Abstract
Little information is known on the rate of repeat gonorrhea infection among U.S. military personnel. We analyzed all gonorrhea cases reported to the Defense Medical Surveillance System during 2006–2012 to determine the rate of repeat infection. During the seven-year study period, 17,602 active duty U.S. Army personnel with a first incident gonorrhea infection were reported. Among the 4987 women with a first gonorrhea infection, 14.4% had at least one repeat infection. Among the 12,615 men with a first gonorrhea infection, 13.7% had at least one repeat infection. Overall, the rate of repeat gonorrhea infection was 44.5 and 48.9 per 1000 person-years for women and men, respectively. Service members aged 17–19 years (hazard ratio [HR] for women = 1.51; HR for men = 1.71), African-American personnel (HR for women = 1.26; HR for men = 2.17), junior enlisted personnel (HR for women = 2.64; HR for men = 1.37), and those with one year or less of service (HR for women = 1.23; HR for men = 1.37) were at higher risk of repeat infection. The findings from this study highlight the need to develop targeted prevention initiatives including education, counseling, and retesting to prevent gonorrhea reinfections among U.S. Army personnel.
Keywords: Gonorrhea, epidemiology, military, sexually transmitted infection, repeat, surveillance
Introduction
In the field of sexually transmitted infections (STIs), it is important to study patients with repeat infections because these individuals, often called “repeaters” or “core group” members, represent a sustained reservoir of infection for STI transmission within a community and their sexual networks.1 According to a systematic review conducted by Fung et al.,2 the median of repeat gonorrhea infection rates is 7.0%. Although this review provides information on the burden of repeat gonorrhea infections in the U.S. civilian population, most of reviewed studies were conducted among high-risk populations, limiting the generalizability of the findings to other populations, for instance, the U.S. military population.
Historically, gonorrhea has been a common STI in the U.S. military (∼22 cases per 10,000 person-years during 2000–2012).3 However, little information is known on the burden of repeat gonorrhea infections among service members. To date, only one study has reported an estimate of the repeat gonorrhea infection rate among U.S. Armed Forces personnel.4 Because of the heterogeneous composition of the U.S. military population, the findings from such a report cannot be generalized across all branches of military service.5 In the Army, the largest branch of the U.S. Armed Forces, the percentage of African-American and female personnel, two groups commonly associated with higher STIs, are higher than in the Navy. In addition, compared to other military branches, the Army has the highest percentage of married personnel.6 In the U.S. military, treatment of uncomplicated gonococcal infections (ceftriaxone 250 mg in a single intramuscular dose plus azithromycin 1 g orally in a single dose) is consistent with the Centers for Disease Control and Prevention (CDC) STI treatment guidelines.7
Given that an understanding of the epidemiology of repeat infections is important to develop individual-level prevention strategies, such as sexual risk-reduction and retesting strategies for STI repeaters, the aim of this study was to determine the rate and determinants of repeat gonorrhea infections among male and female U.S. Army personnel.
Methods
Study design and population
Data for this study were obtained from the Defense Medical Surveillance System (DMSS); details about this military health surveillance system have been published elsewhere.2,5 Briefly, DMSS is the central repository of medical surveillance data for the U.S. Armed Forces. This large relational database contains information on personnel demographics, reportable diseases, deployments, and all inpatient and outpatient medical encounters for U.S. military active duty personnel throughout their careers.
In this population-based, case-series study, the population consisted of all male or female service members in the U.S. Army who had at least one gonorrhea infection reported in the DMSS between 1 January 2006 and 31 December 2012. The gonorrhea case definition was based on the ICD-9-CM codes 098.0x, 098.1x, 098.4x, or 098.8x in either the first or the second diagnostic position for any outpatient or inpatient encounter. Consequently, inpatient and outpatient medical records from all gonorrhea cases were reviewed to determine the occurrence of repeat infections based on the date of their first diagnosis (index date) until the case reached one of two right censoring events: (a) termination of military service or (b) the end of the study. We defined a repeat infection as one for which the diagnosis occurred at least 30 days from the previous diagnosis. This time interval is based on the fact that nucleic acid amplification tests can detect residual bacterial deoxyribonucleic acid for up to three weeks after STI therapy.8
Study variables
Sociodemographic variables were extracted from the DMSS. These variables were: race/ethnicity (white, African-American, Hispanic, and other), education (no high school, high school, and some college or higher), marital status (single, married, and other), rank (officers, lower enlisted ranks [E1–E4], and higher enlisted ranks [E5–E9]), and years of military service (≤1, 2–3, and ≥4). Years of military service is the time in service until the first gonorrhea infection.
Statistical analyses
We calculated the incidence density rate of repeat gonorrhea infection by dividing the number of new repeat infections by the sum of disease-free time that each study participant had during the study period. The log-rank test was used to compare time from the first to the second infection. To analyze the effect of covariates on the risk of repeat events, we applied the Prentice–Williams–Peterson model with gap-time.9 The Prentice–Williams–Peterson model assumes that the occurrence of the first infection influences the occurrence of a subsequent infection. Multivariate analysis for the risk of repeat gonorrhea infection was performed with adjustment for confounding factors such as age, race/ethnicity, and years of military service. All analyses were conducted stratified by gender. All p-values were two-sided and p-values less than 0.05 were considered statistically significant. Data analyses were conducted using Stata version 13.1 (Stata Corporation, College Station, TX, U.S.).
Results
During the study period, 4987 women and 12,615 men with a first gonorrhea infection were registered into the DMSS (Table 1). Among infected women, 89% had a high school diploma, 82% were junior enlisted personnel, 65% were single, 57% were African-Americans, 54% were service members aged 20–24 years, and 42% were service members with ≤1 year of service. Among men, most infections occurred among personnel with a high school diploma (89%), junior enlisted personnel (70%), single marital status (61%), African-Americans (56%), personnel aged 20–24 years (53%), and 22% among service members with ≤1 year of service.
Table 1.
Frequencies of the first incident gonorrhea infection by gender in the U.S. Army personnel.
| Women | Men | |
|---|---|---|
| Feature | Gonorrhea incident cases N (%) | Gonorrhea incident cases N (%) |
| All gonorrhea infections | 4987 (100) | 12,615 (100) |
| Age group (years) | ||
| 17–19 | 1111 (22) | 1129 (9) |
| 20–24 | 2681 (54) | 6742 (53) |
| ≥25 | 1195 (24) | 4744 (38) |
| Race/ethnicity | ||
| White | 1136 (23) | 3620 (29) |
| African-American | 2838 (57) | 7066 (56) |
| Hispanic | 525 (10) | 1153 (9) |
| Othera | 391(7) | 580 (5) |
| Education | ||
| High school | 4456 (89) | 11,192 (89) |
| College or higher | 439 (9) | 1089 (9) |
| Marital status | ||
| Single | 3257 (65) | 7674 (61) |
| Married | 1370 (27) | 4322 (34) |
| Otherb | 352 (7) | 612 (5) |
| Military rank | ||
| Junior enlisted | 4107 (82) | 8828 (70) |
| Senior enlisted | 771 (15) | 3416 (27) |
| Officers | 109 (2) | 371 (3) |
| Years of military service | ||
| ≤1 | 2073 (42) | 2791 (22) |
| 2–3 | 1342 (27) | 3801 (30) |
| ≥4 | 1572 (31) | 6023 (48) |
Other: American Indian/Alaskan Native, Asian/Pacific Islander, and others.
Other: Divorced, widowed, and others.
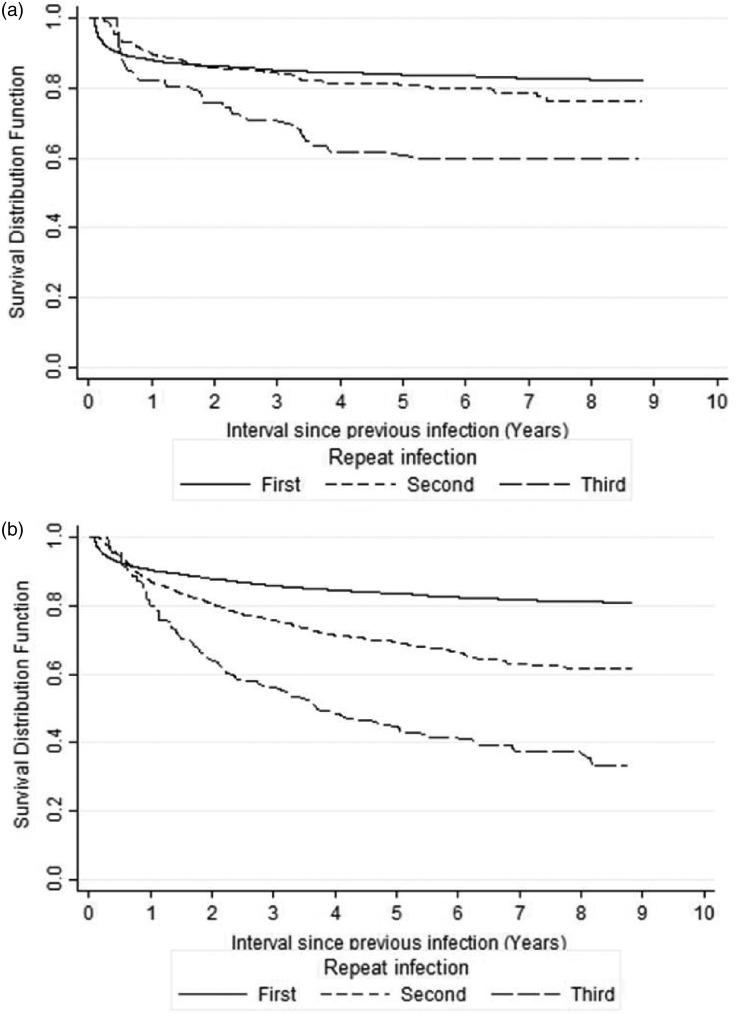
Among the 4987 women with a first gonorrhea infection, 627 (12.6%), 75 (1.5%), and 18 (0.4%) had one, two, and three or more repeat infections, respectively. Among the 12,615 men, 1401 (11.1%), 239 (1.9%), and 89 (0.7%), had one, two, and three or more repeat infections, respectively. The median time from the first to the second infection among men (5.5 months) was higher than that for women (3.0 months), but the survival distributions showed no statistically significantly difference (p-value = 0.237 by the log-rank test). Figure 1(a) and (b) shows that for both genders, the survival time (time to a new reinfection) decreases depending on the number of previous reinfections. That is to say, there is an increase in the hazard ratio with each subsequent repeat infection, in particular, among men with three or more repeat infections. To avoid strata with only a few subjects, only the survival function for the first three repeat infections are shown.
Figure 1.
Survival function estimates for the first three repeat infections by gender. (a) female and (b) male.
Among women, the rate of repeat gonorrhea infection was 44.5 (95% CI = 41.8, 47.9) per 1000 person-years (Table 2). Women with ≤1 year of service had the highest rate of repeat infection followed by junior enlisted personnel, African-Americans, and women aged 20–24 years. Among men, the rate of repeat gonorrhea infection was 48.9 (95% CI = 46.9, 51.0) per 1000 person-years, and this was higher among men aged 17–19 years, men with ≤1year of military service, African-Americans, junior enlisted personnel, and those who were single.
Table 2.
Incidence rates and adjusted hazard ratios for repeat gonorrhea infection by gender among U.S. Army personnel.
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Feature | Repeat infection ratea (95% CI) | Hazard ratiob | (95% CI) | Repeat infection ratea (95% CI) | Hazard ratiob | (95% CI) |
| All subjects | 44.5 (41.8, 47.9) | – | – | 48.9 (46.9, 51.0) | – | – |
| Age group (years) | ||||||
| 17–19 | 42.4 (36.9, 48.5) | 1.51 | (1.22, 1.86)c | 72.1 (63.8, 81.1) | 1.71 | (1.48, 1.97)c |
| 20–24 | 48.1 (43.9, 52.6) | 1.40 | (1.17, 1.69)c | 55.5 (52.5, 58.6) | 1.40 | (1.28, 1.54)c |
| ≥25 | 32.0 (27.1, 37.6) | Ref. | 35.9 (33.2, 38.7) | Ref. | ||
| Race/ethnicity | ||||||
| White | 41.0 (34.9, 47.9) | Ref. | 26.9 (24.1, 30.0) | Ref. | ||
| African-American | 49.5 (45.4, 53.8) | 1.26 | (1.06, 1.50)c | 62.6 (59.6, 65.7) | 2.17 | (1.92, 2.46)c |
| Hispanic | 37.9 (30.0, 47.2) | 0.96 | (0.73, 1.27) | 39.5 (33.7, 45.9) | 1.44 | (1.18, 1.77)c |
| Otherd | 32.9 (24.4, 43.4) | 0.86 | (0.62, 1.20) | 35.9 (33.7, 45.9) | 1.36 | (1.05, 1.76)c |
| Education | ||||||
| High school | 46.7 (43.5, 50.1) | 1.80 | (1.25, 2.58)c | 50.9 (48.7, 53.2) | 1.14 | (0.97, 1.36) |
| College or higher | 20.3 (14.2, 28.3) | Ref. | 33.1 (27.8, 39.1) | Ref. | ||
| Marital status | ||||||
| Single | 45.4 (41.7, 49.3) | 0.90 | (0.76, 1.06) | 54.1 (51.4, 57.0) | 1.07 | (0.97, 1.19) |
| Married | 44.5 (38.8, 50.8) | Ref. | 42.2 (39.0, 45.5) | Ref. | ||
| Othere | 38.0 (28.5, 49.6) | 1.06 | (0.78, 1.44) | 33.2 (26.2, 41.5) | 0.93 | (0.74, 1.16) |
| Military rank | ||||||
| Junior enlisted | 49.5 (46.0, 53.2) | 2.64 | (1.21, 5.74)c | 57.4 (54.7, 60.2) | 1.37 | (1.02, 1.84)c |
| Senior enlisted | 28.6 (23.3, 34.8) | 2.17 | (0.99, 4.74) | 34.1 (31.2, 37.3) | 1.13 | (0.85, 1.51) |
| Officers | 12.8 (4.7, 28.0) | Ref. | 27.7 (20.2, 37.1) | Ref. | ||
| Years of military service | ||||||
| ≤1 | 49.81 (44.9, 55.0) | 1.23 | (1.05, 1.45)c | 62.3 (57.4, 67.4) | 1.37 | (1.23, 1.52)c |
| 2–3 | 44.81 (39.0, 51.1) | 1.09 | (0.91, 1.32) | 53.3 (49.3, 57.5) | 1.21 | (1.09, 1.34)c |
| ≥4 | 38.42 (33.7, 43.6) | Ref. | 40.8 (38.2, 43.4) | Ref. | ||
Rate of infection per 1000 person-years.
Adjusted for age in years, race/ethnicity, and years of military service.
P-value < 0.05.
Other: American Indian/Alaskan Native, Asian/Pacific Islander, and other/unknown.
Other: Divorced, widowed, and others.
CI: confidence interval; Ref: reference for hazard ratio calculation.
Multivariate analysis reported that women aged 17–19 years and 20–24 years old had 1.51 and 1.40 times the risk of repeat infection compared to women aged ≥ 25 years (Table 2). Compared to white women, the risk of repeat infection of African-American women was significantly higher, and among single soldiers was lower, but this was not significant compared to women with married status. Compared to officers, the risk of repeat infection among junior enlisted military women was 2.64. In addition, women with ≤1 year of service had higher risk of repeat infection compared to women with ≥4 years of service.
Among males, the risk for repeat infection was 1.71 and 1.40 times higher for men aged 17–19 years and 20–24 years old when compared to men aged ≥ 25 years, respectively. African-American and Hispanic personnel had 2.17 and 1.44 times the risk of a repeat infection compared to white personnel, respectively. The risk of repeat infection among junior enlisted personnel was statistically significantly 1.37 times higher compared to officer personnel. Men with shorter service time (≤3 years) had an elevated risk compared to those with ≥4 years of service.
Discussion
We found a high rate of repeat gonorrhea infections among U.S. Army personnel, with a slightly higher rate among men compared to women. Although this gender difference has been noted in other studies conducted among STI clinic attendees in the U.S. civilian population,5 we expected to find a higher burden of repeat gonorrhea infections among women than men, since women in the military have two to three times higher gonorrhea infection rates than men since 2000.3 This small gender difference may be associated with the different signs and symptoms of gonorrhea. Gonorrhea usually produces obvious acute symptoms in men. In contrast, acute manifestations of gonorrhea in women are often vague or non-specific.10 Consequently, men may be more likely to seek medical care than women, increasing the detection rate of repeat gonorrhea infections among men.
It is also possible that early detection through gonorrhea screening reduces the number of repeat infections among women service members. In the U.S. military, gonorrhea screening is conducted consistent with the recommendations of the U.S. Preventive Services Task Force (USPSTF).11 The USPSTF recommends annual screening for N. gonorrhoeae in all sexually active women aged <25 years, and older women at increased risk for gonorrhea infection, for instance, women with a previous gonorrhea infection. Further studies are required to evaluate this hypothesis.
We also found a short median gonorrhea reinfection period between the first and the second infection for both men and women. A similar short median reinfection time to first reinfection (4.4 months) has been reported by Bernstein et al.12 However, most civilian studies indicate a longer median time period (9–13 months) for the first gonorrhea reinfection.13–15 Although the reasons for this short reinfection period in our population are worthy of further investigation, our results call for more targeted prevention initiatives including education, counseling, and retesting to prevent gonorrhea reinfections, for both genders. We recommend that all service members with gonorrhea infection should be re-screened 3–6 months after the initial infection to rule out recurrence. Currently, the CDC recommends that women with gonorrhea should be retested approximately 3 months after treatment.16
The finding that service members aged 17–19 years are at higher risk of acquiring a repeat gonorrhea infection is supported by the results of two systematic reviews, both of which found that the risk of repeat infection is strongly associated with younger age in the U.S. civilian population.2,17 Although no specific behavioral factor may predict reinfection for STIs, younger men and women tend to engage in more high-risk sexual practices, and are therefore more prone to repeat infections. In the U.S. military, evidence suggests that sexual behaviors commonly associated with STIs among young service members are inconsistent condom use, multiple sexual partners, and binge drinking.18 It is therefore plausible to assume that these risky behaviors are determinants of repeat gonorrhea infection among service members. Nevertheless, there is the possibility of reinfection from an untreated sexual partner.
Our multivariate analysis also revealed that junior and senior enlisted personnel had much higher risk for repeat gonorrhea infection than officers. These differences may be attributable to young (junior) military personnel being more likely to engage in high-risk sexual behaviors and substance abuse than officer personnel, increasing their risk of acquiring an STI. Based on the 2008 Department of Defense Survey of Health Related Behaviors among active duty military personnel, younger age was associated with a higher prevalence of sexual risk behaviors.17 The high risk of repeat infection among personnel with one year or less of military service compared to more than one year may be associated with the demographic composition of the groups. Over 55% of all service members with less than one year of military service were African-Americans, compared to 20% of members with four or more years of military service.
The finding that female African-American personnel had a lower rate of repeat gonorrhea infection compared to male African-American personnel is consistent with numerous studies from the U.S. civilian population.2 One explanation for this difference may be the fact that African-American women are more responsive to STI prevention efforts than men, mainly due to the presence of more severe medical complications for women (e.g. pelvic inflammatory disease). It is also possible that African-American women engage in less risky sexual behaviors than men, including less chance of exposure to an untreated sexual partner. However, the validity of these arguments should be examined in behavioral research to develop targeted STI control programs in the U.S. Army.
This study had limitations. First, the DMSS was designed to collect medical surveillance data for the U.S. Armed Forces. Therefore, we were unable to control for residual and unmeasured confounding by behavior patterns (e.g. sexual practices). Second, the socio-demographic variables were captured at the time of the first gonorrhea infection, and thus time-dependent variables may have affected our estimates. For example, marital status could have changed over time. Third, because the DMSS is a passive system that relies on reporting from military health centers, gonorrhea events may be unreported to an unknown extent. Finally, the DMSS captures only events that require inpatient or outpatient ambulatory medical care in a military facility, thus service members that sought care at private health facilities were not registered into the system. This might account for some missed gonorrhea infections in our analyses.
In summary, our study expands the literature of gonorrhea infection in the U.S. military and provides evidence-based findings for follow-up recommendations for patients and for further research. The high rate of repeat gonorrhea infections found among U.S. Army personnel indicates the need to develop targeted prevention initiatives including education, counseling, and retesting to prevent reinfections and to decrease the risk of long-term complications, for both women and men.
Acknowledgments
The authors would like to thank Dr. Angelia Cost, a senior managing epidemiologist at the Armed Forces Health Surveillance Branch, for DMSS database extraction and epidemiological support for this study, and to Mr. Sebastian-Santiago for technical assistance.
Authors’ contribution
CB conceived the study, undertook the analysis, and prepared the first draft. All authors contributed in the drafting of the manuscript and approved the final version. WS is currently an epidemiologist at the Jewish General Hospital, Montreal, Quebec, Canada.
Ethics approval
The study was approved by scientific review and institutional review boards at Lancaster University and the Walter Reed Army Institute of Research.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, the US Government, or any organization listed. Some authors are employees of the US government. This work was prepared as part of their official duties and, as such, there is no copyright to be transferred. This report was approved for publication by the US Army Public Health Command.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the US Armed Forces Health Surveillance Branch and its Global Emerging Infectious Surveillance section.
References
- 1.Scott LD, Baster K, Emmett L, et al. Incidence and reinfection rates of genital chlamydial infection among women aged 16-24 years attending general practice, family planning and genitourinary medicine clinics in England: a prospective cohort study by the Chlamydia Recall Study Advisory Group. Sex Transm Infect 2007; 83: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fung M, Scott KC, Kent CK, et al. Chlamydial and gonococcal reinfection among men: a systematic review of data to evaluate the need for retesting. Sex Transm Infect 2007; 83: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armed Forced Health Surveillance Center. Sexually transmitted infections, active component, U.S. Armed Forces, 2000–2012. MSMR 2013; 20: 5–10. [PubMed] [Google Scholar]
- 4.Owings AJ, Clark LL, Rohrbeck P. Incident and recurrent Chlamydia trachomatis and Neisseria gonorrhoeae infections, active component, U.S. Armed Forces, 2010–2014. MSMR 2016; 23: 20–28. [PubMed] [Google Scholar]
- 5.Enewold LR, Zhou J, Devesa SS, et al. Thyroid cancer incidence among active duty U.S. military personnel, 1990–2004. Cancer Epidemiol Biomarkers Prev 2011; 20: 2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2014 Demographic Reports. The U.S. Department of Defense, http://download.militaryonesource.mil/12038/MOS/Reports/2014-Demographics-Report.pdf (accessed 16 November 2016).
- 7.CDC. Updated to CDC’s sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR 2012; 61: 590–594. [PubMed] [Google Scholar]
- 8.Morré SA, Sillekens P, Jacobs MV, et al. RNA amplification by nucleic acid sequence-based amplification with an internal standard enables reliable detection of Chlamydia trachomatis in cervical scrapings and urine samples. J Clin Microbiol 1996; 34: 3108–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Therneau TM and Grambsch PM. Modeling survival data: extending the Cox model (Statistics for Biology and Health). New York: Springer-Verlag, 2010, pp. 439–477.
- 10.Germain A, Holmes KK, Piot P, et al. Reproductive tract infections: Global impact and priorities for women’s reproductive health. New York: Springer-Verlag, 1992, pp.7–35.
- 11.Walker CK, Sweet RL. Gonorrhea infection in women: prevalence, effects, screening, and management. Int J Womens Health 2011; 3: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein KT, Curriero FC, Jennings JM, et al. Defining core gonorrhea transmission utilizing spatial data. Am J Epidemiol 2004; 160: 51–58. [DOI] [PubMed] [Google Scholar]
- 13.De P, Singh AE, Wong T, et al. Predictors of gonorrhea reinfection in a cohort of sexually transmitted disease patients in Alberta, Canada, 1991–2003. Sex Transm Dis 2007; 34: 30–36. [DOI] [PubMed] [Google Scholar]
- 14.Mehta SD, Erbelding EJ, Zenilman JM, et al. Gonorrhoea reinfection in heterosexual STD clinic attendees: longitudinal analysis of risks for first reinfection. Sex Transm Infect 2003; 79: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissinger PJ, Reilly K, Taylor SN, et al. Early repeat Chlamydia trachomatis and Neisseria gonorrhoeae infections among heterosexual men. Sex Transm Dis 2009; 36: 498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6403a1.htm (2015, accessed 5 October 2016).
- 17.Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis 2009; 36: 478–489. [DOI] [PubMed] [Google Scholar]
- 18.Stahlman S, Javanbakht M, Cochran S, et al. Self-reported sexually transmitted infections and sexual risk behaviors in the U.S. Military: how sex influences risk. Sex Transm Dis 2014; 41: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]