Abstract
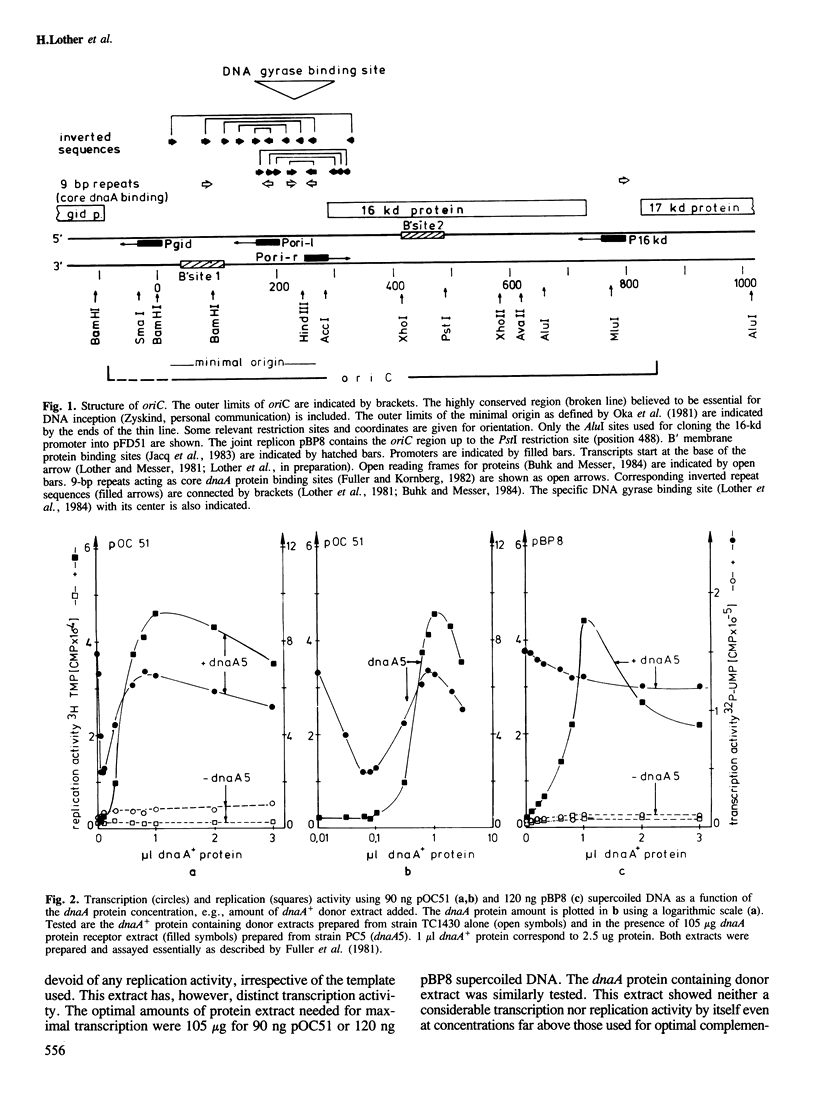
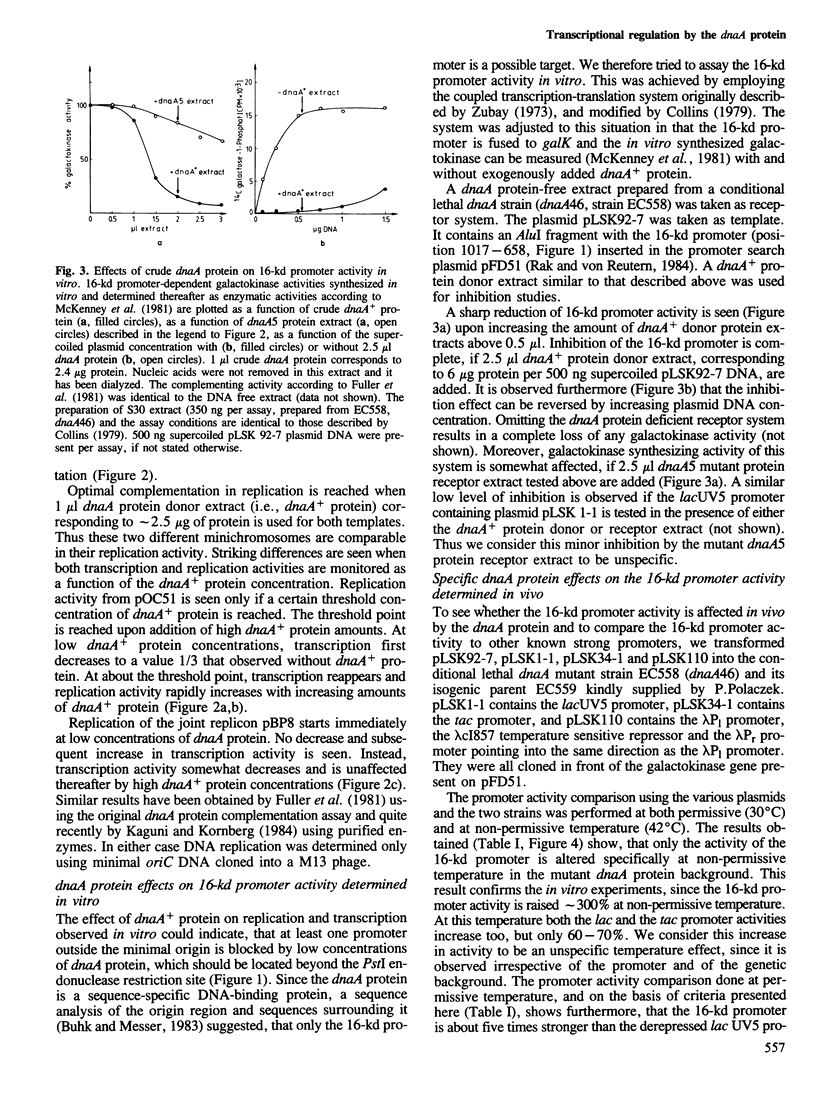
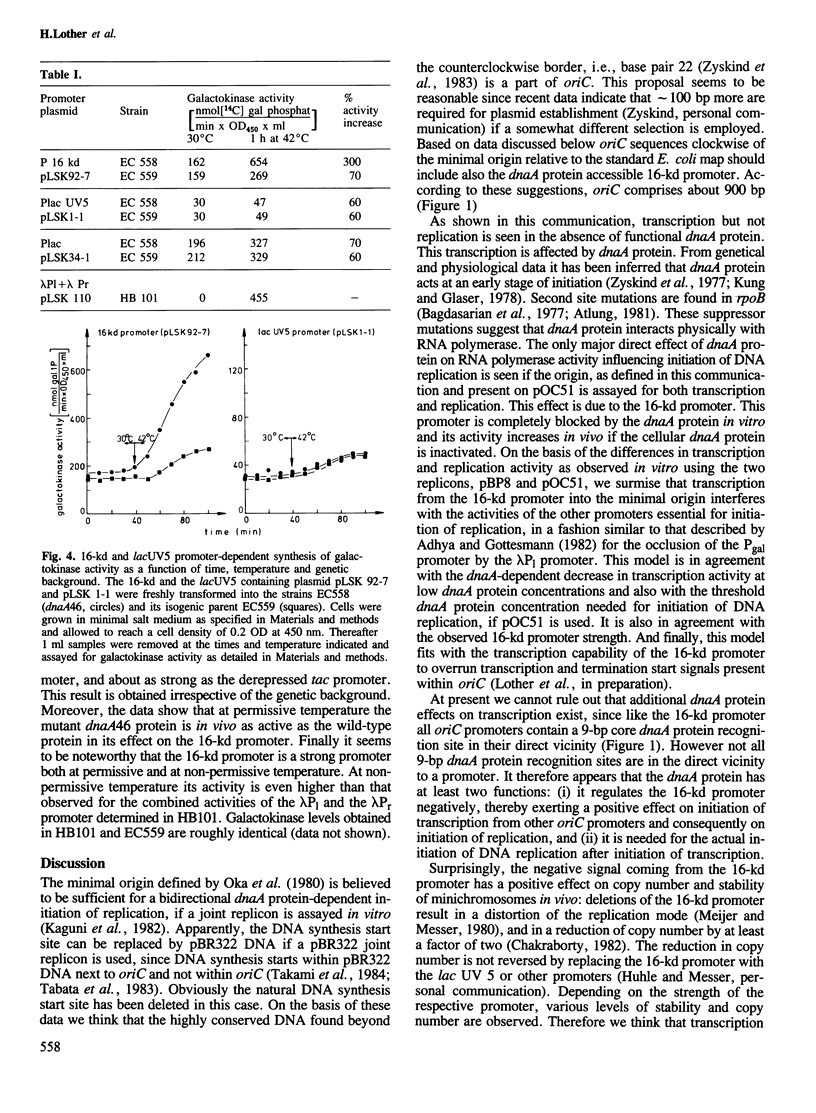
Both initiation of replication and initiation of transcription are influenced by dnaA protein, when minichromosomes are assayed in vitro for dnaA protein complementation. This dnaA protein effect is seen only if minichromosomes are used containing the 16-kd promoter, from which transcription is directed into the minimal origin. Determination of the 16-kd promoter activity both in vivo and in vitro showed that this strong promoter is specifically repressed by dnaA protein. The 16-kd promoter is thus an integral regulatory region of oriC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M. M., Izakowska M., Bagdasarian M. Suppression of the DnaA phenotype by mutations in the rpoB cistron of ribonucleic acid polymerase in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1977 May;130(2):577–582. doi: 10.1128/jb.130.2.577-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buhk H. J., Messer W. The replication origin region of Escherichia coli: nucleotide sequence and functional units. Gene. 1983 Oct;24(2-3):265–279. doi: 10.1016/0378-1119(83)90087-2. [DOI] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Yoshinaga K., Lother H., Messer W. Purification of the E. coli dnaA gene product. EMBO J. 1982;1(12):1545–1549. doi: 10.1002/j.1460-2075.1982.tb01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M., Bird R. E., Caro L. The replication time of the Escherichia coli K12 chromosome as a function of cell doubling time. J Mol Biol. 1975 May 5;94(1):127–132. doi: 10.1016/0022-2836(75)90410-6. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Kornberg A. Purified dnaA protein in initiation of replication at the Escherichia coli chromosomal origin of replication. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5817–5821. doi: 10.1073/pnas.80.19.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. DNA synthesis during the division cycle of three substrains of Escherichia coli B/r. J Mol Biol. 1976 Apr 15;102(3):477–486. doi: 10.1016/0022-2836(76)90329-6. [DOI] [PubMed] [Google Scholar]
- Jacq A., Kohiyama M., Lother H., Messer W. Recognition sites for a membrane-derived DNA binding protein preparation in the E. coli replication origin. Mol Gen Genet. 1983;191(3):460–465. doi: 10.1007/BF00425763. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Fuller R. S., Kornberg A. Enzymatic replication of E. coli chromosomal origin is bidirectional. Nature. 1982 Apr 15;296(5858):623–627. doi: 10.1038/296623a0. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell. 1984 Aug;38(1):183–190. doi: 10.1016/0092-8674(84)90539-7. [DOI] [PubMed] [Google Scholar]
- Kung F. C., Glaser D. A. dnaA acts before dnaC in the initiation of DNA replication. J Bacteriol. 1978 Feb;133(2):755–762. doi: 10.1128/jb.133.2.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Steinmetz D., Hendrickson W. G., Murchie J., King M., Benson A., Schaechter M. Direct evidence for specific binding of the replicative origin of the Escherichia coli chromosome to the membrane. J Bacteriol. 1984 Apr;158(1):313–316. doi: 10.1128/jb.158.1.313-316.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDuca R. J., Helmstetter C. E. Expression of accumulated capacity for initiation of chromosome and minichromosome replication in dnaA mutants of Escherichia coli. J Bacteriol. 1983 Jun;154(3):1371–1380. doi: 10.1128/jb.154.3.1371-1380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lother H., Lurz R., Orr E. DNA binding and antigenic specifications of DNA gyrase. Nucleic Acids Res. 1984 Jan 25;12(2):901–914. doi: 10.1093/nar/12.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lother H., Messer W. Promoters in the E. coli replication origin. Nature. 1981 Nov 26;294(5839):376–378. doi: 10.1038/294376a0. [DOI] [PubMed] [Google Scholar]
- Meijer M., Messer W. Functional analysis of minichromosome replication: bidirectional and unidirectional replication from the Escherichia coli replication origin, oriC. J Bacteriol. 1980 Aug;143(2):1049–1053. doi: 10.1128/jb.143.2.1049-1053.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W., Bergmans H. E., Meijer M., Womack J. E., Hansen F. G., von Meyenburg K. Mini-chromosomes: plasmids which carry the E. coli replication origin. Mol Gen Genet. 1978 Jul 4;162(3):269–275. doi: 10.1007/BF00268852. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Polaczek P., Cieśla Z. Effect of altered efficiency of the RNA I and RNA II promoters on in vivo replication of ColE1-like plasmids in Escherichia coli. Mol Gen Genet. 1984;194(1-2):227–231. doi: 10.1007/BF00383521. [DOI] [PubMed] [Google Scholar]
- Rak B., von Reutern M. Insertion element IS5 contains a third gene. EMBO J. 1984 Apr;3(4):807–811. doi: 10.1002/j.1460-2075.1984.tb01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Stuitje A. R., Meijer M. Maintenance and incompatibility of plasmids carrying the replication origin of the Escherichia coli chromosome: evidence for a control region of replication between oriC and asnA. Nucleic Acids Res. 1983 Aug 25;11(16):5775–5791. doi: 10.1093/nar/11.16.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata S., Oka A., Sugimoto K., Takanami M., Yasuda S., Hirota Y. The 245 base-pair oriC sequence of the E. coli chromosome directs bidirectional replication at an adjacent region. Nucleic Acids Res. 1983 May 11;11(9):2617–2626. doi: 10.1093/nar/11.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Yamaguchi M., Tomizawa J. Incompatibility of plasmids containing the replication origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5347–5351. doi: 10.1073/pnas.79.17.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Hirota Y. Cloning and mapping of the replication origin of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5458–5462. doi: 10.1073/pnas.74.12.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]
- Zyskind J. W., Cleary J. M., Brusilow W. S., Harding N. E., Smith D. W. Chromosomal replication origin from the marine bacterium Vibrio harveyi functions in Escherichia coli: oriC consensus sequence. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1164–1168. doi: 10.1073/pnas.80.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Deen L. T., Smith D. W. Isolation and mapping of plasmids containing the Salmonella typhimurium origin of DNA replication. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3097–3101. doi: 10.1073/pnas.76.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Smith D. W. NOVEL Escherichia coli dnaB mutant: direct involvement of the dnaB252 gene product in the synthesis of an origin-ribonucleic acid species during initiaion of a round of deoxyribonucleic acid replication. J Bacteriol. 1977 Mar;129(3):1476–1486. doi: 10.1128/jb.129.3.1476-1486.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Riise E., Bergmans H. E., Meijer M., Messer W. Origin of replication, oriC, of the Escherichia coli K12 chromosome: genetic mapping and minichromosome replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):121–128. doi: 10.1101/sqb.1979.043.01.018. [DOI] [PubMed] [Google Scholar]



