Abstract
Background: Parenteral nutrition (PN) covering the need for carbohydrates, amino acids, and lipids can either be compounded from single nutrients or purchased as an industrially manufactured ready-to-use regimen. This study compares a commercially available 3-chamber bag (study group) with a conventionally compounded monobag regarding nutrition efficacy, safety, and regimen preparation time. Materials and Methods: This prospective, randomized, single-blind study was conducted at 5 Chinese hospitals from October 2010–October 2011. Postsurgical patients requiring PN for at least 6 days were randomly assigned to receive the study or control regimen. Plasma concentrations of prealbumin and C-reactive protein (CRP), regimen preparation time, length of hospital stay (LOS), 30-day mortality, safety laboratory parameters, and adverse events (AEs) were recorded. Results: In total, 240 patients (121 vs 119 in study and control groups) participated in this study. Changes in prealbumin concentrations during nutrition support (ΔPrealb(StudyGroup) = 2.65 mg/dL, P < .001 vs ΔPrealb(ControlGroup) = 0.27 mg/dL, P = .606) and CRP values were comparable. Regimen preparation time was significantly reduced in the study group by the use of 3-chamber bags (t(StudyGroup) = 4.90 ± 4.41 minutes vs t(ControlGroup) = 12.13 ± 5.62 minutes, P < .001). No differences were detected for LOS, 30-day mortality, safety laboratory parameters, and postoperative AEs (37 vs 38 in study and control groups). Conclusion: The PN regimen provided by the 3-chamber bag was comparable to the compounded regimen and safe in use. Time savings during regimen preparation indicates that use of 3-chamber bags simplifies the process of regimen preparation.
Keywords: parenteral nutrition, 3-chamber bags, multichamber bags, compounding, efficacy, patient, parenteral nutrition solutions, nutritional support, treatment outcome
Surgery-induced metabolic changes like catabolic processes and increased energy expenditure lead to nutrition depletion during the postoperative period.1 In consequence, surgical patients are at risk of postoperative malnutrition in case of insufficient caloric intake. It is well recognized that malnutrition is associated with higher infection and complication rates, impaired wound healing, prolonged hospital stay, and increased morbidity and mortality.2 For patients undergoing abdominal surgery, appropriate nutrition support has beneficial effects on principal outcome parameters.3 To prevent malnutrition and to maintain the nutrition status, postoperative nutrition support is recommended for patients who cannot meet their caloric requirements orally for >7 days.4 If nutrition support via the enteral route is not possible, macronutrients (carbohydrates, amino acids, and lipids) and micronutrients (electrolytes, vitamins, and trace elements) may be administered intravenously as parenteral nutrition (PN). PN regimens are complex formulas that contain a variety of substances and are composed according to established clinical guidelines providing carbohydrates, amino acids, and lipids in a defined ratio.5 The formulation of PN regimens can be individualized to meet specific requirements or standardized to cover nutrition needs of a larger patient population. Standardized PN regimens are either mixed from single nutrients to all-in-one (AIO) admixtures (monobags) or purchased as industrially manufactured ready-to-use 3-chamber bags.
Few studies are published that compare the use of compounded monobags with industrially manufactured 3-chamber bags. They indicate benefits of multichamber bags in comparison to compounded AIO admixtures in terms of cost reduction and reduced risk of bloodstream infections.6-9
This study was set up to further investigate advantages of commercially available 3-chamber bags. Use of 3-chamber bags and conventionally compounded monobags was compared in a population of postsurgical patients requiring PN for at least 6 days focusing on nutrition efficacy, safety, and time-saving aspects during regimen preparation.
Materials and Methods
Study Design
This study was a prospective, multicenter, randomized, comparative, single-blind study with 2 parallel groups conducted at 5 Chinese hospitals from October 2010 until October 2011 in accordance with the principles of the Declaration of Helsinki and requirements for Good Clinical Practice. Study protocol and informed consent form were approved by the Chinese State Food and Drug Administration as well as by the Ethic Committees of the Peking Union Medical College Hospital and the other participating sites. Informed consent was obtained from all participating patients. This study was sponsored by B. Braun Medical (Shanghai; study registration: ClinicalTrials.gov [NCT01247740]).
Patient Population
Male and female adult patients aged between 18 and 85 years admitted to the hospital for elective open abdominal surgery with an expected need of postoperative PN for at least 6 consecutive days were considered for study participation. Eligible patients weighed between 50 and 70 kg, had a Nutrition Risk Screening (NRS) score ≥3, and had nutrition requirements not exceeding 2100 kcal/d. Patients with contraindications for PN or infusion therapy and those who received PN within 7 days or chemotherapy/radiation within 30 days prior to study enrollment were excluded. The following diagnoses also led to exclusion from study participation: liver surgery, laparoscopic surgery, expected volume of blood transfusion during surgery >1000 mL, infectious diseases like human immunodeficiency virus, hyperglycemia requiring insulin doses >6 U/h, diabetic ketoacidosis within 7 days prior to study enrollment, intrahepatic cholestasis, amino acid and/or fat metabolism disorders, acute thromboembolism/fat embolism, sepsis and septic shock, severe pancreatitis, severe liver dysfunction, renal dysfunction, severe blood coagulation function disorders, hemodynamic failure for any origin, and hemoglobin <8 g/dL before surgery. Other reasons for exclusion were participation in another clinical trial, pregnancy and lactation, and known or suspected drug abuse.
Nutrition Treatment and Interventions
Patients were randomly assigned to receive the study regimen, a commercially available 3-chamber bag (NuTRIflex Lipid peri; B. Braun Melsungen AG, Melsungen, Germany), or the control regimen that was conventionally compounded and provided by monobags. Total caloric and amino acid intakes of both groups were set to 24 kcal/kg body weight/d and 1.2 g/kg body weight/d, respectively. Both mixtures provided comparable amounts of carbohydrates, amino acids, lipids, and electrolytes (Table 1). Lipid composition differed between study and control regimens: while the study regimen contained a mixture of medium-chain triglycerides (MCTs) and long-chain triglycerides (LCTs) in a 1:1 ratio, the control regimen provided LCT only. Both regimens were prepared at the compounding unit of the hospital. Three-chamber bags were mixed according to the manufacturer’s instructions while compounding of monobags followed hospital routine. Vitamins (10 mL Soluvit N and 10 mL Vitalipid) and trace elements (10 mL Addamel N) were added to the infusion bag as required. All supplements were purchased from Fresenius Kabi (Bad Homburg, Germany). To ensure blinding, infusion bags were covered with an opaque bag and labeled with the randomization number that was assigned according to a software-generated master randomization list. Randomization was stratified for study centers. Both regimens were suited for central as well as peripheral administration and were delivered intravenously via central venous catheters, peripherally inserted central catheters (PICCs), or peripheral lines at room temperature over a period of 12–20 hours. The maximum rate of infusion was 2.5 mL/kg body weight/h. Up to 1 bag of PN was administered corresponding to a volume of 1875 mL and 1886.5 mL for study and control regimen, respectively. Oral and/or enteral nutrition (EN) covered up to 20% of the daily caloric intake. Laboratory parameters were determined on postoperative days (PODs) 1 and 7 at the laboratory of each center and included hematological parameters (hemoglobin, red blood cell count, white blood cell count, platelet count, percentage of granulocyte and hematocrit), parameters of blood coagulation function (prothrombin time and activated partial thromboplastin time), liver function parameters (alanine aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase, total bilirubin, serum albumin), blood chemistry and electrolytes (triglycerides, total cholesterol, blood glucose, serum urea nitrogen, creatinine, sodium, potassium, chloride, and phosphate), and C-reactive protein (CRP) and prealbumin (as short-term marker for nutrition status10). The change of prealbumin levels between POD 1 (ie, before administration of PN) and POD 7 (ie, after administration of PN for 6 consecutive days), ΔPrealb, as the primary variable was used as an indicator for the efficacy of both PN regimens, with ΔPrealb = 0 indicating maintenance of nutrition status. Compliance of PN therapy was assessed via the ratio volumeadministered/volumeprescribed. Poor compliance was assumed for ratios <0.8 or >1.2. Time needed for PN preparation was recorded on PODs 1 and 5 to assess the workload associated with regimen preparation. Length of hospital stay (LOS) and 30-day mortality were determined via a follow-up call around POD 30. All adverse events (AEs) were recorded to assess the incidence of postoperative complications. AEs were classified according to intensity, frequency, action taken, causal relationship with the study medication, seriousness, and outcome. AEs were coded according to MedDRA system organ class (SOC) and lowest level term (LLT).
Table 1.
Composition of Parenteral Nutrition Regimens.
| Component (per Bag) | Three-Chamber Bag (NuTRIflex Lipid Peri) | Compounded Monobag |
|---|---|---|
| Carbohydrates (glucose), g | 120 | 120 |
| Amino acid, g | 60 | 61 |
| Total nitrogen, g | 8.6 | 8.7 |
| Lipids | ||
| LCT, g | 37.5 | 75 |
| MCT, g | 37.5 | 0 |
| Electrolytes | ||
| Sodium, mmol/mEq | 75/75 | 77/77 |
| Potassium, mmol/mEq | 45/45 | 30/30 |
| Magnesium, mmol/mEq | 4.5/9 | 4/8 |
| Calcium, mmol/mEq | 4.5/9 | 2.2/4.4 |
| Chloride, mmol/mEq | 72/72 | 107/107 |
| Zinc, mmol/mEq | 0.045/0.089 | 0 |
| Phosphate, mmol/mEq | 11.25/33.75 | 0.75/2.25 |
| Acetate, mmol/mEq | 60/60 | 0 |
| Total energy, kJ (kcal) | 6000 (1435) | 6015 (1439) |
| Volume, mL | 1875 | 1886.5 |
| Osmolarity, mOsm/L | 840 | 818 |
LCT, long-chain triglyceride; MCT, medium-chain triglyceride.
Statistics
The study was designed to show noninferiority of the commercial 3-chamber bag in comparison to the compounded monobag. Following the Chinese regulations for registration trials for import products, sample size was set to 120 patients per group, including an estimated dropout rate of 20%. Statistical analysis was performed for both per protocol set (PPS, all eligible patients without major protocol violations) and full analysis set (FAS, all eligible patients who received study medication at least once) using the software package SAS (SAS Institute, Cary, NC). Since PPS and FAS analyses were comparable for all variables, data from FAS are presented in the Results section. Solely analyses of the primary variable ΔPrealb are displayed for PPS and FAS to take account of the noninferiority study design. Safety analyses were based on data of all patients who received study medication at least once (safety analysis set). Data are presented as mean ± standard deviation (SD) or median with first and third quartiles (Q1, Q3). The primary variable was analyzed using paired t test and analysis of covariance (ANCOVA) to compare the variance of prealbumin level on POD 7 and its variance relative to baseline. For secondary variables, quantitative data were analyzed using the Wilcoxon rank-sum test, and qualitative data were analyzed using the χ2 test. All statistical tests were 2-sided; P values <.05 were considered statistically significant.
Results
Study Population
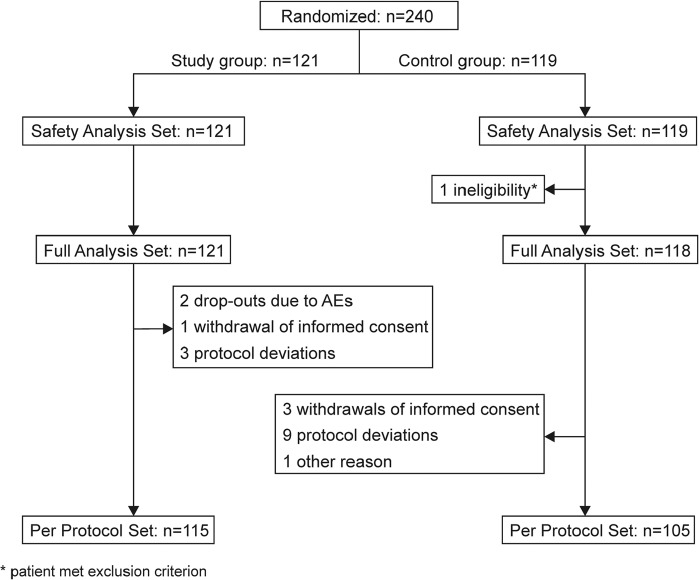
A total of 240 patients who underwent open abdominal surgery (eg, radical surgery for gastric cancer, colon cancer, rectal carcinoma resection, partial small intestine resection, and anastomosis) were randomized and received study medication at least once (121 in the study and 119 in the control group). This group was used for safety analysis. One patient in the control group met exclusion criteria and was omitted from FAS. Nineteen further patients were excluded from PPS due to study discontinuation or protocol deviation (PPS: 115 and 105 patients for study and control groups, respectively; see Figure 1 for details). Groups were well balanced for age, sex, height, body weight, NRS score, and medical history (Table 2).
Figure 1.
Participant flow. Flowchart giving the number of patients randomized, allocated to study and control groups, and included for analysis. AE, adverse event.
Table 2.
Characteristics of Study Population.
| Item | Study Group (n = 121) | Control Group (n = 118)a | P Value |
|---|---|---|---|
| Age, mean ± SD, y | 59.83 ± 12.42 | 59.75 ± 12.56 | .902 |
| Male/female, No. | 67/54 | 62/56 | .661 |
| Height, mean ± SD, cmb | 165.58 ± 6.59 | 163.85 ± 7.15 | .066 |
| Weight, mean ± SD, kg | 61.39 ± 6.40 | 60.80 ± 6.63 | .557 |
| Nutrition Risk Screening score, mean ± SD | 3.45 ± 0.90 | 3.36 ± 0.66 | .806 |
| Other diseases, no/yes, No. | 43/78 | 46/72 | .582 |
| Medication history, no/yes, No. | 86/35 | 86/32 | .756 |
Of the 119 in the control group, 1 patient met exclusion criterion.
nstudy group = 120 and ncontrol group = 117.
Treatment Compliance
Patients received PN either via peripheral (20%, both groups) or central veins (80%, both groups). Average infusion rate was about 2.2 mL/kg body weight/h, and average infusion duration was about 14 hours for both groups. Compliance for both regimens was good for all patients. Median volume administered corresponded to the volume of 1 infusion bag (eg, median volume administered on POD 1: 1875 mL [Q1, Q3: 1865 mL, 1891 mL] and 1887 mL [Q1, Q3: 1886 mL, 1902 mL] in study and control groups, respectively).
Efficacy of Nutrition Treatment
Prealbumin levels determined before nutrition treatment at POD 1 were comparable between groups in PPS (16.60 ± 5.02 mg/dL and 16.39 ± 5.01 mg/dL in study and control groups, respectively, P = .467) and FAS (16.48 ± 5.07 mg/dL and 16.37 ± 5.15 mg/dL in study and control groups, respectively, P = .510). During nutrition treatment, prealbumin levels increased significantly in the study group by 2.70 ± 5.69 mg/dL and 2.59 ± 5.61 mg/dL in PPS and FAS (P < .001), respectively, while they remained stable in the control group (ΔPrealb PPS = 0.36 ± 4.69 mg/dL, P = .465 and ΔPrealb FAS = 0.29 ± 4.95 mg/dL, P = .606; see Figure 2). After nutrition treatment at POD 7, prealbumin levels in the study and control groups were significantly different in PPS (19.43 ± 6.02 mg/dL and 16.73 ± 5.20 mg/dL in study and control groups, respectively, P < .001) and FAS (19.13 ± 6.02 mg/dL and 16.64 ± 5.38 mg/dL in study and control groups, respectively, P < .001). Concurrently, CRP levels decreased significantly between PODs 1 and 7 in both groups without differences in the intergroup comparison. PPS and FAS analyses thus show good nutrition efficacy of both regimens and noninferiority of the study treatment. Prealbumin and CRP values are listed in Table 3.
Figure 2.
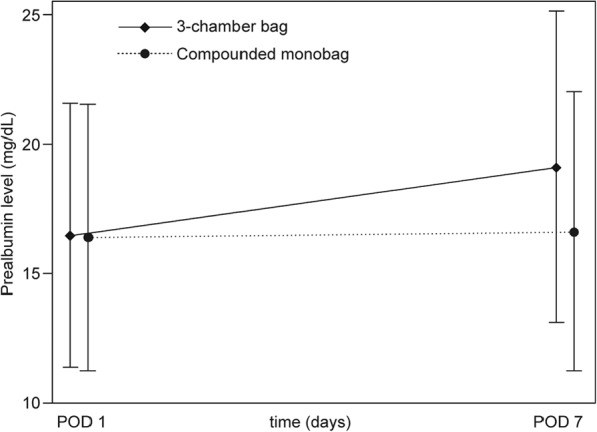
Changes of prealbumin levels during parenteral nutrition (full analysis set). Prealbumin levels determined at postoperative days (PODs) 1 and 7 significantly increased during parenteral nutrition with 3-chamber bags (P < .001) and remained stable during treatment with conventionally compounded monobags.
Table 3.
Prealbumin and CRP Levels Before and After Parenteral Nutrition Treatment (Full Analysis Set).
| Characteristic | Study Group, Mean ± SD | Control Group, Mean ± SD | P Value |
|---|---|---|---|
| Prealbumin, mg/dLa | |||
| POD 1 | 16.48 ± 5.07 | 16.37 ± 5.15 | .510 |
| POD 7 | 19.13 ± 6.02 | 16.64 ± 5.38 | <.001 |
| CRP, mg/Lb | |||
| POD 1 | 52.32 ± 35.30 | 50.13 ± 36.25 | .519 |
| POD 7 | 37.24 ± 28.39 | 41.28 ± 33.13 | .576 |
CRP, C-reactive protein; POD, postoperative day.
POD 1: nstudy group = 120, ncontrol group = 115; POD 7: nstudy group = 121, ncontrol group = 118.
POD 1: nstudy group = 117, ncontrol group = 115; POD 7: nstudy group = 116, ncontrol group = 115.
LOS in Hospital and 30-Day Mortality
No difference was detected for LOS or 30-day mortality. Median LOS after surgery was 9 days for the study and control groups (Q1, Q3study group: 8 days, 12 days; Q1, Q3control group: 8 days, 11 days). Thirty-day mortality rate in both groups was 0%.
PN Regimen Preparation Time
Time needed for preparation of PN regimens at the compounding unit was significantly shorter for 3-chamber bags on PODs 1 and 5 in all 5 participating sites (Table 4). On average, preparation of the 3-chamber bag was about 7 minutes shorter than preparation of the monobag (t = 4.90 ± 4.41 minutes and t = 12.13 ± 5.62 minutes for study and control regimen, respectively, P < .001, POD 1).
Table 4.
Preparation Time of Parenteral Nutrition Regimens.
| Characteristic | Three-Chamber Bag, Mean ± SD |
Compounded Monobag, Mean ± SD |
P Value |
|||
|---|---|---|---|---|---|---|
| POD 1 | POD 5 | POD 1 | POD 5 | POD 1 | POD 5 | |
| Preparation time, min | ||||||
| Center 1a | 2.25 ± 0.53 | 2.17 ± 1.64 | 9.04 ± 2.29 | 8.13 ± 1.36 | <.001 | <.001 |
| Center 2b | 6.77 ± 5.52 | 5.60 ± 2.69 | 13.36 ± 7.36 | 12.27 ± 4.06 | <.001 | <.001 |
| Center 3c | 3.00 ± 0.00 | 3.00 ± 0.00 | 10.00 ± 0.00 | 10.29 ± 1.69 | <.001 | <.001 |
| Center 4d | 5.67 ± 7.06 | 6.33 ± 5.85 | 18.33 ± 4.08 | 22.33 ± 5.68 | .028 | <.001 |
| Center 5e | 9.88 ± 1.64 | 10.88 ± 0.83 | 19.50 ± 2.51 | 20.67 ± 3.27 | <.001 | <.001 |
| Centers 1–5 | 4.90 ± 4.41 | 4.56 ± 3.15 | 12.13 ± 5.62 | 11.77 ± 4.79 | <.001 | <.001 |
POD, postoperative day.
POD 1: nstudy group = 24, ncontrol group = 24; POD 5: nstudy group = 23, ncontrol group = 24.
POD 1 + 5: nstudy group = 47, ncontrol group = 44.
POD 1: nstudy group = 36, ncontrol group = 36; POD 5: nstudy group = 35, ncontrol group = 35.
POD 1 + POD 5: nstudy group = 6, ncontrol group = 6.
POD 1: nstudy group = 8, ncontrol group = 8; POD 5: nstudy group = 8, ncontrol group = 6.
Safety of Nutrition Treatment
A total of 75 AEs were recorded between PODs 1 and 7 affecting 24 patients in the study group (37 AEs) and 27 patients in the control group (38 AEs). Gastrointestinal diseases (25% of all AEs, inter alia nausea, vomiting, diarrhea, gastric hypomotility, and gastoplegia), deviating laboratory values (17% of all AEs, inter alia positive urine glucose and abnormal hemogram), and systemic diseases/reaction at the administration site (16% of all AEs, inter alia fever, chills, and phlebitis at the infusion site) occurred most frequently. Electrolyte imbalances (normal values before and abnormal values after nutrition treatment) were recorded for sodium (9 patients in study group and 5 in control group), potassium (9 patients in study group and 7 in control group), chloride (2 patients in study group and 3 in control group), and phosphate (18 patients in study group and 20 in control group). Electrolyte imbalances were clinically significant in 1 case of decreased potassium (study group), and administration of study medication was discontinued. Four AEs were related to the nutrition regimen—namely γ-glutamyltransferase elevation (1 case in the study group), positive urine glucose (1 case in the study group), and phlebitis at the infusion site (after peripheral administration, 1 case in the study group and 1 in the control group). All of these AEs were considered mild, and administration of the nutrition regimen was not adjusted. One patient in the study group and 5 patients in the control group experienced the following serious adverse events (SAEs) that were rated as unlikely related or not related to the nutrition treatment: syncope and angina, postoperative gastric cancer and gastric motility disorder, wound dehiscence and anastomotic fistula, hepatitis B and liver dysfunction, wound infection, and gastric motility disorder. Administration of the nutrition regimen was discontinued for the patient with syncope and angina. All other SAEs did not lead to discontinuation of the nutrition treatment. Incidence and severity of AEs and SAEs were comparable between study and control groups, and refeeding syndrome, feeding intolerance, or bloodstream infections were not reported. No patient died during this study.
All mean laboratory parameters determined at POD 1 were within the normal reference range in both groups. At POD 7, all mean laboratory parameters were comparable between groups except for platelet count (213.04 ± 69.04 × 109/L and 253.42 ± 89.26 × 109/L in study and control groups, respectively, P < .001), prothrombin time (12.34 ± 1.04 seconds and 12.83 ± 1.30 seconds in study and control groups, respectively, P = .008), alkaline phosphatase (89.56 ± 71.83 U/L and 72.44 ± 28.59 U/L in study and control groups, respectively, P = .015), triglycerides (1.55 ± 0.92 mmol/L and 1.15 ± 0.68 mmol/L in study and control groups, respectively, P < .001), and total cholesterol (3.97 ± 0.85 mmol/L and 3.53 ± 0.77 mmol/L in study and control groups, respectively, P < .001), as well as potassium (4.23 ± 0.50 mmol/L and 3.97 ± 0.40 mmol/L in study and control groups, respectively, P < .001), chloride (102.40 ± 2.86 mmol/L and 104.30 ± 3.33 mmol/L in study and control groups, respectively, P < .001), and phosphate concentrations (1.18 ± 0.22 mmol/L and 1.09 ± 0.25 mmol/L in study and control groups, respectively, P = .007). Although group differences of mean laboratory values at POD 7 were statistically significant, they were without clinical relevance as all parameters remained within the normal ranges.
Discussion
Postsurgical nutrition support intends to attenuate the postsurgical stress response and to prevent postoperative catabolism.11 The aim of this study was to compare a standardized PN regimen provided by 3-chamber bags with compounded monobags regarding efficacy of nutrition support and clinical outcome parameters in abdominal surgery patients. Inclusion and exclusion criteria for participation in this clinical study ensured that PN support was used according to current guidelines.4,5 Total caloric intake was calculated based on standard estimates of energy requirements. Since calculating caloric requirements may severely overestimate nutrition requirements of obese patients and underestimate requirements of patients with very low body weight,5 only normal-weight participants (average body mass, Asian population: 57.7 kg12) were considered for study participation. Because of that, assessment of nutrition efficacy of study and control regimens in this study was restricted to normal-weight patients undergoing abdominal surgery, and transferability to other patient populations, especially obese or underweight patients, might be limited.
Prealbumin changes during nutrition therapy as a primary variable remained stable in the control group and increased by ~2.6 mg/dL in the study group. Reliability of prealbumin as a biomarker for nutrition state, however, is limited since prealbumin is a negative acute phase protein and serum levels are influenced not only by nutrition but also by the inflammatory state.11 It is now recognized that visceral proteins such as serum albumin, prealbumin, and transferrin reflect the dynamic and catabolic response to surgery, injury, or infection in postoperative patients and are not suitable to measure adequacy of nutrition support.13 At the time of protocol development, prealbumin was a commonly used marker for nutrition status due to its small pool size and short half-life. To increase the validity of prealbumin measurements, the change in prealbumin levels during nutrition treatment was investigated together with CRP levels, as suggested by Raguso et al.10 CRP levels revealed a comparable inflammatory state in both groups, indicating that surgery-induced inflammatory processes were comparable in study and control groups. Nevertheless, prealbumin data presented have to be interpreted carefully. Of note, study and control nutrition regimens differed in terms of lipid composition: MCTs/LCTs in the study group vs LCTs in the control group. MCTs are known to undergo faster clearance and oxidation and to allow better energy provision and nitrogen sparing than LCTs.14 The increase in prealbumin of ~2.6 mg/dL observed in the study group could probably be attributed to the MCT content of the study regimen.15 The different lipid compositions administered in study and control group can also be considered a limitation of this study.
The clinical outcome reflected by the secondary outcome parameters of LOS and 30-day mortality was similar between both regimens.
The use of 3-chamber bags intends to obviate time-consuming and demanding compounding procedures by simple mixing of solutions already contained in the bag. This was confirmed by a significantly shorter preparation time for 3-chamber bags at all participating sites with saving of time ranging between 6 and 16 minutes depending on the clinical setting. By simplifying processes, use of 3-chamber bags could thus contribute to reduce workload and manpower costs, especially at the hospital pharmacy. This is in line with other studies that demonstrated differences in preparation time for monobags and 3-chamber bags in a comparable magnitude (eg, time saving using 3-chamber bags ~11 min6 and 25.9 min7) and showed that 3-chamber bags are more economical than compounded monobags in Swiss6 and Spanish hospitals.7 A detailed cost analysis was not in the scope of the present clinical trial. This would be an interesting aspect to be included in further studies.
In addition to the simplification of regimen preparation, 3-chamber bags offer further advantages. Although providing a standardized PN regimen, 3-chamber bags allow safe modification by addition of supplements directly to the infusion bag. Furthermore, 3-chamber bags ease the PN regimen supply: while AIO admixtures have to be stored refrigerated and administered within 30 days, 3-chamber bags can be stored at room temperature with a shelf-life of 2 years. A particular advantage of the 3-chamber bag used in this study is its osmolarity that allows for central as well as peripheral administration and thereby contributes to further facilitate nutrition support.
Several studies indicate that use of industrially manufactured PN regimens may reduce the risk of bloodstream infections compared with compounded regimens.8,9,16 Three-chamber bags could therefore contribute to increase patient safety during PN therapy. In this study, no bloodstream infections were recorded and reported as AEs in the study and control groups. Both regimens were well tolerated and appeared safe as revealed by safety laboratory parameters and the comparable rate of electrolyte imbalances and other AEs/SAEs in the study and control groups.
In conclusion, this study showed that nutrition efficacy of a standardized PN regimen provided by 3-chamber bags was comparable to the conventionally compounded regimen. Use of 3-chamber bags was safe and well tolerated. Preparation of the standardized PN regimen was significantly shorter for 3-chamber bags than for compounded monobags. Three-chamber bags therefore have the potential to reduce manpower costs. Taking into account potential safety advantages, the central or peripheral venous administration of the 3-chamber bag used in this study should be considered for patients with nutrition requirements that can be covered using standardized PN regimens.
Acknowledgments
We thank Shi Pan, PhD, B. Braun Medical Shanghai, and Yim Yung, B. Braun Medical Shanghai, for planning and coordination of all study activities and wish to acknowledge medical writing services provided by Katharina Achilles, PhD. We also thank Kang Weiming, MD; Ma Zhiqiang, MD; Liang Bin, MD; and Guo Shuli for their contributions to this research.
Footnotes
Financial disclosure:The study was sponsored by B. Braun Medical Shanghai, China. Jianchun Yu has received medical writing support, which was funded by B. Braun Melsungen AG, Melsungen, Germany. Jianchun Yu has signed an agreement with the sponsor of the research, which requires approval of the sponsor before the manuscript is published.
Conflicts of interest:None declared.
This article originally appeared online on May 24, 2017.
Statement of Authorship: J. Yu contributed to the conception and design of the research; contributed to the acquisition, analysis, and interpretation of the data; and drafted the manuscript. G. Wu, Y. Tang, Y. Ye, and Z. Zhang contributed to the design of the research. G. Wu contributed to the acquisition and interpretation of the data. Y. Tang, Y. Ye, and Z. Zhang contributed to the acquisition of the data. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
References
- 1. Ward N. Nutrition support to patients undergoing gastrointestinal surgery. Nutr J. 2003;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8(2):514-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26:698-709. [DOI] [PubMed] [Google Scholar]
- 4. Weimann A, Ebener Ch, Holland-Cunz S, et al. Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine. Surgery and transplantation—Guidelines on Parenteral Nutrition, Chapter 18. Ger Med Sci. 2009;7:Doc10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F; ESPEN. ESPEN guidelines on parenteral nutrition: surgery. Clin Nutr. 2009;28(4):378-386. [DOI] [PubMed] [Google Scholar]
- 6. Pichard C, Schwarz G, Frei A, et al. Economic investigation of the use of three-compartment total parenteral nutrition bag: prospective randomized unblinded controlled study. Clin Nutr. 2000;19(4):245-251. [DOI] [PubMed] [Google Scholar]
- 7. Berlana D, Sabin P, Gimeno-Ballester V, et al. Cost analysis of adult parenteral nutrition systems: three-compartment bag versus customized. Nutr Hosp. 2013;28(6):2135-2141. [PubMed] [Google Scholar]
- 8. Pontes-Arruda A, Zaloga G, Wischmeyer P, Turpin R, Liu FX, Mercaldi C. Is there a difference in bloodstream infections in critically ill patients associated with ready-to-use versus compounded parenteral nutrition? Clin Nutr. 2012;31(5):728-734. [DOI] [PubMed] [Google Scholar]
- 9. Turpin RS, Canada T, Rosenthal V, et al. ; IMPROVE Study Group. Bloodstream infections associated with parenteral nutrition preparation methods in the United States: a retrospective, large database analysis. JPEN J Parenter Enteral Nutr. 2012;36(2):169-176. [DOI] [PubMed] [Google Scholar]
- 10. Raguso CA, Dupertuis YM, Pichard C. The role of visceral proteins in the nutritional assessment of intensive care unit patients. Curr Opin Clin Nutr Metab Care. 2003;6(2):211-216. [DOI] [PubMed] [Google Scholar]
- 11. Torgersen Z, Balters M. Perioperative nutrition. Surg Clin North Am. 2015;95(2):255-267. [DOI] [PubMed] [Google Scholar]
- 12. Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med. 2016;44(2):390-438. [DOI] [PubMed] [Google Scholar]
- 14. Bach AC, Frey A, Lutz O. Clinical and experimental effects of medium-chain-triglyceride-based fat emulsions—a review. Clin Nutr. 1989;8(5):223-235. [DOI] [PubMed] [Google Scholar]
- 15. Chen FM, Wang JY, Sun LC, Juang RF, Huang TJ, Hsieh JS. Efficacy of medium-chain triglycerides compared with long-chain triglycerides in total parenteral nutrition in patients with digestive tract cancer undergoing surgery. Kaohsiung J Med Sci. 2005;21(11):487-494. [DOI] [PubMed] [Google Scholar]
- 16. Turpin RS, Canada T, Liu FX, Mercaldi CJ, Pontes-Arruda A, Wischmeyer P. Nutrition therapy cost analysis in the US: pre-mixed multi-chamber bag vs compounded parenteral nutrition. Appl Health Econ Health Policy. 2011;9(5):281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]