Abstract
Purpose
To systematically evaluate the efficacy and safety of Cytokine-induced killer cells/dendritic cells-cytokine induced killer cells (CIK/DC-CIK) immunotherapy in treating advanced colorectal cancer (CRC) patients.
Results
29 trials including 2,610 CRC patients were evolved. Compared with chemotherapy alone, the combination of chemotherapy with CIK/DC-CIK immunotherapy significantly prolonged the overall survival rate (OS) and disease-free survival rate (DFS) (1–5 year OS, P < 0.01; 1-, 2-, 3- and 5-year DFS, P < 0.01). The combined therapy also improved patients’ overall response, disease control rate and life quality (P < 0.05). After immunotherapy, lymphocyte subsets percentages of CD3+, CD3−CD56+, CD3+CD56+ and CD16+CD56+ (P < 0.01) and cytokines levels of IL-2 and IFN-γ (P < 0.05) were increased, while CD4+, CD8+ and CD4+CD25+ and IL-6 and TNF-α did not show significant change (P > 0.05).
Materials and Methods
Clinical trials reporting response or safety of CIK/DC-CIK immunotherapy treating advanced CRC patients and published before September 2016 were searched in Cochrane Library, EMBASE, PubMed, Wanfang and CNKI database. Research quality and heterogeneity were evaluated before analysis. Pooled analyses were performed using random or fixed-effect models.
Conclusions
The combination of CIK/DC-CIK immunotherapy and chemotherapy prolong CRC patients’ survival time, enhanced patients’ immune function and alleviates the adverse effects caused by chemotherapy.
Keywords: cytokine-induced killer cells, dendritic cells, colorectal cancer, immunotherapy, meta-analysis
INTRODUCTION
Colorectal cancer (CRC) is currently the third common malignant tumor [1, 2]. In recent years, CRC incidence in newly developed or economically transitional countries have been significantly raised, and the 5-year survival rate of IV stage CRC patients is lower than 10% [2]. Surgery, radiotherapy and chemotherapy are the most widely used therapeutic methods for CRC [2], but their curative effects on CRC patients were poor. Researchers found these methods were not able to thoroughly remove small lesions and metastatic cells, which raises the probability of cancer recurrence [3]. Application of these treatments is also limited by drug resistance and adverse effects [3, 4]. Therefore, a more effective and safer therapeutic method is urgently required.
Immunotherapy is an emerging approach for CRC treatment, especially adoptive cell therapy by cytokine-induced killer cells (CIK) [5], tumor-infiltrating lymphocytes [6] and other immune cells [7, 8]. CIK cells, which consist primarily of the CD3+CD56+ subset, are induced by IFN-γ, anti-CD3 monoclonal antibodies and IL-2 in vitro [4]. Compared with other immune cells, CIK cells have greater proliferative capability, broader anti-tumor spectrum and stronger anti-tumor ability [3, 9]. CIK cells’ tumoricidal ability relies on inducing tumor cell apoptosis through direct contact and secretion of cytokines such as IL-2, TNF-a and IFN-γ [10], and the cytotoxicity of CIK cells is not affected by immune inhibitors such as CsA and FK506 [11]. Dendritic cells (DC) are the most potent antigen-presenting cells [12]. Studies have shown that DC play an important role in CIK activation, proliferation, phenotype expression and cytokine secretion by direct contact and secreted IL-12, IFN-γ, and TNF-a [13–16]. Co-culture of DC and CIK showed increased levels of cytokines such as IL-2, IFN-γ, TNF-a, and IL-12, but downregulated negative regulatory factors including TGF-β and IL-10. Upon DC and CIK co-culture, the proportion of CD3+CD56+ cells, which are the main effector cells enhancing CIK cytotoxicity, were increased; whereas CD4+CD25+ regulatory T cells, which inhibit CIK anti-tumor activity, where decreased [13–15]. On the other hand, CIK cells promote DC maturation and expression of co-stimulatory molecules such as CD40, CD80 and CD86 [3, 13], and the combination of DC and CIK provides a remarkably increased cytotoxic activity [17].
Immunotherapy using CIK/DC-CIK was widely reported to be effective in treating various malignancies, without causing serious adverse reactions [18–28]. In CRC treatment, CIK/DC-CIK immunotherapy combined with chemotherapy had better therapeutic effects than treated by chemotherapy alone (Supplementary Table 1) [5, 18, 29–55]. To investigate the efficacy and safety of CIK/DC-CIK immunotherapy for CRC and thus provide scientific evidence for future clinical trials, we performed a systematic review and meta-analysis of published literature on recent CIK/DC-CIK and chemotherapy combined clinical trials for CRC. This review evaluates the patient survival, clinical responses, safety, and immune functions in this combined therapeutic method.
RESULTS
Search results
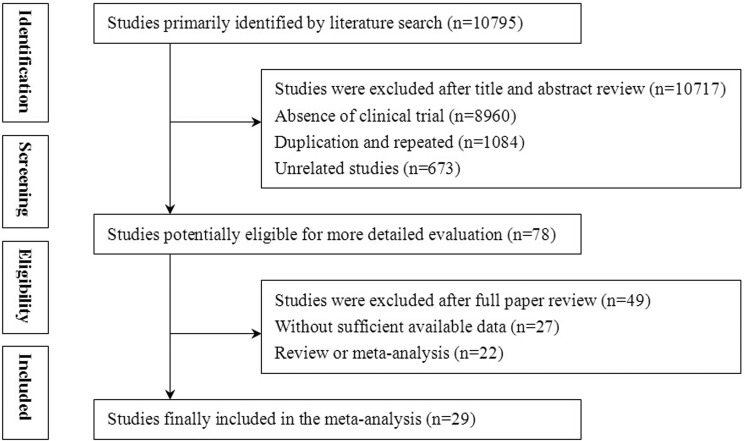
In our initial retrieve, 10,795 articles were identified, but 10,717 were excluded for lacking of clinical trial (n = 8,960), duplication (n = 1084), and unrelated studies (n = 673). After a detailed assessment of full texts, 27 papers with insufficient data and 22 reviews or meta-analyses were excluded. Finally, 29 trials that included a total of 2,610 patients were eligible for inclusion in this meta-analysis (Figure 1).
Figure 1. Flow diagram of the selection process.
Patient characteristics
All of the involved trials were conducted in mainland China. In total, 1300 patients were treated by CIK/DC-CIK in combination with chemotherapy, while 1310 patients were treatment by chemotherapy alone. Detailed clinical information on the trials is presented in Supplementary Table 1. DC and CIK cells used in the 29 trials were all obtained from autologous peripheral blood. DC-CIK immunotherapy was applied in 20 trials, whereas only CIK cells were used in the other 9 trials. Tumor size and injection modes were not analyzed in this article because of insufficient data.
Quality assessment
Bias risk assessment is shown in Figure 2A and 2B. According to the assessment, quality of involved studies was moderate or high. 21 studies had low bias risk, and the remaining 8 studies lacked a clear description of randomization process. The allocation, performance and detection risks were low. 2 studies were regarded as an unclear risk due to the absence of follow-up. 12 trials were considered as unclear risk owing to selective reporting, while the other 8 studies were considered as high risk because of the lack of primary outcome data.
Figure 2.
(A) Risk of bias summary: review of authors’ judgments about each risk of bias item for included studies. (B) Risk of bias graph: review of authors’ judgments about each risk of bias item presented as percentages across all included studies.
Prognosis evaluation
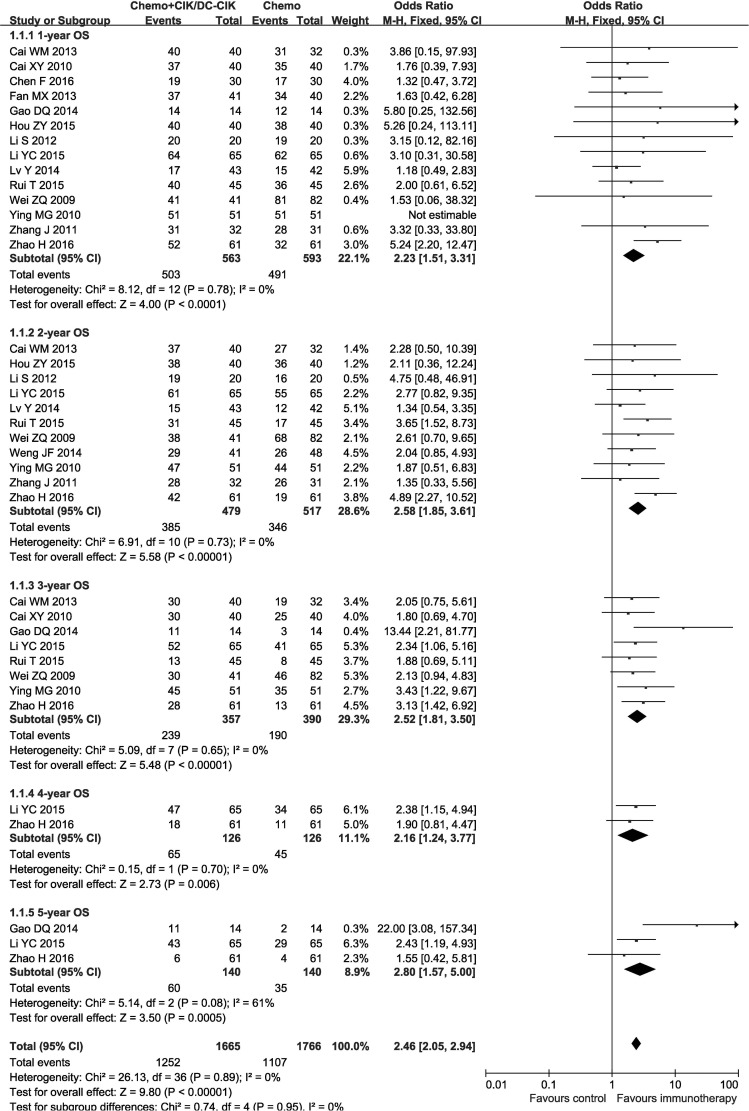
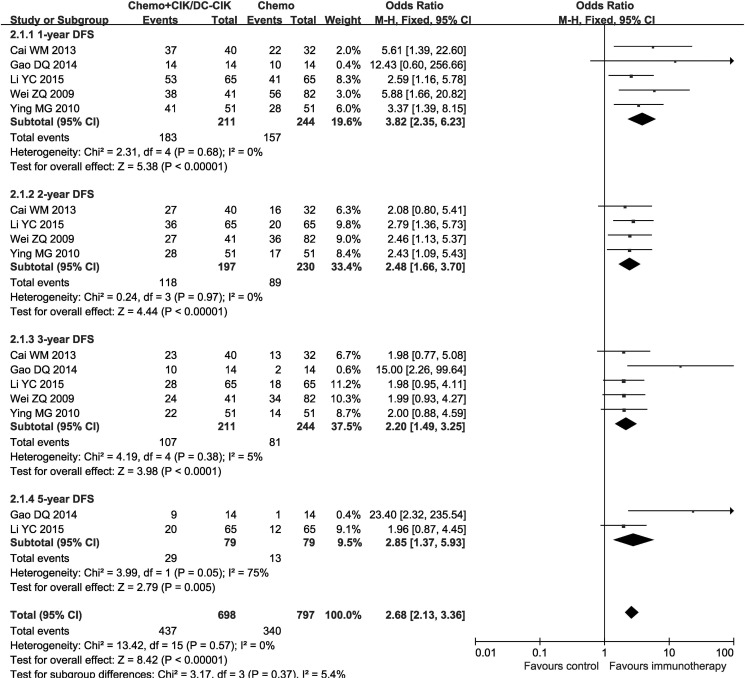
In the 29 studies, patients treated by combined therapy (combined group) have higher overall survival rate (OS) and disease free survival rate (DFS) than those treated by chemotherapy alone (chemo-alone group) (Figures 3 and 4, 1-year OS: OR = 2.23, CI = 1.51–3.31, P < 0.0001; 2-year OS: OR = 2.58, CI = 1.85–3.61, P< 0.00001; 3-year OS: OR = 2.52, CI = 1.81–3.50, P < 0.00001; 4-year OS: OR = 2.16, CI = 1.24–3.77, P = 0.006; 5-year OS: OR = 2.80, CI = 1.57–5.00, P = 0.0005; 1-year DFS: OR = 3.82, CI = 2.35–6.23, P < 0.00001; 2-year DFS: OR = 2.48, CI = 1.66–3.70, P < 0.00001; 3-year DFS: OR = 2.20, CI = 1.49–3.25, P < 0.0001; 5-year DFS: OR = 2.85, CI = 1.37–5.93, P = 0.005). Fixed-effects model were applied in this analysis considering slightly significant heterogeneity.
Figure 3. Forest plot of the comparison of overall survival (OS).
CI, confidence interval; Chemo, chemotherapy; CIK/DC-CIK, CIK/DC-CIK immunotherapy. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Figure 4. Forest plot of the comparison of disease free survival (DFS).
CI, confidence interval; Chemo, chemotherapy; CIK/DC-CIK, CIK/DC-CIK immunotherapy. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Efficacy assessments
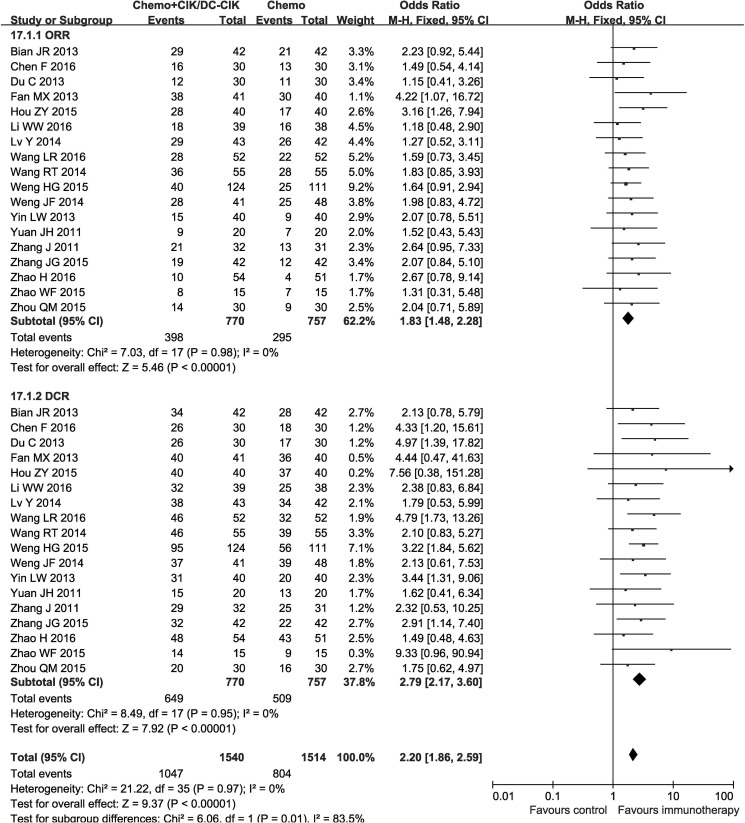
Pooled analysis indicated patients in combined group had significantly higher complete response rates (CR), partial response rates (PR), overall response rate (ORR) and disease control rate (DCR) (CR: OR = 1.96, CI = 1.22−3.15, P = 0.005; PR: OR = 1.53, CI = 1.22−1.92, P = 0.0003; ORR: OR = 1.83, CI = 1.48−2.28, P < 0.00001; DCR: OR = 2.79, CI = 2.17−3.60, P < 0.00001) and significantly lower progressive disease rates (PD) (OR = 0.36, CI = 0.27−0.47, P < 0.00001), whereas the stable disease rates (SD) did not show significant difference from chemo-alone group (OR = 1.25, CI = 0.98−1.59, P = 0.07). Fixed-effect models were used to analyze the OR rate because of low heterogeneity (Figure 5, Table 1 and Supplementary Figure 1).
Figure 5. Forest plot of the comparison of overall response rate (ORR) and disease control rate (DCR).
CI, confidence interval; Chemo, chemotherapy; CIK/DC-CIK, CIK/DC-CIK immunotherapy. The fixed-effects meta-analysis model (Mantel– Haenszel method) was used.
Table 1. Comparison of CR, PR, SD, PD, ORR and DCR between the chemotherapy+CIK/DC-CIK and chemotherapy alone groups.
| Parameter | Chemo+CIK/DC-CIK | Chemo | Analysis method | Heterogeneity | Odds Ratio (OR) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|
| No. patients (n) | No. patients (n) | I2 (%) | P-value | |||||
| CR | 689 | 669 | Fixed | 0 | 0.94 | 1.96 | 1.22 to 3.15 | 0.005 |
| PR | 689 | 669 | Fixed | 0 | 0.99 | 1.53 | 1.22 to 1.92 | 0.0003 |
| SD | 689 | 669 | Fixed | 42 | 0.04 | 1.25 | 0.98 to 1.59 | 0.07 |
| PD | 689 | 669 | Fixed | 0 | 0.92 | 0.36 | 0.27 to 0.47 | < 0.00001 |
| ORR | 770 | 757 | Fixed | 0 | 0.98 | 1.83 | 1.48 to 2.28 | < 0.00001 |
| DCR | 770 | 757 | Fixed | 0 | 0.95 | 2.79 | 2.17 to 3.60 | < 0.00001 |
CR: complete response rates; PR: partial response rates; SD: stable disease rates; PD: progressive disease rates; ORR: overall response rate; DCR: disease control rate. Chemo: chemotherapy.
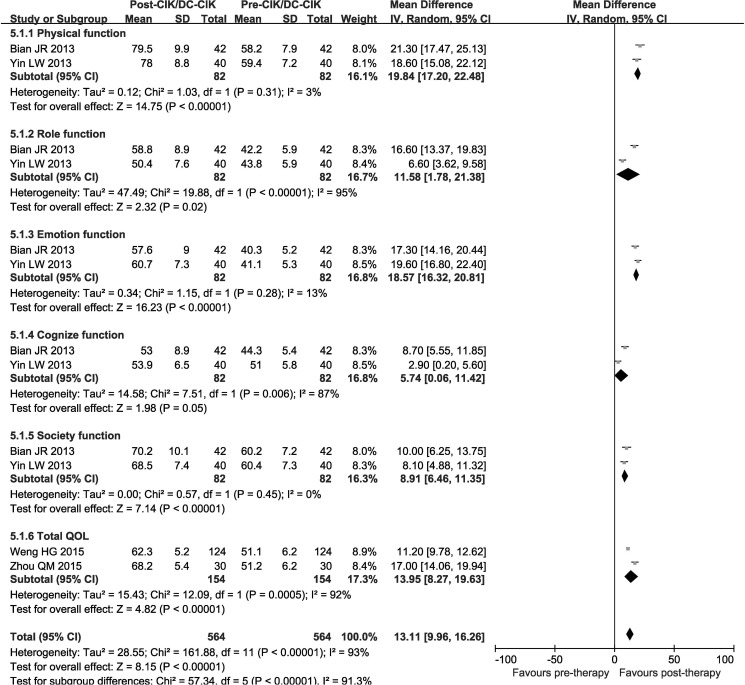
Quality of life (QOL) assessment
QOL was evaluated before and after therapy and analyzed with random-effect models. QOL assessment shows that in combined group, patients after CIK/DC-CIK immunotherapy showed significantly improved physical function (OR = 19.84, CI = 17.20−22.48, P < 0.00001), emotion function (OR = 18.57, CI = 16.32−20.81, P < 0.00001), cognize function (OR = 5.74, CI = 0.06−11.42, P = 0.05), society function (OR = 8.91, CI = 6.46−11.35, P < 0.00001) and but role function (OR = 11.58, CI = 1.78−21.38, P = 0.02). Taken together, the overall QOL was significantly improved (Total QOL: OR = 13.95, CI = 8.27−19.63, P < 0.00001) (Figure 6).
Figure 6. Forest plot of the comparison of QQL.
CI, confidence interval; Chemo, chemotherapy; CIK/DC-CIK, CIK/DC-CIK immunotherapy. The random effects meta-analysis model (Mantel–Haenszel method) was used.
Immune function evaluation
The immune status of patients was examined before and after treatment. After CIK/DC-CIK treatment, percentages of CD3+, CD3−CD56+, CD3+CD56+ and CD16+CD56+ cells and CD4+/CD8+ ratio were significantly increased (Table 2 and Supplementary Figure 2, CD3+: OR = 5.46, CI = 3.18−7.74, P < 0.00001; CD3−CD56+: OR = 4.08, CI = 0.82−7.34, P = 0.01; CD3+CD56+: OR = 2.16, CI = 0.15−4.16, P = 0.03; CD4+/CD8+: OR = 0.39, CI = 0.24−0.53, P < 0.00001; CD16+CD56+: OR = 5.62, CI = 2.78−8.47, P = 0.0001), whereas proportions of CD4+, CD8+ and CD4+CD25+ were not significantly changed (CD4+: OR = 0.40, CI = −4.81−5.61, P = 0.88; CD8+: OR = 0.07, CI = −2.98−3.12, P = 0.97; CD4+CD25+: OR = −0.70, CI = −1.52–0.12, P = 0.10). Levels of IFN-γ and IL-2 were significantly increased after CIK/DC-CIK immunotherapy (Table 2 and Supplementary Figure 3, IFN-γ: OR = 5.28, CI = 2.21−8.36, P = 0.0008; IL-2: OR = 1.60, CI = 0.26−2.95, P = 0.02), while no obvious change was found in IL-6 and TNF-α expression (IL-6: OR = −1.30, CI = −2.85−0.26, P = 0.10; TNF-α: OR = 1.21, CI = −1.06−3.48, P = 0.30).
Table 2. Comparison of lymphocyte subsets and cytokines before CIK/DC-CIK treatment and after CIK/DC-CIK therapy.
| Parameter | CIK/DC-CIK after treatment (No.) | CIK/DC-CIK before treatment (No.) | Analysis method | Heterogeneity | Odds Ratio (OR) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | |||||||
| CD3+ | 860 | 860 | Random | 94 | < 0.00001 | 5.46 | 3.18 to 7.74 | <0.00001 |
| CD4+ | 860 | 860 | Random | 99 | < 0.00001 | 0.40 | −4.81 to 5.61 | 0.88 |
| CD8+ | 808 | 808 | Random | 98 | < 0.00001 | 0.07 | −2.98 to 3.12 | 0.97 |
| CD4+/CD8+ | 391 | 391 | Random | 87 | < 0.00001 | 0.39 | 0.24 to 0.53 | <0.00001 |
| CD3−CD56+ | 441 | 441 | Random | 95 | < 0.00001 | 4.08 | 0.82 to 7.34 | 0.01 |
| CD3+CD56+ | 429 | 429 | Random | 99 | < 0.00001 | 2.16 | 0.15 to 4.16 | 0.03 |
| CD4+CD25+ | 393 | 393 | Random | 81 | 0.0001 | −0.70 | −1.52 to 0.12 | 0.10 |
| CD16+CD56+ | 204 | 204 | Random | 97 | < 0.00001 | 5.62 | 2.78 to 8.47 | 0.0001 |
| IFN-γ | 429 | 429 | Random | 99 | < 0.00001 | 5.28 | 2.21 to 8.36 | 0.0008 |
| IL-2 | 429 | 429 | Random | 92 | < 0.00001 | 1.60 | 0.26 to 2.95 | 0.02 |
| IL-6 | 92 | 92 | Random | 93 | < 0.00001 | −1.30 | −2.85 to 0.26 | 0.10 |
| TNF-α | 337 | 337 | Random | 97 | < 0.00001 | 1.21 | −1.06 to 3.48 | 0.30 |
Adverse events assessment
No serious adverse events or death occurrence was reported in patients receiving CIK/DC-CIK immunotherapy. Compared with patients in chemo-alone group, those in combined group suffered milder leucopenia, thrombocytopenia, nausea and vomiting, liver dysfunction, myelosuppression, peripheral neurotoxicity and gastrointestinal adverse reactions, except the effects on anemia [Table 3 and Supplementary Figures 4–9, leucopenia: OR = 0.51, CI = 0.32–0.82, P = 0.005 (leucopenia I: OR = 1.02, CI = 0.67–1.56, P = 0.92; leucopenia II: OR = 0.81, CI = 0.42–1.56, P = 0.53; leucopenia III: OR = 0.40, CI = 0.21–0.75, P = 0.004; leucopenia IV: OR = 0.55, CI = 0.26–1.19, P = 0.13); thrombocytopenia: OR = 0.54, CI = 0.29–0.99, P = 0.05 (thrombocytopenia I: OR = 0.69, CI = 0.42–1.13, P = 0.14; thrombocytopenia II: OR = 0.76, CI = 0.35–1.69, P = 0.50; thrombocytopenia III: OR = 0.24, CI = 0.10–0.54, P = 0.0006; thrombocytopenia IV: OR = 0.90, CI= 0.22–3.63, P = 0.88); nausea and vomiting: OR = 0.55, CI = 0.31–0.98, P = 0.04 (nausea and vomiting I: OR = 1.06, CI = 0.49–2.29, P = 0.88; nausea and vomiting II: OR = 0.61, CI = 0.42–0.89, P = 0.01; nausea and vomiting III: OR = 0.48, CI = 0.21–1.08, P = 0.08; nausea and vomiting IV: OR = 0.45, CI = 0.08–2.45, P = 0.35); liver dysfunction: OR = 0.55, CI = 0.38–0.80, P = 0.002 (liver dysfunction I: OR = 0.59, CI = 0.33–1.06, P = 0.08; liver dysfunction II: OR = 0.52, CI = 0.31–0.88, P = 0.01; liver dysfunction III: OR = 0.90, CI = 0.26–3.14, P = 0.87; liver dysfunction IV: OR = 0.53, CI = 0.13–2.26, P = 0.39); myelosuppression: OR = 0.21, CI = 0.11–0.39, P < 0.00001; peripheral neurotoxicity: OR = 0.31, CI = 0.18–0.55, P < 0.00001; gastrointestinal adverse reaction: OR = 0.26, CI = 0.15–0.45, P < 0.00001; anemia: OR = 0.52, CI = 0.26–1.05, P = 0.07 (anemia I: OR = 0.52, CI = 0.27–1.00, P = 0.05; anemia II: OR = 0.71, CI = 0.44–1.14, P = 0.15; anemia III: OR = 0.60, CI = 0.22–1.63, P = 0.32; anemia IV: OR = 0.45, CI = 0.13–1.51, P = 0.19)].
Table 3. Comparison of adverse events between the chemotherapy+CIK/DC-CIK and chemotherapy alone groups.
| Adverse events | Chemo+CIK/DC-CIK | Chemo | Analysis method | Heterogeneity | Odds Ratio (OR) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|
| No. patients (n) | No. patients (n) | I2 (%) | P-value | |||||
| Leucopenia | 412 | 384 | Random | 38 | 0.15 | 0.51 | 0.32 to 0.82 | 0.005 |
| Leucopenia I | 339 | 312 | Random | 0 | 0.48 | 1.02 | 0.67 to 1.56 | 0.92 |
| Leucopenia II | 339 | 312 | Random | 63 | 0.05 | 0.81 | 0.42 to 1.56 | 0.53 |
| Leucopenia III | 339 | 312 | Random | 23 | 0.28 | 0.40 | 0.21 to 0.75 | 0.004 |
| Leucopenia IV | 339 | 312 | Random | 0 | 1.00 | 0.55 | 0.26 to 1.19 | 0.13 |
| Anemia | 382 | 354 | Random | 75 | 0.003 | 0.52 | 0.26 to 1.05 | 0.07 |
| Anemia I | 339 | 312 | Random | 59 | 0.06 | 0.52 | 0.27 to 1.00 | 0.05 |
| Anemia II | 339 | 312 | Random | 0 | 0.55 | 0.71 | 0.44 to 1.14 | 0.15 |
| Anemia III | 339 | 312 | Random | 0 | 0.53 | 0.60 | 0.22 to 1.63 | 0.32 |
| Anemia IV | 339 | 312 | Random | 0 | 0.68 | 0.45 | 0.13 to 1.51 | 0.19 |
| Thrombocytopenia | 412 | 384 | Random | 63 | 0.02 | 0.54 | 0.29 to 0.99 | 0.05 |
| Thrombocytopenia I | 339 | 312 | Random | 23 | 0.27 | 0.69 | 0.42 to 1.13 | 0.14 |
| Thrombocytopenia II | 339 | 312 | Random | 55 | 0.08 | 0.76 | 0.35 to 1.69 | 0.50 |
| Thrombocytopenia III | 339 | 312 | Random | 0 | 0.96 | 0.24 | 0.10 to 0.54 | 0.0006 |
| Thrombocytopenia IV | 339 | 312 | Random | 0 | 0.88 | 0.90 | 0.22 to 3.63 | 0.88 |
| Nausea, vomiting | 427 | 399 | Random | 65 | 0.009 | 0.55 | 0.31 to 0.98 | 0.04 |
| Nausea, vomiting I | 339 | 312 | Random | 67 | 0.03 | 1.06 | 0.49 to 2.29 | 0.88 |
| Nausea, vomiting II | 339 | 312 | Random | 0 | 0.86 | 0.61 | 0.42 to 0.89 | 0.01 |
| Nausea, vomiting III | 339 | 312 | Random | 0 | 0.57 | 0.48 | 0.21 to 1.08 | 0.08 |
| Nausea, vomiting IV | 339 | 312 | Random | 0 | 1.00 | 0.45 | 0.08 to 2.45 | 0.35 |
| Liver dysfunction | 401 | 374 | Random | 0 | 0.44 | 0.55 | 0.38 to 0.80 | 0.002 |
| Liver dysfunction I | 298 | 272 | Fixed | 0 | 0.96 | 0.59 | 0.33 to 1.06 | 0.08 |
| Liver dysfunction II | 298 | 272 | Fixed | 0 | 0.57 | 0.52 | 0.31 to 0.88 | 0.01 |
| Liver dysfunction III | 298 | 272 | Fixed | 36 | 0.21 | 0.90 | 0.26 to 3.14 | 0.87 |
| Liver dysfunction IV | 298 | 272 | Fixed | 0 | 0.47 | 0.53 | 0.13 to 2.26 | 0.39 |
| Myelosuppression | 159 | 159 | Random | 0 | 0.90 | 0.21 | 0.11 to 0.39 | < 0.00001 |
| Peripheral neurotoxicity | 196 | 195 | Random | 0 | 0.42 | 0.31 | 0.18 to 0.55 | < 0.00001 |
| Gastrointestinal AE | 153 | 152 | Random | 0 | 0.53 | 0.26 | 0.15 to 0.45 | < 0.00001 |
AE: adverse reaction.
Sensitivity analysis
Firstly, a sensitivity analysis was conducted and 8 trials [18, 30, 32, 38, 44, 49, 52, 55] were excluded because Folfox/Xelox/Folfiri regimen was not applied to all patients. The results of this analysis were similar to those obtained from the overall analysis of the pooled trials.
Secondly, CRC patients were treated by DC-CIK immunotherapy in 20 trials and CIK alone in the other 9 trials. Studies were grouped according to different immunotherapy strategies (CIK or DC-CIK), and pooled results were compared. The comparison showed both CIK and DC-CIK were effective in treating CRC, and no obvious difference was observed in most pooled analyses. (Tables 4 and 5, Supplementary Figures 10–13).
Table 4. Meta-analysis of OS, DFS, ORR and DCR in Chemo-DC-CIK and Chemo-CIK subgroups.
| Immunotherapy type (subgroup) | Parameters | Chemo+CIK/DC-CIK | Chemo | Analysis method | Heterogeneity | Odds Ratio (OR) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| No. patients (n) | No. patients (n) | I2 (%) | P-value | ||||||
| DC-CIK | 1-year OS | 273 | 313 | Fixed | 0 | 0.94 | 2.02 | 1.05 to 3.89 | 0.04 |
| 2-year OS | 270 | 317 | Fixed | 0 | 0.90 | 2.43 | 1.55 to 3.83 | 0.0001 | |
| 3-year OS | 151 | 192 | Fixed | 26 | 0.25 | 2.73 | 1.64 to 4.55 | 0.0001 | |
| 1-year DFS | 106 | 147 | Fixed | 0 | 0.60 | 4.54 | 2.26 to 9.11 | < 0.0001 | |
| 2-year DFS | 92 | 133 | Fixed | 0 | 0.98 | 2.45 | 1.40 to 4.28 | 0.002 | |
| 3-year DFS | 106 | 147 | Fixed | 50 | 0.13 | 2.41 | 1.43 to 4.08 | 0.001 | |
| ORR | 547 | 539 | Fixed | 0 | 0.99 | 1.85 | 1.44 to 2.37 | < 0.00001 | |
| DCR | 547 | 539 | Fixed | 0 | 0.94 | 2.94 | 2.20 to 3.93 | < 0.00001 | |
| CIK | 1-year OS | 290 | 280 | Fixed | 20 | 0.29 | 2.36 | 1.44 to 3.86 | 0.0006 |
| 2-year OS | 209 | 200 | Fixed | 35 | 0.21 | 2.78 | 1.70 to 4.54 | < 0.0001 | |
| 3-year OS | 206 | 198 | Fixed | 0 | 0.83 | 2.37 | 1.53 to 3.65 | 0.0001 | |
| 1-year DFS | 105 | 97 | Fixed | 0 | 0.35 | 3.17 | 1.59 to 6.33 | 0.001 | |
| 2-year DFS | 105 | 97 | Fixed | 0 | 0.63 | 2.51 | 1.41 to 4.46 | 0.002 | |
| 3-year DFS | 105 | 97 | Fixed | 0 | 1.00 | 1.98 | 1.11 to 3.52 | 0.02 | |
| ORR | 223 | 218 | Fixed | 0 | 0.53 | 1.79 | 1.16 to 2.78 | 0.009 | |
| DCR | 223 | 218 | Fixed | 0 | 0.64 | 2.33 | 1.37 to 3.97 | 0.002 | |
Table 5. Meta-analysis of immunophenotype in Chemo-DC-CIK and Chemo-CIK subgroups.
| Immunotherapy type (subgroup) | Parameters | CIK/DC-CIK after treatment (No.) | CIK/DC-CIK Before treatment (No.) | Analysis method | Heterogeneity | Odds Ratio (OR) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | ||||||||
| DC-CIK | CD3+ | 661 | 661 | Random | 80 | < 0.00001 | 4.33 | 2.15 to 6.50 | < 0.0001 |
| CD4+ | 661 | 661 | Random | 99 | < 0.00001 | −0.48 | −8.03 to 7.07 | 0.90 | |
| CD8+ | 609 | 609 | Random | 93 | < 0.00001 | 0.27 | −2.08 to 2.61 | 0.82 | |
| CD4+/CD8+ | 252 | 252 | Random | 91 | < 0.00001 | 0.34 | 0.19 to 0.50 | < 0.0001 | |
| CD3+CD56+ | 369 | 369 | Random | 99 | < 0.00001 | 1.16 | −1.05 to 3.38 | 0.30 | |
| CD16+CD56+ | 108 | 108 | Random | 55 | 0.11 | 6.95 | 5.67 to 8.23 | <0.00001 | |
| CIK | CD3+ | 199 | 199 | Random | 97 | < 0.00001 | 7.85 | 3.54 to 12.17 | 0.0004 |
| CD4+ | 199 | 199 | Random | 95 | < 0.00001 | 3.62 | 0.10 to 7.13 | 0.04 | |
| CD8+ | 199 | 199 | Random | 99 | < 0.00001 | −0.47 | −6.82 to 5.87 | 0.88 | |
| CD4+/CD8+ | 139 | 139 | Random | 3 | 0.36 | 0.70 | 0.36 to 1.03 | < 0.0001 | |
| CD3+CD56+ | 60 | 60 | Random | 0 | 0.36 | 4.66 | 3.86 to 5.46 | < 0.00001 | |
| CD16+CD56+ | 96 | 96 | Random | 99 | < 0.00001 | 3.26 | −2.72 to 9.23 | 0.29 | |
Publication bias
Funnel plots drawn for the studies on the OS, ORR, DCR and lymphocyte subsets percentages of CD3+, CD4+, CD8+ and CD4+/CD8+ were symmetrical in general, indicating passably controlled publication bias (Supplementary Figure 14). In addition, publication bias was further analyzed by both Begg's and Egger's regression asymmetry tests (Table 6). Bias was observed in 3-year OS (Egger: 0.016; Begg: 0.386) and percentages of CD8+ subsets (Egger: 0.008; Begg: 0.284), but not in other pooled-analyses. We further evaluated whether the observed publication bias significantly influenced the pooled risk using trim and filled method. The adjusted OR indicated same trend with the result of the primary analysis (3-year OS: before: P < 0.00001, after: P < 0.0001; CD8+: before: P = 0.966, after: P = 0.966). This publication bias analysis confirmed the reliability of our primary conclusions, except those based on few numbers of trials.
Table 6. Publication bias on OS, ORR, DCR and lymphocyte subsets (CD3+, CD4+, CD8+ and CD4+/CD8+).
| Publication Bias | 1-year OS | 2-year OS | 3-year OS | ORR | DCR | CD3 | CD4 | CD8 | CD4/CD8 |
|---|---|---|---|---|---|---|---|---|---|
| Begg | 0.100 | 0.640 | 0.386 | 0.289 | 0.256 | 0.880 | 0.028 | 0.284 | 0.474 |
| Egger | 0.630 | 0.390 | 0.016 | 0.198 | 0.195 | 0.554 | 0.282 | 0.008 | 0.679 |
Parameters discussed in over 8 papers were conducted bias analyses.
DISCUSSION
In recent years, there have been several clinical trials using CIK/DC-CIK immunotherapy to treat CRC [5, 18]. However, the exact therapeutic effects remain unclear because of sample sizes variability and unstandardized clinical trial protocols. To address this problem, we performed an extensive online search followed by rigorous data analysis. We studied 29 clinical trials including 1300 CRC patients who received CIK/DC-CIK immunotherapy. Our meta-analysis revealed that the combination of CIK/DC-CIK immunotherapy and chemotherapy for CRC could improve OS, ORR, DCR, patients’ life quality and immune function, and alleviates the adverse events caused by chemotherapy.
This meta-analysis confirmed the safety of CIK/DC-CIK immunotherapy for CRC, and its side effects were tolerated by all patients without causing serious adverse events or death after therapy. The adverse events caused by chemotherapy, including leucopenia, thrombocytopenia, nausea and vomiting, liver dysfunction, myelosuppression, peripheral neurotoxicity and gastrointestinal adverse reaction were obviously alleviated by CIK/DC-CIK immunotherapy (P < 0.05). The combination therapy also improved the quality of life of patients reflected by enhanced physical, role, emotion, cognize and society function (P < 0.05). Furthermore, CIK/DC-CIK immunotherapy also enhanced the efficiency of chemotherapy for CRC. Compare to patients treated by chemotherapy alone, patients with combined therapy showed markedly increased OS, DFS, ORR and DCR.
The immunosuppressed status of cancer patients has been reported previously [3]. Therefore, immune system reconstruction is one of the key factors to effectively treat malignant tumors. Our analysis showed significantly increased percentages of CD3+, CD4+/CD8+, CD3−CD56+, CD3+CD56+ and CD16+CD56+ T cells upon CIK/DC-CIK treatment, indicating that immune function of CRC patients was improved after CIK/DC-CIK immunotherapy [56, 57]. However, no significant difference was shown in percentages of CD4+, CD8+ and CD4+CD25+ T cells between pre and post immunotherapy. This may be related to the different time points of T-lymphocyte subset were tested in these trials [19] and the various CIK/DC-CIK transfusion dosages may also cause different immune responses. Th1 cytokines, including IFN-γ, IL-2, and TNF-α, enhance the cytotoxicity of CIK cells, whereas Th2 cytokines like IL-6 and IL-10 is associated with tumor immune escape [3, 58]. The balance between Th1 and Th2 cells is crucial in immunotherapy [3, 4]. Our analysis showed that after CIK/DC-CIK immunotherapy for CRC patients, IFN-γ and IL-2 levels were significantly increased, whereas no significant differences were observed in TNF-α and IL-6, indicating a more important role of Th1 than Th2 cytokines.
The determination of optimal therapeutic strategy is valuable for CRC treatment. To optimized the therapeutic protocol, there are several aspects need to be considered. First of all, the phenotype of in vitro cultured CIK cells is associated with treatment outcomes of immunotherapy. Low percentages of CD3+CD4+ subset and high percentages of CD3+CD8+ and CD3+CD56+ subset were reported associated with improved OS in CRC patients [56]. Therefore, the immune phenotype of CIK cells could be an important criterion for CIK/DC-CIK immunotherapy. However, relevant studies were insufficient and need more research evidence to support this conclusion. Secondly, to determine the usage of CIK or DC-CIK, the difference between their therapeutic effects should be evaluated. In our analysis, CIK and DC-CIK subgroup analyses showed that both were effective in treating CRC. In vitro experiments showed that DC-CIK had a better antitumor effect than CIK alone, but no obvious difference was observed in most pooled analysis in treating CRC patients. Moreover, the choose of chemotherapy regimen is also important for the determination of optimal therapeutic strategy. Folfox, Xelox and Folfiri are the three most common chemotherapy regimens with similar therapeutic effects and have been considered as first-line treatments for CRC [59–61]. Sensitivity analysis was conducted in this research and trials in which Folfox, Xelox, or Folfiri were not applied to all patients were excluded. The results of this analysis indicated that Folfox/Xelox/Folfiri combined with CIK/DC-CIK immunotherapy is effective for treating CRC. To summarize, we expect that our study will be valuable for the design of more comprehensive clinical trials in the future.
There are some limitations in our study. Firstly, the investigated 29 trials were conducted in the Chinese population, and this analysis did not go through the open external evaluation, which may lead to an overestimation of treatment effects. Furthermore, insufficient information of some patients, the small total sample sizes and other variables may introduce bias into conclusions. Moreover, the therapeutic effects of CIK/DC-CIK immunotherapy are affected by numerous factors such as injection modes, tumor stage and transfer cycles [5, 17, 30, 45]. Further detailed analyses need to be conducted based on papers with complete information, standardized therapeutic regimens and restrict patients involving criterion.
Taken together, this research demonstrated that the combination of CIK/DC-CIK immunotherapy and chemotherapy was safe and applicable for CRC patients. It markedly prolongs survival time, enhances immune function, and improves the efficacy of the treatment of CRC patients. Therefore, CIK/DC-CIK immunotherapy is an effective therapy for CRC treatment.
MATERIALS AND METHODS
Search strategy and selection criteria
Literatures were searched across Cochrane Library, EMBASE, PubMed, Wanfang and CNKI databases with key terms “dendritic cells”, “immunotherapy”, “cytokine-induced killer cells” or “DC-CIK” combined with “colorectal cancer”. No language limits were applied. The initial search was performed in February 2016 and updated in September 2016.
Trials meet the following criteria were selected into this analysis: (1) Studies were concerned with advanced CRC. (2) Patients in experimental group underwent chemotherapy combined with CIK /DC-CIK immunotherapy, and patients in control group were treated by chemotherapy alone.
Data collection and quality assessment
Data were extracted from all selected literatures independently by two investigators, and discrepancy was resolved by discussing with a third investigator. Collected information includes: name of first author; years of publication; numbers of subjects; patient ages; tumor stages; experiment regimens; chemotherapy regimens; in vitro cell culture conditions, and utilized immune cells numbers.
The quality of the included articles was evaluated according to Cochrane Handbook [62].
Curative effects
Clinical responses include prognosis and treatment efficacy. Prognosis was estimated by OS and DFS. Treatment efficacy was assessed in terms of the CR, PR, SD, PD, ORR (ORR = CR+PR), DCR (DCR = CR+PR+SD) and patients’ QOL. OS was defined as the length of time from the start of treatment to death from any cause [63]. DFS was defined as length of time from the start of treatment to the first recurrence evidence or death [3]. The immune function of CRC patients before and after treatment was determined by lymphocyte subsets percentages (CD3+, CD4+, CD8+, CD3−CD56+, CD3+CD56+, CD16+CD56+ and CD4+CD25+) and cytokines secretion levels (IFN-γ, IL-2, IL-4, IL-6 and TNF-α). Adverse events in included trials were also taken into assessment.
Statistical analysis
Review Manager (version 5.2, Nordic Cochran Centre, Copenhagen, Denmark) and Stata Statistical Software (version 12.0, Stata Corp., College Station, TX, USA) was used for analyses. P < 0.05 was considered statistically significant. Heterogeneity among the involved trials was assessed to determine the most suitable model [64]. A random-effects method would be applied when heterogeneity existed; otherwise, a fixed-effects method would be used. Cochran's Q test was performed to determine the homogeneity of our results; homogeneity was considered at I2 < 50% or P > 0.1. Odds ratios (OR) were the principal measures of effect and were presented with a 95% confidence interval (CI).
Publication bias was analyzed by Begg's and Egger's regression asymmetry tests [65], and visually evaluated using the funnel plot. When publication bias existed, trim-and-fill method was applied to adjust the pooled estimates of potentially unpublished studies. Corrected results were compared with the original pooled OR. Sensitivity analysis was conducted and 8 trials [18, 30, 32, 38, 44, 49, 52, 55] were excluded because Folfox/Xelox/Folfiri regimen was not applied to all patients.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
This research was supported by grants from the Natural Science Foundation of Shandong (No. 2015ZRA15027 to Changhui Zhou), National Natural Science Foundation of China (No. 81600087 to Shaoda Ren), Natural Science Foundation of Shandong (No. ZR2016HB45 to Shaoda Ren), Medical Science Foundation of Shandong (No. 2014WS0300 to Weihua Wang), and Wu Jieping Medical Foundation (No. 320.6750.15043 to Weihua Wang).
Footnotes
Authors’ contributions
Changhui Zhou and Shaoda Ren conceived and designed the experiments. Lei Zhang, Ying Mu, Anqi Zhang and Shuangfeng Chen performed the experiments. Jiaping Xie, Fang Xu, Weihua Wang and Ying-xin Zhang analyzed the data. Lei Zhang and Ying Mu drafted the manuscript.
CONFLICTS OF INTEREST
The authors have declared that no conflicts of interest exits.
REFERENCES
- 1.Zhang SW, Zhang D, Yang ZD, Zhang XP. Tumor Budding, Micropapillary Pattern, and Polyploidy Giant Cancer Cells in Colorectal Cancer: Current Status and Future Prospects. Stem Cells Int. 2016;2016:4810734. doi: 10.1155/2016/4810734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JS, Kim YG, Park EJ, Kim B, Lee HK, Hong JT, Kim Y, Han SB. Cell-based Immunotherapy for Colorectal Cancer with Cytokine-induced Killer Cells. Immune Netw. 2016;16:99–108. doi: 10.4110/in.2016.16.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mu Y, Zhou CH, Chen SF, Ding J, Zhang YX, Yang YP, Wang WH. Effectiveness and safety of chemotherapy combined with cytokine-induced killer cell /dendritic cell-cytokine-induced killer cell therapy for treatment of gastric cancer in China: A systematic review and meta-analysis. Cytotherapy. 2016;18:1162–77. doi: 10.1016/j.jcyt.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Mu Y, Wang WH, Xie JP, Zhang YX, Yang YP, Zhou CH. Efficacy and safety of cord blood-derived dendritic cells plus cytokine-induced killer cells combined with chemotherapy in the treatment of patients with advanced gastric cancer: a randomized Phase II study. Onco Targets Ther. 2016;9:4617–27. doi: 10.2147/OTT.S107745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H, Wang Y, Yu JP, Wei F, Cao S, Zhang XW, Dong N, Li H, Ren XB. Autologous Cytokine-Induced Killer Cells Improves Overall Survival of Metastatic Colorectal Cancer Patients: Results From a Phase II Clinical Trial. Clin Colorectal Canc. 2016;15:228–35. doi: 10.1016/j.clcc.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Gardini A, Ercolani G, Riccobon A, Ravaioli M, Ridolfi L, Flamini E, Ridolfi R, Grazi GL, Cavallari A, Amadori D. Adjuvant, adoptive immunotherapy with tumor infiltrating lymphocytes plus interleukin-2 after radical hepatic resection for colorectal liver metastases: 5-year analysis. J Surg Oncol. 2004;87:46–52. doi: 10.1002/jso.20066. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Yang XJ, Li JL, Ren YJ, Zhang TY, Zhang CZ, Zhang JT, Li J, Pang Y. Immune response, safety, and survival and quality of life outcomes for advanced colorectal cancer patients treated with dendritic cell vaccine and cytokine-induced killer cell therapy. Biomed Res Int. 2014;2014:603871. doi: 10.1155/2014/603871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barkholt L, Alici E, Conrad R, Sutlu T, Gilljam M, Stellan B, Christensson B, Guven H, Bjorkstrom NK, Soderdahl G, Cederlund K, Kimby E, Aschan J, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1:753–64. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- 9.Jakel CE, Schmidt-Wolf IG. An update on new adoptive immunotherapy strategies for solid tumors with cytokine-induced killer cells. Expert Opin Biol Th. 2014;14:905–16. doi: 10.1517/14712598.2014.900537. [DOI] [PubMed] [Google Scholar]
- 10.Li GX, Zhao SS, Zhang XG, Wang WH, Liu J, Xue KW, Li XY, Guo YX, Wang LH. Comparison of the proliferation, cytotoxic activity and cytokine secretion function of cascade primed immune cells and cytokine-induced killer cells in vitro. Mol Med Rep. 2015;12:2629–35. doi: 10.3892/mmr.2015.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta BA, Schmidt-Wolf IG, Weissman IL, Negrin RS. Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3+ CD56+ killer cells. Blood. 1995;86:3493–9. [PubMed] [Google Scholar]
- 12.Zhong R, Han B, Zhong H. A prospective study of the efficacy of a combination of autologous dendritic cells, cytokine-induced killer cells, and chemotherapy in advanced non-small cell lung cancer patients. Tumour Biol. 2014;35:987–94. doi: 10.1007/s13277-013-1132-1. [DOI] [PubMed] [Google Scholar]
- 13.Wei XC, Yang DD, Han XR, Zhao YA, Li YC, Zhang LJ, Wang JJ. Bioactivity of umbilical cord blood dendritic cells and anti-leukemia effect. Int J Clin Exp Med. 2015;8:19725–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Ren XB, Zhang P, An XM, Liu H, Hao XS. [Dendritic cells reduce the number and function of CD4+CD25+ cells in cytokine-induced killer cells]. [Article in Chinese] Zhonghua Yi Xue Za Zhi. 2005;85:3134–8. [PubMed] [Google Scholar]
- 15.Schmidt J, Eisold S, Buchler MW, Marten A. Dendritic cells reduce number and function of CD4+CD25+ cells in cytokine-induced killer cells derived from patients with pancreatic carcinoma. Cancer Immunol Immu. 2004;53:1018–26. doi: 10.1007/s00262-004-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge W, Li CH, Zhang W, Han Q, Deng WM, Chen L, You SG, Zhao CH. [Coculture of dendritic cell with cytokine-induced killer results in a significant increase in cytotoxic activity of CIK to tumor cells in vitro and in vivo]. [Article in Chinese] Zhonghua Xue Ye Xue Za Zhi. 2004;25:277–80. [PubMed] [Google Scholar]
- 17.Wang ZX, Cao JX, Wang M, Li D, Cui YX, Zhang XY, Liu JL, Li JL. Adoptive cellular immunotherapy for the treatment of patients with breast cancer: a meta-analysis. Cytotherapy. 2014;16:934–45. doi: 10.1016/j.jcyt.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Gao DQ, Li CY, Xie XH, Zhao P, Wei XF, Sun WH, Liu HC, Alexandrou AT, Jones J, Zhao RH, Li JJ. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS One. 2014;9:e93886. doi: 10.1371/journal.pone.0093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi LR, Zhou Q, Wu J, Ji M, Li GJ, Jiang JT, Wu CP. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immu. 2012;61:2251–9. doi: 10.1007/s00262-012-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Wang LP, Luo ZZ, Zhao X, Huang JM, Li H, Yang SN, Zhao XL, Zhang L, Li LX, Wang F, Huang L, Zhang Y. Efficacy and safety of cord blood-derived cytokine-induced killer cells in treatment of patients with malignancies. Cytotherapy. 2015;17:1130–8. doi: 10.1016/j.jcyt.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhu XP, Xu YH, Zhou J, Pan XF. A clinical study evaluating dendritic and cytokine-induced killer cells combined with concurrent radiochemotherapy for stage IIIB non-small cell lung cancer. Genet Mol Res. 2015;14:10228–35. doi: 10.4238/2015.August.28.6. [DOI] [PubMed] [Google Scholar]
- 22.Mao Q, Li L, Zhang C, Sun Y, Liu S, Cui S. Clinical effects of immunotherapy of DC-CIK combined with chemotherapy in treating patients with metastatic breast cancer. Pak J Pharm Sci. 2015;28:1055–8. [PubMed] [Google Scholar]
- 23.Schmidt-Wolf IG, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, Heuft HG, Prange G, Korte M, Takeya M, Dorbic T, Neubauer A, Wittig B, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999;81:1009–16. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 25.Linn YC, Niam M, Chu S, Choong A, Yong HX, Heng KK, Hwang W, Loh Y, Goh YT, Suck G, Chan M, Koh M. The anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignancies. Bone Marrow Transpl. 2012;47:957–66. doi: 10.1038/bmt.2011.202. [DOI] [PubMed] [Google Scholar]
- 26.Introna M, Borleri G, Conti E, Franceschetti M, Barbui AM, Broady R, Dander E, Gaipa G, D'Amico G, Biagi E, Parma M, Pogliani EM, Spinelli O, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92:952–9. doi: 10.3324/haematol.11132. [DOI] [PubMed] [Google Scholar]
- 27.Introna M, Pievani A, Borleri G, Capelli C, Algarotti A, Mico C, Grassi A, Oldani E, Golay J, Rambaldi A. Feasibility and safety of adoptive immunotherapy with CIK cells after cord blood transplantation. Biol Blood Marrow Tr. 2010;16:1603–7. doi: 10.1016/j.bbmt.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Tr. 2005;11:181–7. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Bian JR. Study on chemotherapy combined with DC-CIK on colorectal cancer. Chin J Surg Onco. 2013;5:306–309. [Google Scholar]
- 30.Cai WM, Li WW. Clinical study of autologous CIK cells combined with synchronous radiotherapy and chemotherapy in the treatment of elderly rectal cancer patients. Modern Preventive Medicine. 2013;40:1564–1567. [Google Scholar]
- 31.Cai XY, Xiong W, Li YF, Shen T. Clinical research of the treatment on patients of mid-low locally rectal cancer afteroperation with cytokine induced killers. The Practical Journal of Cancer. 2010;25:37–39. [Google Scholar]
- 32.Chen F, Jiang QQ, Jiao L, He Y, Zhai FL, Qu LF. Dendritic cell - cytokine induced killer cells combined with chemotherapy and targeted therapy in treatment of advanced colon cancer patients. Modern Oncology. 2016;23:1686–1690. [Google Scholar]
- 33.Du C, Liu ZZ, Ding ZY, Guo F, Ma DC, Xie XD. Autologous cytokine-induced killer cells combined with chemotherapy in the treatment of advanced colorectal cancer: a randomized control study. The Chinese-German Journal of Clinical Oncology. 2013;12:487–491. [Google Scholar]
- 34.Fan MX, Ye W, Yao J, Li X. [Clinical analysis of cytokine-induced killer cells combined with concurrent chemoradiotherapy in the treatment of local recurrence of rectal neoplasms]. [Article in Chinese] Chin J Postgrad Med. 2013;36:21–25. [Google Scholar]
- 35.Hou ZY. [Clinical study of DC-CIK combined with chemotherapy in the treatment of colon cancer]. [Article in Chinese] Guide of China Medicine. 2015;13:64–65. [Google Scholar]
- 36.Li S, Li Y, Liang J, Liu XL. [The study of clinical application of DC-CIK combined with chemotherapy on colon cancer]. [Article in Chinese] Chinese Journal Of Immunology. 2012;28:835–839. [Google Scholar]
- 37.Li WW, Zhang HP, Wu M. [Study on DC-CIK cells combined with intravenous chemotherapy for advanced colorectal cancer with diffuse hepatic metastasis]. [Article in Chinese] Modern Journal of Integrated Traditional Chinese and Western Medicine. 2016;25:1942–1945. [Google Scholar]
- 38.Li YC, Jin AS, Chen S, Song C, Zhang GR. [Efficacy of adjuvant chemotherapy combined with CIK cell immunotherapy in 130 patients with postoperative colorectal cancer]. [Article in Chinese] Journal of Chinese Oncology. 2015;21:843–847. [Google Scholar]
- 39.Lin T, Song C, Chuo DY, Zhang H, Zhao J. Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study. Tumour Biol. 2016;37:4367–72. doi: 10.1007/s13277-015-3957-2. [DOI] [PubMed] [Google Scholar]
- 40.Lv Y, Shi Y, Wang ZK, Mao H, Dai GH. A randomized study of cytokine-induced killer cells combined with chemotherapy for advanced colorectal cancer. Oncology Progress. 2014;12:505–510. [Google Scholar]
- 41.Rui T, Wu GQ, Xu J, Zheng AH, Wu H, Ye ZY. [Activated DC combined with CIK immunotherapy for patients with advanced colorectal cancer]. [Article in Chinese] Zhejiang Medical Journal. 2015;18:1505–1509. [Google Scholar]
- 42.Wang LR, Zhao D, Cao Y, Li RH. The application of DC-CIK biological immunotherapy combined with chemotherapy and targeted therapy in advanced colon cancer. International Medicine And Health Guidance News. 2016;22:42–44. [Google Scholar]
- 43.Wang R, Yi M, Yang SR, Chai LX, Hua M. [The influence of DC-CIK in advanced colorectal cancer patients on T lymphocyte subsets and cytokines]. [Article in Chinese] Journal of Hainan Medical University. 2016;22:1580–1583. [Google Scholar]
- 44.Wang RT, Meng MY, Li P, Li RH, Zhao WS, Hou ZL. [Effects of IL-2- and IL-15-induced CIK cells combined with chemotherapy treatment for colorectal cancer]. [Article in Chinese] Journal of Kunming Medical University. 2014;35:97–101. [Google Scholar]
- 45.Wei ZQ, Yang JW, Chen LC, Zheng QH, Ying MG. [A retrospective analysis of postsurgical treatment for rectal cancer by chemotherapy and radiotherapy combined with dentritic cells]. [Article in Chinese] Journal Of Fujian Medical University. 2009;43:483–487. [Google Scholar]
- 46.Weng HG, Shen D, Mao WD, Han LX. [Clinical efficacy of DC-CIK immunotherapy combined with chemotherapy in treatment of advanced colorectal cancer]. [Article in Chinese] Zhejiang Medical Journal. 2015;8:626–629. [Google Scholar]
- 47.Weng JF, Yu LW, Li YP, Shi YQ, Pu YD, Cao ZY. [Clinical observation of Co-treatment with CIK cells and dendritic cells for advanced rectal cancer]. [Article in Chinese] Chin J Clin Oncol Rehabil. 2014;21:1040–1043. [Google Scholar]
- 48.Yin LW, Wang SP, Zhang L, Wang Y, Ji J, Wang HS, Guo X, Wang XH, Ma SB, Du XH, Ma HY. [Efficacy of dendritic cells /cytokine induced killer cells adoptive immunotherapy combined with chemotherapy in treatment of metastatic colorectal cancer]. [Article in Chinese] Chin J Cancer Biother. 2013;20:217–224. [Google Scholar]
- 49.Ying MG, Wei ZQ, Yang JW, Chen LC, Zheng QH. Retrospective analysis of pos-operative chemo-radiotherapy combined with DC-CIK in the treatment of patients with colorectal cancer. The Practical Journal of Cancer. 2010;25:274–276. [Google Scholar]
- 50.Yuan JH, Peng DW, Li JW, Wang MQ. [Clinical research of dendritic cells combined with cytokine induced killer cells therapy for advanced colorectal cancer]. [Article in Chinese] Chinese General Practice. 2011;36:4139–4141. [Google Scholar]
- 51.Zhang J, Geng J, Han ZX, Gao XY. [Chemotherapy combined with CIK/DC cells in the treatment of advanced colorectal cancer: a clinical observation]. [Article in Chinese] Acta Academiae Medicinae Xuzhou. 2011;31:457–459. [Google Scholar]
- 52.Zhang JG, Ye ZF, Huang L, Huang T, Zhang ZD. [The effect of DC-CIK adoptive immunotherapy combined with chemotherapy in the treatment of advanced colorectal cancer]. [Article in Chinese] Zhejiang Clinical Medical Journal. 2015;17:2105–2109. [Google Scholar]
- 53.Zhang JY, Zhu LJ, Zhang Q, He X, Yin YM, Gu YH, Guo RH, Lu KH, Liu LK, Liu P, Shu YQ. Effects of cytokine-induced killer cell treatment in colorectal cancer patients: a retrospective study. Biomed Pharmacother. 2014;68:715–20. doi: 10.1016/j.biopha.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Zhao WF, Chen XJ, Wen YY, Wang CJ, Chang SD, Bai B, Fu L. [To explore the effect of DC-CIK combine with chemotherapy plus targeted therapy for advanced colorectal cancer]. [Article in Chinese] China Continuing Medical Education. 2015;7:93–95. [Google Scholar]
- 55.Zhou QM. [Observation effect of biological immune method in the treatment of patients with advanced colorectal cancer]. [Article in Chinese] China Modern Medicine. 2015;22:37–39. [Google Scholar]
- 56.Pan K, Wang QJ, Liu Q, Zheng HX, Li YQ, Weng DS, Li JJ, Huang LX, He J, Chen SP, Ke ML, Zeng YX, Xia JC. The phenotype of ex vivo generated cytokine-induced killer cells is associated with overall survival in patients with cancer. Tumour Biol. 2014;35:701–7. doi: 10.1007/s13277-013-1096-1. [DOI] [PubMed] [Google Scholar]
- 57.Cho MY, Joh YG, Kim NR, Jung SI, Bae JW, Kim YC, Koo BH, Whang CW, Suh SO. T-lymphocyte subsets in patients with AJCC stage III gastric cancer during postoperative adjuvant chemotherapy. American Joint Committee on Cancer. Scand J Surg. 2002;91:172–7. doi: 10.1177/145749690209100207. [DOI] [PubMed] [Google Scholar]
- 58.Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P, Punt CJ, Jacobs JF, Schalken JA, Oosterwijk E, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35:169–78. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- 59.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Carteni G, Agostara B, Pezzella G, Manzione L, Borsellino N, Misino A, Romito S, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–75. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y, Xiong BH, Zhang T, Cheng Y, Ma L. XELOX vs. FOLFOX in metastatic colorectal cancer: An updated meta-analysis. Cancer Invest. 2016;34:94–104. doi: 10.3109/07357907.2015.1104689. [DOI] [PubMed] [Google Scholar]
- 61.Chen YX, Yang Q, Kuang JJ, Chen SY, Wei Y, Jiang ZM, Xie DR. Efficacy of adding bevacizumab in the first-line chemotherapy of metastatic colorectal cancer: evidence from seven randomized clinical trials. Gastroenterol Res Pract. 2014;2014:594930. doi: 10.1155/2014/594930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 63.Han RX, Liu X, Pan P, Jia YJ, Yu JC. Effectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e108958. doi: 10.1371/journal.pone.0108958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31:3805–20. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000;4:1–115. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.