Abstract
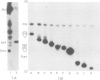
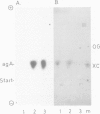
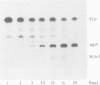
We showed that phosphorus-containing metabolites of crown gall tissues were all taken up by appropriate pTi+ agrobacteria. All but one were also taken up by pTi- bacteria. This one compound, produced by nopaline-, but not by octopine-type tumours, was the only phosphorylated organic compound actively secreted by healthy crown gall cells, and it appears to be agrocinopine A. Testing crown gall cell exudates may be a general procedure for the identification of opines by transformed plant cells.
Keywords: Agrobacterium, agrocin 84, exudate, phosphorus, plant tumour
Full text
PDF
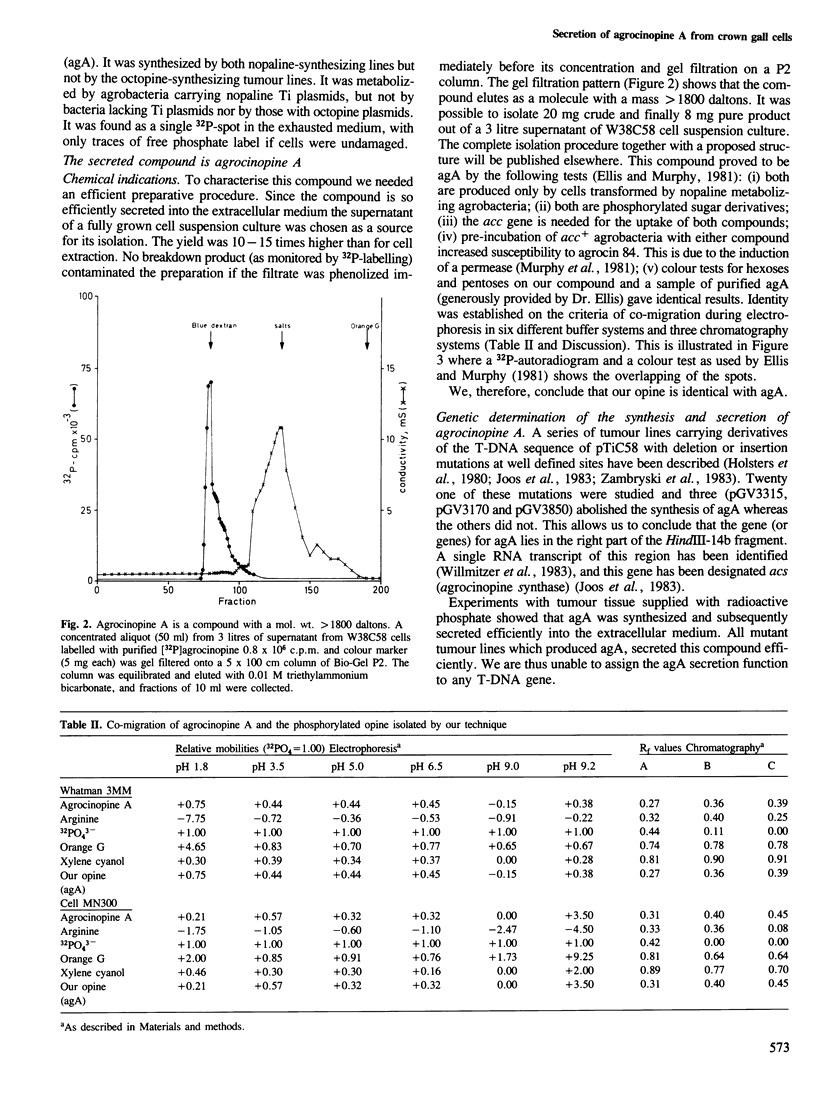




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binns A. N., Sciaky D., Wood H. N. Variation in hormone autonomy and regenerative potential of cells transformed by strain A66 of Agrobacterium tumefaciens. Cell. 1982 Dec;31(3 Pt 2):605–612. doi: 10.1016/0092-8674(82)90316-6. [DOI] [PubMed] [Google Scholar]
- Braun A. C., Wood H. N. Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci U S A. 1976 Feb;73(2):496–500. doi: 10.1073/pnas.73.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Chen C. M., Adams B. R., Trost B. M. Leucinopine, a characteristic compound of some crown-gall tumors. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3573–3576. doi: 10.1073/pnas.80.12.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton W. S., Tempé J., Matzke M., Chilton M. D. Succinamopine: a new crown gall opine. J Bacteriol. 1984 Feb;157(2):357–362. doi: 10.1128/jb.157.2.357-362.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler G., Holsters M., Van Montagu M., Schell J., Hernalsteens J. P., Schilperoort Agrocin 84 sensitivity: a plasmid determined property in Agrobacterium tumefaciens. Mol Gen Genet. 1975 Jul 10;138(4):345–349. doi: 10.1007/BF00264804. [DOI] [PubMed] [Google Scholar]
- Hernalsteens J. P., Thia-Toong L., Schell J., Van Montagu M. An Agrobacterium-transformed cell culture from the monocot Asparagus officinalis. EMBO J. 1984 Dec 20;3(13):3039–3041. doi: 10.1002/j.1460-2075.1984.tb02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Joos H., Inzé D., Caplan A., Sormann M., Van Montagu M., Schell J. Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell. 1983 Apr;32(4):1057–1067. doi: 10.1016/0092-8674(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Kemp J. D. Octopine as a marker for the induction of tumorous growth by agrobacterium tumefaciens strain B6. Biochem Biophys Res Commun. 1976 Apr 5;69(3):816–822. doi: 10.1016/0006-291x(76)90948-7. [DOI] [PubMed] [Google Scholar]
- Leemans J., Deblaere R., Willmitzer L., De Greve H., Hernalsteens J. P., Van Montagu M., Schell J. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J. 1982;1(1):147–152. doi: 10.1002/j.1460-2075.1982.tb01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers M., De Beuckeleer M., Holsters M., Zambryski P., Depicker A., Hernalsteens J. P., Van Montagu M., Schell J. Internal organization, boundaries and integration of Ti-plasmid DNA in nopaline grown gall tumours. J Mol Biol. 1980 Dec 15;144(3):353–376. doi: 10.1016/0022-2836(80)90095-9. [DOI] [PubMed] [Google Scholar]
- MacGregor A. N., Alexander M. Formation of tumor-like structures on legume roots by Rhizobium. J Bacteriol. 1971 Mar;105(3):728–732. doi: 10.1128/jb.105.3.728-732.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Márton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol. 1973 Jul 4;244(131):29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Murai N., Skoog F., Doyle M. E., Hanson R. S. Relationships between cytokinin production, presence of plasmids, and fasciation caused by strains of Corynebacterium fascians. Proc Natl Acad Sci U S A. 1980 Jan;77(1):619–623. doi: 10.1073/pnas.77.1.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. J., Tate M. E., Kerr A. Substituents at N6 and C-5' control selective uptake and toxicity of the adenine-nucleotide bacteriocin, agrocin 84, in Agrobacteria. Eur J Biochem. 1981 Apr;115(3):539–543. doi: 10.1111/j.1432-1033.1981.tb06236.x. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. A., Huisman T. H. Hemoglobin Russ or alpha-2-51-arg-beta-2. Biochim Biophys Acta. 1966 Dec 28;130(2):541–543. [PubMed] [Google Scholar]
- Ryder M. H., Tate M. E., Jones G. P. Agrocinopine A, a tumor-inducing plasmid-coded enzyme product, is a phosphodiester of sucrose and L-arabinose. J Biol Chem. 1984 Aug 10;259(15):9704–9710. [PubMed] [Google Scholar]
- Salomon F., Deblaere R., Leemans J., Hernalsteens J. P., Van Montagu M., Schell J. Genetic identification of functions of TR-DNA transcripts in octopine crown galls. EMBO J. 1984 Jan;3(1):141–146. doi: 10.1002/j.1460-2075.1984.tb01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M. E., Murphy P. J., Roberts W. P., Keer A. Adenine N6-substituent of agrocin 84 determines its bacteriocin-like specificity. Nature. 1979 Aug 23;280(5724):697–699. doi: 10.1038/280697a0. [DOI] [PubMed] [Google Scholar]
- Virtanen A. I., Laine T. Investigations on the root nodule bacteria of leguminous plants: The excretion products of root modules. The mechanism of N-fixation. Biochem J. 1939 Apr;33(4):412–427. doi: 10.1042/bj0330412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Dhaese P., Schreier P. H., Schmalenbach W., Van Montagu M., Schell J. Size, location and polarity of T-DNA-encoded transcripts in nopaline crown gall tumors; common transcripts in octopine and nopaline tumors. Cell. 1983 Apr;32(4):1045–1056. doi: 10.1016/0092-8674(83)90289-1. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Joos H., Genetello C., Leemans J., Montagu M. V., Schell J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983;2(12):2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]