in the past some centres were reluctant to consider patients with necrotizing pneumonia for ECLS. This view has been challenged over the past decade, and in many institutions, staphylococcal necrotizing pneumonia is not a contraindication for ECLS support anymore.
Air leak is a typical problem seen in cases of necrotizing pneumonia. At our institution, chest drains in patients supported by ECLS are only ever inserted by a specialist intensivist or cardiac surgeon, and we use a percutaneous Seldinger technique rather than blunt dissection. Our patient did not encounter major bleeding complications from chest drain insertions (though hemoserous drain losses were seen), which is consistent with our previous experience with ECLS patients who require drain insertions (chest or abdomen).
Our case illustrates the importance of aggressive source control, which may be achieved even while on ECLS. During the incision and drainage of abscesses (particularly related to bone drilling in the mastoid), we encountered bleeding that necessitated transfusion but was not life limiting nor ongoing beyond 24 h post procedure. We paused all anticoagulation for the incision and drainage procedures according to our protocol described previously.11
We faced unique challenges in the treatment of our patient for a number of reasons, mainly the paucity of data for a case of the severity of illness our patient encountered, and how to manage the unique triad of local source control, pulmonary embolism and pulmonary necrosis and abscesses. Even though case reports suggest partial pneumectomy to be a successful treatment for necrotizing pneumonia, we managed our patient without pulmonary surgical source control.
Conclusion
Our case highlights the unique treatment challenges in extreme staphylococcal infections manifesting as Lemierre’s syndrome and severe necrotizing staphylococcal pneumonia. The case demonstrates the successful use of ECLS which – combined with aggressive infective source control – led to a positive outcome for our patient.
Acknowledgments
A.C.M. drafted the initial manuscript and approved the final manuscript as submitted. S.P. contributed and edited the technical aspects of the ECMO run. J.C. edited and contributed to the infectious disease component of the paper. R.L. contributed and edited the orthopaedic component of the paper. H.B. contributed and edited the ENT component of the paper. L.J.S. contributed to and edited every section of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series. However, our HREC requires us to obtain a consent from the parents and/or patient for case reports. For the case report that we submitted we have written consent from the parents and the patient approving of the publication in an international, peer-reviewed journal.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
References
- 1. Schlapbach LJ, Straney L, Alexander J, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis 2015; 15(1): 46–54. [DOI] [PubMed] [Google Scholar]
- 2. MacLaren G, Butt W, Best D. Pediatric septic shock guidelines and extracorporeal membrane oxygenation management. Crit Care Med 2009; 37(6): 2143–2144; author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 3. MacLaren G, Butt W, Best D, et al. Central extracorporeal membrane oxygenation for refractory pediatric septic shock. Pediatr Crit Care Med 2011; 12(2): 133–136. [DOI] [PubMed] [Google Scholar]
- 4. Maclaren G, Butt W, Best D, et al. Extracorporeal membrane oxygenation for refractory septic shock in children: one institution’s experience. Pediatr Crit Care Med 2007; 8(5): 447–451. [DOI] [PubMed] [Google Scholar]
- 5. Kamath SS, Mason K. Extra-corporeal membrane oxygenation in a patient with Fusobacterium sepsis: a case report and review of literature. Ann Thorac Cardiovasc Surg 2011; 17(4): 397–399. [DOI] [PubMed] [Google Scholar]
- 6. Carre AC, David JS, Mahr A, et al. Use of ECMO as a salvage therapy for refractory hypoxia secondary to a Lemierre’s syndrome. Ann Fr Anesth Reanim 2011; 30(6): 512–515. [DOI] [PubMed] [Google Scholar]
- 7. Chanin JM, Marcos LA, Thompson BM, et al. Methicillin-resistant Staphylococcus aureus USA300 clone as a cause of Lemierre’s syndrome. J Clin Microbiol 2011; 49(5): 2063–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41(2): 580–637. [DOI] [PubMed] [Google Scholar]
- 9. Noah MA, Dawrant M, Faulkner GM, et al. Panton-Valentine leukocidin expressing Staphylococcus aureus pneumonia managed with extracorporeal membrane oxygenation: experience and outcome. Crit Care Med 2010; 38(11): 2250–2253. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz KL, Nourse C. Panton-Valentine leukocidin-associated Staphylococcus aureus necrotizing pneumonia in infants: a report of four cases and review of the literature. Eur J Pediatr 2012; 171(4): 711–717. [DOI] [PubMed] [Google Scholar]
- 11. Prabhu S, Mattke AC, Anderson B, et al. Repair of congenital diaphragmatic hernia during extracorporeal life support: experience with six neonates. ANZ J Surg 2016; 86(9): 711–716. [DOI] [PubMed] [Google Scholar]