Abstract
The 2009 Oxford Classification of immunoglobulin A (IgA) nephropathy (IgAN) identifies four histological features as predictors of renal prognosis: mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), and tubular atrophy/interstitial fibrosis (T). However, the clinical and prognostic significance of crescent formation still remains controversial. Therefore, we performed a meta-analysis to evaluate the association between crescents and kidney outcome in IgAN. A total of 20 studies published from January 2009 to July 2016 involving 5,285 patients were included after systematic searches of PubMed and EMBASE databases. Pooled results showed that crescent lesions were associated with kidney failure (HR, 1.93; 95% CI, 1.49-2.50; P < 0.001). IgAN patients with crescents had lower eGFR levels (SMD, -0.21; 95% CI, -0.40--0.03; P = 0.023); higher proteinuria levels (SMD, 0.87; 95% CI, 0.11-1.63; P = 0.024); a larger number of patients with M1 (RR, 1.22; 95% CI, 1.07-1.40; P = 0.003), E1 (RR, 4.83; 95% CI, 3.04-7.66;P < 0.001), S1 (RR, 1.76; 95% CI, 1.11-2.80; P = 0.016) and T1/2 (RR, 2.74; 95% CI, 2.10-3.57; P < 0.001) lesions; and received immunosuppressive therapy more frequently (RD, 0.17; 95% CI, 0.11-0.23; P < 0.001). Our results suggest that crescent formation represents an efficient prognostic factor associated with progression to kidney failure and thus could be considered into the new Oxford Classification.
Keywords: Immunoglobulin A (IgA) nephropathy, Oxford classification, crescent lesions, meta-analysis
INTRODUCTION
Immunoglobulin A (IgA) nephropathy (IgAN) is the most common glomerulonephritis worldwide and recognized to progress to end-stage kidney disease (ESKD) in approximately 20 to 40% of patients within 10 to 20 years from onset [1, 2]. IgAN is characterized by IgA deposition in the glomerular mesangium with widely variable clinical presentations, including impaired estimate glomerular filtration rate (eGFR), arterial hypertension and proteinuria, and pathological features of renal biopsy specimens [1–3]. Histologic classification of IgAN is essential for evaluating the severity of lesions and guiding appropriate therapeutic strategies during clinical practice [2, 4]. In 2009, the Oxford Classification of IgAN was developed by an international working group to establish a consensus on identifying the specific pathologic features reliably predicting the risk of IgAN progression. By analyzing renal biopsy specimens from a cohort of 265 patients from 11 countries in four continents, this study proposed four pathological features—mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), and tubular atrophy and interstitial fibrosis (T)—as the histopathologic predictor of renal outcome of IgAN, independently of clinical indicators [5, 6].
In 2014, the VALIGA study (validation of IgA nephropathy), including a cohort of 1,147 patients from 13 European countries with unrestricted criteria at entry, provided a validation of the independent predictive value of the Oxford Classification [7]. The presence of crescents in both studies was not identified as a prognostic variable, mainly due to their low prevalence in the enrolled cohort [5–7]. However, the predictive value of crescent lesions in IgAN is still debated. Several studies with the cohorts differed from Oxford and VALIGA have described crescent formation as a predictive factor in the prognosis of IgAN [8–18]. In 2013, a new Japanese histologic classification (JHC) of IgAN proposed cellular/fibrocellular crescents as significant histologic prognostic variables [19]. Therefore, our study aimed to conduct a systematic review and meta-analysis to investigate crescent formation as a predictive marker in the progression of IgAN.
RESULTS
Study selection and characteristics
562 full-text articles were downloaded as potential studies, and 162 publications of which were excluded due to duplication. After detailed evaluation, 380 more were excluded according to the inclusion and exclusion criteria. Eventually, 20 studies published from 2009 to 2016 involving a total of 5,285 patients were included in this meta-analysis (Figure 1). Eleven studies [8–18] including 2,703 patients with 426 end point events evaluated the association of crescent formation with kidney survival in IgAN. Nine studies [6, 11, 13, 17, 18, 20–23] showed comparison of kidney function between IgA with C0 and C1 (defined as absence and any presence of crescents, respectively, in the Oxford Classification; eGFR in 7 and urinary protein excretion in 3), 4 studies [6, 11, 20, 24] reported the two main treatments (immunosuppressive therapy in 4 and renin-angiotensin system blockades, abbreviated as RASBs, in 3), and 4 studies [17, 25–27] reported the association with other four pathologic lesions (M, E, S and T). Characteristics of the included studies are summarized in Table 1.
Figure 1. Flow chart for study selection.
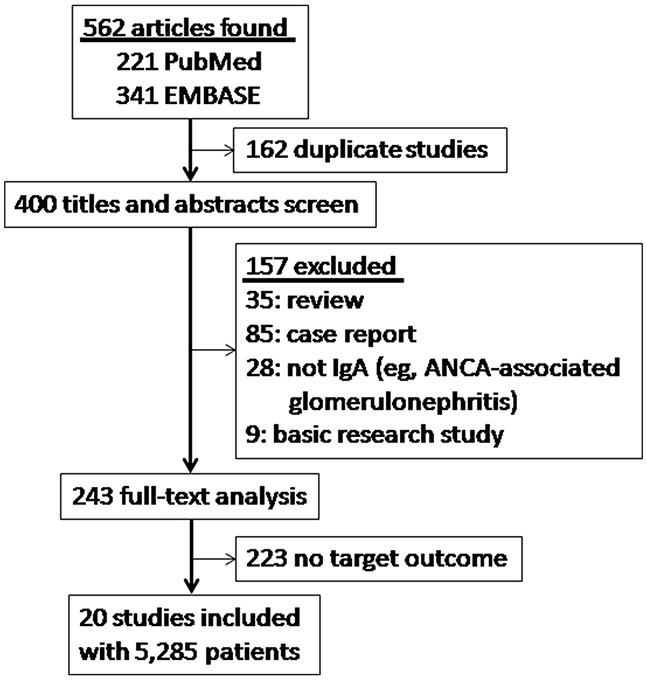
562 articles were downloaded as potential studies, and 162 publications of which were excluded due to duplication. After detailed evaluation, 380 more were excluded according to the inclusion and exclusion criteria. Eventually, 20 studies involving a total of 5,285 patients were included.
Table 1. Characteristics of included studies in this systematic review.
| Validation cohort study | ||||||||
|---|---|---|---|---|---|---|---|---|
| Oxford cohort[6] | Joh[8] | Bazzi[22] | Walsh[9] | Katafuchi[10] | Shi[11] | Shima[12] | Edstrom Halling[13] | |
| Country | Multicountry | Japan | Italy | Canada | Japan | China | Japan | Sweden |
| Ethnicity | ||||||||
| White | 66% | — | NA | NA | — | — | — | NA |
| Asian | 27% | 100% | NA | NA | 100% | 100% | 100% | NA |
| African | 3% | — | NA | NA | — | — | — | NA |
| other | 4% | — | NA | NA | — | — | — | NA |
| Designa | Multi | Single | Single | Multi | Single | Single | Multi | Single |
| No. pts | 265 | 233 | 168 | 146 | 702 | 410 | 161 | 99 |
| F/U (mo) | 69 | 127 | 60±40 | 69.6 | 62 | 38 | 54 | 156 |
| Age (y) | 30 (4-73) | 36 (18-70) | NA | NA | 30 (8-82) | 31 | 11.7 | 9.7 |
| Age < 18 y | 22.30% | 0% | NA | 0% | NA | 0% | 100% | 100% |
| M:F | 2.6:1 | 01:01 | NA | 1.74:1 | 01:01.2 | 01:01 | 1.7:1 | 1.4:1 |
| Proteinuria (g/d) | 1.7 | 1.3 | NA | NA | 0.85b | 1.7 | 0.7 | 2 |
| eGFR (mL/min/1.73m2) | 83 | 78 | NA | NA | 82 | 85.8 | 103 | 100 |
| CKD1/2/3/4/5c | 36/38/26/0/0 | 27/50/23/0/0 | NA | NA | 37/37/21/3/1 | 43/38/19/0/0 | NA | 75/16/4/3/2 |
| MAP (mm Hg) | 98 | 94 | NA | NA | 92 | 94 | 79 | 85.4 |
| HTN | 31% | 9% | 54% | NA | NA | NA | NA | NA |
| RAAS blockade | 74% | 77% | NA | 46% | 37% | 86.10% | NA | 86% |
| Immunosuppressive therapy | 29% | 35% | NA | 5.10% | 32%d | 42.70% | 16% | 43% |
| End point definition | 50% decline in eGFR or ESKD; slope of eGFR | 50% decline in eGFR or ESKD; slope of eGFR | Doubling of SCr or ESRD | Doubling of SCr, ESRD or death | ESKD | ESKD | CKD stages 3-5 | 50% decline in eGFR or ESKD |
| No. end point events | NA | 58 | NA | 40 | 84 | 30 | 7 | 18 |
| No. of pathologists | 5, blinded | NA | NA | 1, blinded | NA | 2, blinded | NA | 1, blinded |
| Lesion | ||||||||
| M0/M1 | 20/80 | NA | NA | NA | 88/12 | 44/56 | 36/64 | 69/31 |
| E0/E1 | 58/42 | NA | NA | NA | 58/42 | 43/57 | 42/58 | 90/10 |
| S0/S1 | NA | NA | NA | NA | 21/79 | 25/75 | 92/8 | 77/23 |
| T0/T1/T2 | NA | NA | NA | NA | 71/18/12 | 78/14/8 | 99/1/0 | 84/12/3 |
| C0/C1 | 55/45 | NA | 75/25 | 75/25 | 37/63 | 40/60 | 48/52 | 82/18 |
| Crescent %e | 9f | NA | 19 | 5-60f | 4.2 (0-73.3) f | 16f | 9.2 | 4.5 |
| Adjusted factors | Initial GFR, MAP, proteinuria; initial GFR, F/U MAP, proteinuria | Initial GFR, MAP, proteinuria; initial GFR, F/U MAP, proteinuria | None | Age, gender, initial SBP, proteinuria, creatinine | Initial GFR, MAP, proteinuria, therapy (steroid or not), M,E,S,T | Initial GFR, MAP, proteinuria | Initial proteinuria | Initial proteinuria, 1 y-F/U proteinuria |
| Validation Cohort Study | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kataoka[14] | Le[20] | Zeng[21] | Lv[15] | Zhang[25] | Rafalska[16] | Lee[17] | Kaneko[18] | Chan[24] | Li[23] | Stangou[26] | Stefan[27] |
| Japan | China | China | China | China | Poland | Korea | Japan | China | China | Greece | Romania |
| — | — | — | — | — | NA | — | — | — | — | NA | NA |
| 100% | 100% | 100% | 100% | 100% | NA | 100% | 100% | 100% | 100% | NA | NA |
| — | — | — | — | — | NA | — | — | — | — | NA | NA |
| — | — | — | — | — | NA | — | — | — | — | NA | NA |
| Single | Multi | Multi | Multi | NA | Single | Single | Single | NA | Single | Single | NA |
| 43 | 218 | 1,026 | 113 | 539 | 52 | 430 | 314 | 115 | 24 | 50 | 121 |
| 120 | 56 | 53 | 23.7 | 36 | >6 | 61 | 106 | NA | NA | 68 | NA |
| 40 | 14 (2-17.9) | 34 | 36.3 | NA | 32.5 | 34.9 | 36 | NA | NA | 40.7 | 40.1 |
| 0% | 100% | 0% | NA | NA | 0% | 0% | NA | NA | NA | NA | 0% |
| 1.5:1 | 1.9:1 | 01:01 | 1.4:1 | NA | 1.4:1 | 1.1:1 | 0.92:1 | 0.69:1 | NA | 2.6:1 | 2.2:1 |
| 1.8 | 1.5 | 1.3 | 4.44 | NA | 1.35 | 0.8b | 1.05 | NA | NA | 1.5 | NA |
| 78.3 | 134 | 85 | NA | NA | 74.7 | 80.5 | 80.3 | NA | NA | 64.3 | 47 |
| NA | NA | 41/35/24/0/0 | NA | NA | NA | 36.7/42.8/19.3/1.2 | NA | NA | NA | NA | NA |
| 102.6 | 88 | 98 | 118.1 | NA | 97 | NA | 91.1 | NA | NA | NA | NA |
| NA | NA | 30.50% | 69.90% | NA | NA | 21.90% | NA | 39.10% | NA | 86% | NA |
| 58.10% | 61.50% | 89% | 69.00% | NA | NA | 83.00% | 59.55% | NA | NA | NA | 98% |
| 51.20% | 56% | 31% | 47.80% | NA | NA | 2.30% | 60.19% | 43.50% | NA | NA | 49% |
| >150% increase of baseline SCr | 50% decline in eGFR or ESKD | 50% decline in eGFR or ESKD; slope of eGFR | ESRD | ESRD | >50% increase of SCr | 50% decline in eGFR | 50% decline in eGFR or ESKD | Doubling of SCr or starting of RRT | NA | ESRD | Doublingof SCr or ESRD |
| 16 | 24 | 159 | 63 | 56 | 8 | 59 | 43 | NA | NA | 8 | 34 |
| 2, blinded | 2 | 2 | NA | NA | NA | 1, blinded | 5 | NA | NA | NA | 1 |
| 19/81 | 55/45 | 57/43 | NA | NA | NA | 41.6/58.4 | 57/43 | NA | NA | 38/62 | NA |
| 47/53 | 77/23 | 89/11 | NA | NA | NA | 86.3/13.7 | 80/20 | NA | NA | 74/26 | NA |
| 19/81 | 38/62 | 17/83 | NA | NA | NA | 42.3/57.7 | 33/67 | NA | NA | 76/24 | NA |
| NA | 93/6/1 | 72.7/24/3.3 | NA | NA | 95.2/4.8 | 63.3/26.5/10.2 | 93/5/2 | NA | NA | 64/20/16 | NA |
| 47/53 | 56/44 | 52/48 | NA | 58/42 | 83.3/16.7 | 81.2/18.8 | 59/41 | 78/22 | 58/42 | 82/18 | 69/31 |
| NA | 9f | 8f | 66.4 | NA | NA | NA | NA | NA | NA | 5-30f | NA |
| Age, sex, eGFR | Initial GFR, MAP, proteinuria | Initial GFR, MAP, proteinuria; initial GFR, F/U MAP, proteinuria | None | NA | Initial eGFR, MAP, proteinuria, tonsillectomy, S,T | Sex, age, initial eGFR, MAP, proteinuria, M,E,S,T | Iinitial eGFR, MAP, proteinuria, M,E,S,T, arteriolar hyalinosis | Sex, age, initial creatinine, proteinuria, HTN, DM, therapy (RASBs, steroid) | None | None | NA |
Note: Values for continuous variables are given as mean or mean (range).
Abbreviations: CKD: chronic kidney disease; DM: diabetes mellitus; E: endocapillary hypercellularity; eGFR: estimated glomerular filtration rate; ESKD: end-stage kidney disease; F/U: follow-up; HTN: hypertension; M: mesangial hypercellularity; MAP: mean arterial pressure; NA: not available; pts: patients; RAAS: renin-angiotensin-aldosteronesystem; RRT: renal replacement therapy; S: segmental glomerulosclerosis; SCr: serum creatinine; T: tubular atrophy/interstitial fibrosis.
aNumber of centers.
bGrams of protein per gram of creatinine.
cPercentage number indicates CKD stage.
dSteroid.
eThe percentage of glomeruli with crescents.
fThese values are given as median, range or median (range).
Primary outcome
Kidney survival
Eleven studies (8 Asian, 2 European and 1 North American) with 2,703 patients and 426 end point events reported the association of crescent formation with kidney survival. These studies showed that a score of C1 was associated strongly with progression to kidney failure in a fixed-effects model (C0 as reference; hazard ratio, abbreviated as HR, 1.93; 95% confidence interval, abbreviated as 95% CI, 1.49-2.50; P < 0.001; Figure 2), with no evidence of heterogeneity (I2 = 40.9%; P = 0.076).
Figure 2. Hazard ratios (HR) for kidney failure for patients with versus without cellular/fibrocellular crescents.
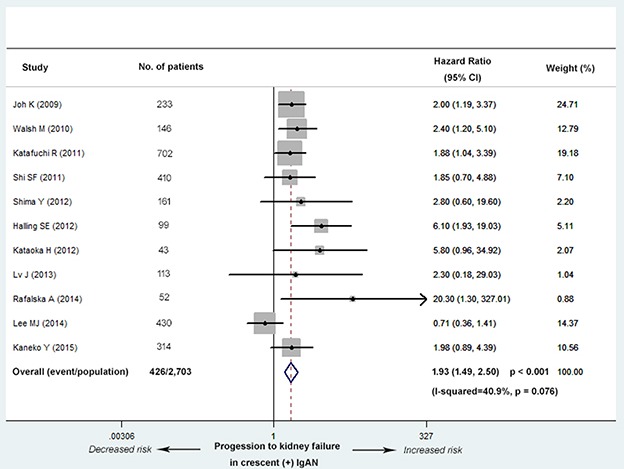
The IgAN patients with crescents had an increased risk of worse kidney outcome (C0 as reference; HR, 1.93; 95% CI, 1.49-2.50; P < 0.001), with no evidence of heterogeneity (I2 = 40.9%; P = 0.076).
The analyses for Asian and European populations were also conducted separately. The result indicated that ethnicity had no obvious impact on our conclusion (C0 as reference; Asia, HR, 1.69; 95% CI, 1.27-2.25; P < 0.001; Europe and North America, HR, 3.42; 95% CI, 1.88-6.21; P < 0.001; Supplementary Figure 1), with no evidence of heterogeneity separately (Asia, I2 = 23.1%; P = 0.246; Europe and North America, I2 = 42.8%; P = 0.174). Meanwhile, based on 3 confounders from Supplementary Table 1 (age, the percentage of patients under the age of 18 and use of immunosuppressive therapy), eight studies with more consistent baseline characteristics were included in a new meta-analysis. This analysis also verified the conclusion above (C0 as reference; HR, 2.13; 95% CI, 1.59-2.85; P < 0.001; Supplementary Figure 2), with no evidence of heterogeneity (I2 = 0.0%; P = 0.757).
Secondary outcome
eGFR and proteinuria levels
Seven studies (5 Asian and 2 European) with 2,762 patients reported a difference in eGFR levels between IgAN with and without crescents. These studies showed that the eGFR level was significantly decreased in C1 group (standard mean difference, abbreviated as SMD, -0.21; 95% CI, -0.40--0.03; P = 0.023; Figure 3). Evidence of high heterogeneity lay in the magnitude of the effect across the included studies (I2 = 78.0%; P < 0.001). Univariate meta-regression analysis revealed the presence of heterogeneity mainly in the effect of design type (multi-center or single-center cohort, coefficient= 0.41; 95% CI, 1.02-2.24; P = 0.042) and ethnicity (Asian or not, coefficient= -0.65; 95% CI, 0.29-0.93; P = 0.033) (Table 2).
Figure 3. Standard mean differences (SMD) for the level of eGFR for patients with versus without cellular/fibrocellular crescents.
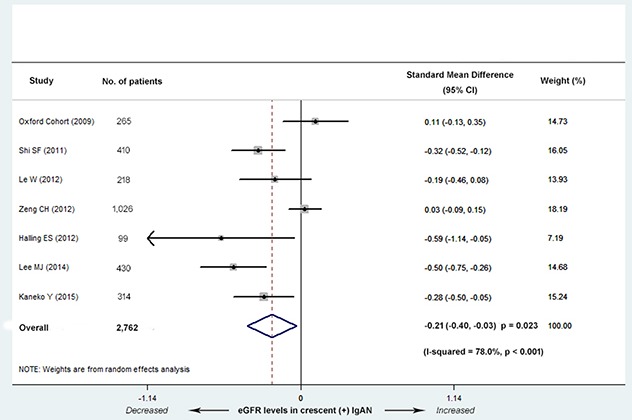
The IgAN patients with crescents had decreased eGFR levels (SMD, -0.21; 95% CI, -0.40--0.03; P = 0.023), with high heterogeneity (I2 = 78%; P < 0.001).
Table 2. Univariate metaregression analysis of possible sources of heterogeneity across studies.
| Possible source of heterogeneity | Adjusted-R2 (%)a | Pb |
|---|---|---|
| No. of patients | 0.3 | 0.301 |
| Ethnicity (Asian or not) | 61.78 | 0.033 |
| Study design (multi- or single-center cohort) | 87.86 | 0.042 |
| Age | −9.30 | 0.310 |
| Age < 18 y (%) | −28.77 | 0.627 |
| Follow-up time (months) | 14.74 | 0.152 |
| Male/female | −10.84 | 0.465 |
| Hypertension (%) | −14.07 | 0.281 |
| Immunosuppressive therapy (%) | −35.70 | 0.926 |
| Treatment with RASBs (%) | −36.04 | 0.682 |
| End point eventc number | −2.84 | 0.351 |
aProportion of between-study variance explained by covariates.
bP value derived from the joint test for all covariates with Knapp–Hartung modification.
cEnd-stage kidney disease (ESRD), >50% decrease in estimated glomerular filtration rate (eGFR), or doubling of serum creatinine concentration.
Three studies (2 Asian and 1 European) with 486 patients reported a difference in the proteinuria level between C0 and C1 group. These studies showed that the proteinuria level was significantly increased in C1 group (SMD, 0.87; 95% CI, 0.11-1.63; P = 0.024; Figure 4). Evidence of high heterogeneity was noted in the magnitude of the effect across the included three studies (I2 = 89.2%; P < 0.001). The number of included studies was too small to perform the univariate meta-regression analysis.
Figure 4. Standard mean differences (SMD) for the level of proteinuria for patients with versus without cellular/fibrocellular crescents.
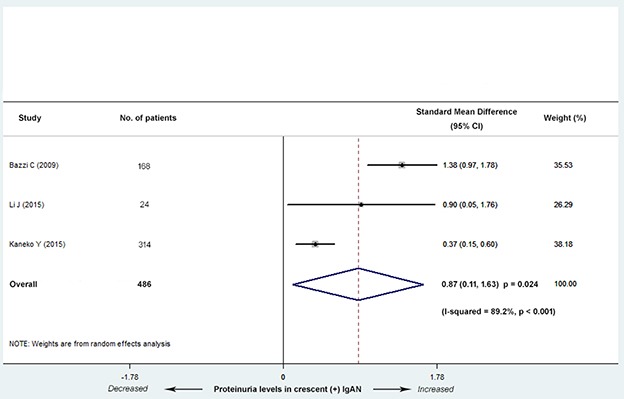
The IgAN patients with crescents had increased proteinuria levels (SMD, 0.87; 95% CI, 0.11-1.63; P = 0.024), with high heterogeneity (I2 = 89.2%; P < 0.001).
Use of immunosuppressive therapy and RASBs
Four studies (3 Asian and 1 European) with 1,008 patients reported the different use of immunosuppressive therapy between C0 and C1 group. These studies showed that patients with C1 were more likely received immunosuppressive treatment in a fixed-effects model (risk difference, abbreviated as RD, 0.17; 95% CI, 0.11-0.23; P < 0.001; Figure 5), with no evidence of heterogeneity (I2 = 48.9%; P = 0.118).
Figure 5. Risk differences (RD) for immunosuppressive therapy for patients with versus without cellular/fibrocellular crescents.
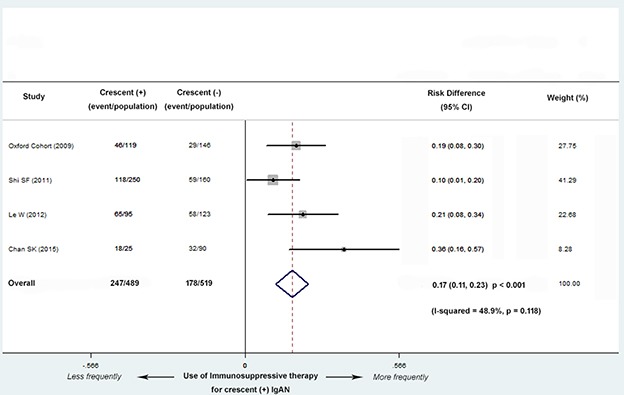
The IgAN patients with crescents were more likely received immunosuppressive treatment (RD, 0.17; 95% CI, 0.11-0.23; P < 0.001), with no evidence of heterogeneity (I2 = 48.9%; P = 0.118).
Three studies (2 Asian and 1 European) with 893 patients reported the different use of RASBs treatment between C0 and C1 group. There were no significant differences in the use of RASBs between the two groups (RD, 0.09; 95% CI, -0.01-0.19; P = 0.071; Figure 6), with moderate evidence of heterogeneity (I2 = 69.0%; P = 0.040).
Figure 6. Risk differences (RD) for RASBs treatment for patients with versus without cellular/fibrocellular crescents.
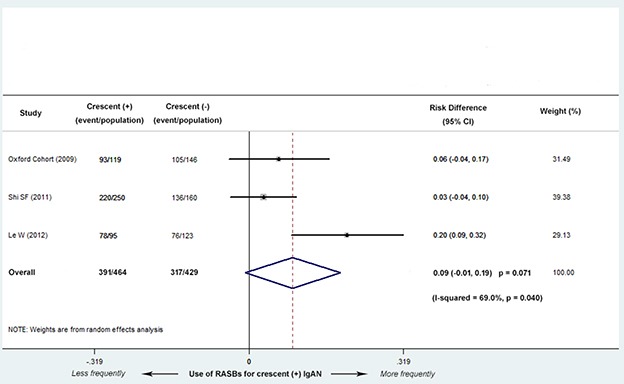
There were no significant differences in the use of RASBs between the two groups (RD, 0.09; 95% CI, -0.01-0.19; P = 0.071), with evidence of heterogeneity (I2 = 69.0%; P = 0.040).
Association with other lesions
Four studies (3 Asian and 1 European) with 1,140 patients reported the different proportions of the four Oxford Classification pathologic lesions (M, E, S and T) between C0 and C1 group. These studies demonstrated that the number of patients with M1 (defined as M score > 0.5; risk ratio, abbreviated as RR, 1.22; 95% CI, 1.07-1.40; P = 0.003; Figure 7), E1 (defined as any E lesion present; RR, 4.83; 95% CI, 3.04-7.66; P < 0.001; Figure 7), S1 (defined as any S lesion present; RR, 1.76; 95% CI, 1.11-2.80; P = 0.016; Figure 7), and T1/2 (defined as > 25% but < 50% and > 50% T lesion present; RR, 2.74; 95% CI, 2.10-3.57; P < 0.001; Figure 7) were significantly larger in C1 group. There was no evidence of heterogeneity in the magnitude of the effect in M, E, T (M1, I2 = 24.0%; P = 0.267; E1, I2 = 64.8%; P = 0.059;I2 = 17.4%; P= 0.271), and high heterogeneity in S1 (I2 = 87.4%; P < 0.001). The number of included studies in S was not sufficient to perform the univariate meta-regression analysis.
Figure 7. Risk ratios (RR) for other lesions for patients with versus without cellular/fibrocellular crescents.
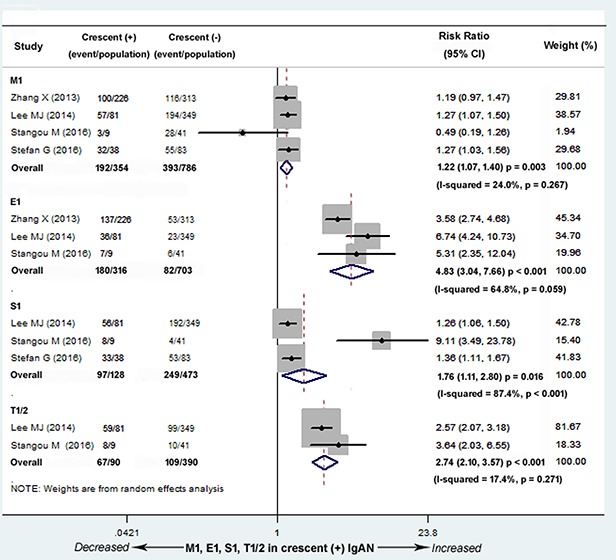
The numbers of patients with M1 (RR, 1.22; 95% CI, 1.07-1.40; P = 0.003), E1 (RR, 4.83; 95% CI, 3.04-7.66; P < 0.001), S1 (RR, 1.76; 95% CI, 1.11-2.80; P = 0.016), and T1/2 (RR, 2.74; 95% CI, 2.10-3.57; P < 0.001) were significantly larger in the IgAN patients with crescents, with no evidence of heterogeneity in M, E, T (M1, I2 = 24.0%; P = 0.267; E1, I2 = 64.8%; P = 0.059; I2 = 17.4%; P = 0.271), and high heterogeneity in S1 (I2 = 87.4%; P < 0.001). M1 defined as M score > 0.5, E1 defined as any E lesion present, S1 defined as any S lesion present, T1/2 defined as > 25% but < 50% and > 50% T lesion present.
Publication bias
No obvious asymmetry was observed in the funnel plot (Figure 8). Furthermore, the results of Begg's test (P = 0.119) and Egger's test (P = 0.100) indicated no obvious publication bias among the 11 studies accessing the association of crescent formation in IgAN with kidney survival.
Figure 8. Funnel plot for testing the publication bias of the 11 studies evaluating the association between crescent formation and kidney survival.
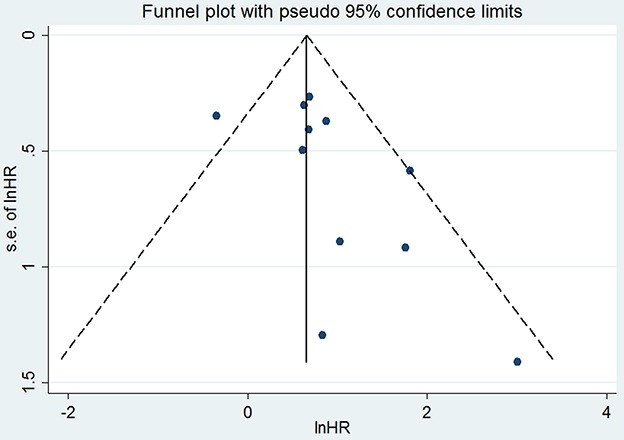
No obvious publication bias affected the association of crescent formation in IgAN with kidney survival. HR: hazard ratio; s.e.: standard error.
DISCUSSION
In the 2009 Oxford Classification, four pathologic lesions were proposed as independent prognostic markers in IgAN: mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), and tubular atrophy and interstitial fibrosis (T). This classification has been validated in numerous studies with different ethnic populations [7, 10–14, 16, 18, 28]. However, the predictive value of crescent lesions for worse kidney survival was not determined mostly due to the low prevalence of crescents in included patients and the lack of end point events [6].
Systematic review and meta-analysis is an appropriate strategy to overcome the limitations noted above. In the present study with 2,703 patients and 426 end point events, we found that crescents were associated strongly with the progression to kidney failure. Compared with C0 group, C1 group had an increased risk of worse kidney outcome, with no evidence of heterogeneity across all included studies. A large number of crescent lesions (>50% of all glomeruli) often lead to rapidly progressive renal failure in IgAN patients [6, 29, 30]. In addition, the prognostic value of moderate crescent formation (<50% of all glomeruli) in IgAN remains controversial [31, 32]. In this meta-analysis, we were unable to perform a subgroup analysis to explore the predictive significance of mild, moderate or severe crescent formation in IgAN respectively. It was because only 5 of the 20 included studies [10, 12, 16–18] had the exact definitions of C1 according to the percentage of glomeruli with crescent lesions, and the definitions were inconsistent among these studies. The JHC 2013 of IgAN confirmed crescent formation as the predictive marker of ESKD and developed four histological grades (HGs) established corresponding to the percentage of glomeruli exhibiting cellular or fibrocellular crescents, global sclerosis, segmental sclerosis or fibrous crescents, respectively [19], but we were unable to determine the precise proportion of crescent lesions in each grade. Therefore, the accurate degree of crescent formation with prognostic value in IgAN should still be further explored.
It is notable that our study also explored some secondary clinical outcomes not included in other similar meta-analyses [28]. First, the patients with crescents had a significantly increased proteinuria level and reduced eGFR level than those without this lesion. Using meta-regression we identified the interaction among study design (multi-center or single-center cohort), ethnicity (Asian or not) and eGFR level. This finding might be attributed to variable baseline levels of renal biomarkers in different ethnic populations. Second, the patients with crescents received immunosuppressive therapy more frequently. However, whether crescent lesions were more responsive to immunosuppressant or RASBs treatment is not known due to the limited number of studies [11, 18, 33]. Finally, the numbers of patients with M1, E1, S1 and T1/2 lesions were considerably increased in C1 group. These results suggested crescent formation as a predictive factor of worse renal outcome from another clinical aspect.
This meta-analysis has some strengths. On one hand, a wide range of IgAN populations with crescents were included. The number of patients was approximately 20-fold increased compared with that of the 2009 Oxford cohort and supported the use of crescent lesions in the classification of IgAN. On the other hand, some renal biomarkers, M, E, S, T and treatment strategies were observed as secondary outcomes of crescent formation in IgAN, which had not been described in previous meta-analyses. There are also some limitations in the present study. First, all the included studies were retrospective. This study was not an individual patient-data meta-analysis, so we could not evaluate the interaction between crescent lesions and their response to two main treatments. Second, the number of included studies for evaluating secondary renal outcomes was too small. This limitation could explain the high heterogeneity among the included studies for evaluating the level of eGFR, proteinuria and the number of patients with S1 lesions. Finally, the quality of the included studies was variable. Unlike randomized clinical trials, a reliable and robust tool is not currently available to evaluate the risk of bias in nonrandomized studies. Thus, we could not evaluate the effect of study quality on our pooled data. To reduce the possibility of publication bias, we also searched conference proceedings and consulted experts to identify unpublished studies.
In conclusion, our study confirms that crescent lesions in IgAN are associated strongly with progression to kidney failure. In addition, IgAN patients with crescents exhibit increased proteinuria levels, reduced eGFR levels, and a larger number of patients with M1, E1, S1 and T1/2 lesions and received immunosuppressive therapy more frequently. Therefore, our finding supports the addition of crescent lesions to the new Oxford Classification to identify IgAN patients with an increased rate of progression to ESKD.
MATERIALS AND METHODS
Data sources, search strategy, and selection criteria
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) was used as a guide in our study to ensure a standard approach for transparent and complete reporting of systematic reviews and meta-analyses [34]. Relevant studies were identified by searching PubMed and EMBASE (from January 2009 to July 2016) with medical subject heading terms and text words as follows in multiple combinations: “IGA Nephropathy,” “IGA Type Nephritis,” “Nephropathy, Immunoglobulin A,” “Immunoglobulin A Nephropathy,” “IGA Glomerulonephritis,” “Glomerulonephritides, IGA,” or “Berger Disease”; “Extracapillary proliferation,” “Extracapillary hypercellularity,” or “Crescent”. No language or region restrictions were imposed. In addition, other relevant studies were also identified through manual searches of the reference lists and bibliographies.
All published articles that met the following inclusion criteria were included in this meta-analysis: primary IgAN classified as C0 and C1; study end points included ESKD, >50% decrease in eGFR, or doubling of serum creatinine concentration; reports showed available data of kidney outcomes including the level of eGFR (ml/min/1.73m2), urinary protein excretion (g/d) and the number of patients with other lesions (defined as M, E, S and T in the Oxford Classification); reports disclosed the number of patients using the immunosuppressive therapy or RASBs. Studies meeting the following criteria were excluded: duplication, basic research studies, case reports, nonoriginal studies including reviews, commentaries, letters and editorials, and studies that did not investigate crescent formation as a variable or clinical characters outcomes. The literature search, data extraction, and validity assessment were performed by 2 coauthors (B.L. and L.C.).
Data extraction and quality assessment
Published reports were obtained for eligible studies, and standardized information was presented in Table 1. The extracted data were as follows: participants’ characteristics, follow-up duration, mean arterial blood pressure (MAP), renal function measurements (eGFR and 24-hour urine protein excretion), number and percentage of patients receiving treatment (immunosuppressive therapy and RASBs), number of end point events, pathologic methodology, characteristics of the Oxford Classification pathologic lesions (M, E, S and T) and crescent lesions, and statistical methodology. Disagreements about the extracted data were adjudicated by a third reviewer (X.S.) to make a final decision.
Statistical analysis
All of the statistical analyses were performed using Stata, version 12.0 (StataCorp LP). Individual-study HRs, RRs and RDs with 95% CIs were calculated or extracted from each study for dichotomous outcomes and SMD for continuous outcomes. The heterogeneity among the studies was examined with the I2 statistics. The potential heterogeneity in the predictive value of crescent formation was explored by comparing the summary results obtained from the studies: number of patients, ethnicity, study design, age, follow-up time, gender, hypertension, immunosuppressive and RASBs treatment, and number of end point events. Fixed-effects models (Inverse Variance or Mantel-Haenszel) were used for low heterogeneity, otherwise, the random effect models were used. Moreover, publication bias was assessed by Egger's test and Begg's test among the 11 studies evaluating the association of crescent formation with kidney survival. A two-tailed P < 0.05 was considered statistically significant for all analyses.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
This study received no specific funding. The authors thank all the participants for their support in this study.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Koyama A, Igarashi M, Kobayashi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research Group on Progressive Renal Diseases. Am J Kidney Dis. 1997;29:526–32. doi: 10.1016/s0272-6386(97)90333-4. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–37. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–14. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 4.Roufosse CA, Cook HT. Pathological predictors of prognosis in immunoglobulin A nephropathy: a review. Curr Opin Nephrol Hypertens. 2009;18:212–9. doi: 10.1097/MNH.0b013e328329605c. [DOI] [PubMed] [Google Scholar]
- 5.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D'Agati V, D'Amico G, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–56. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 6.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D'Agati V, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 7.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts IS, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–36. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joh K, Hashiguchi A, Okonogi H, Miyazaki Y, Utsunomiya Y, Kawamura T. Histological evaluation on IgA nephropathy in 233 Japanese adult patients: retrospective analysis [abstract; poster no.12]. Oral and poster presentation inWorld Congress of Nephrology 2009 Satellite Symposium on IgA Nephropathy; May 22-26, 2009; Milan, Italy. [Google Scholar]
- 9.Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, Hemmelgarn B. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5:425–30. doi: 10.2215/CJN.06530909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H. Validation study of Oxford classification of IgA nephropathy: the significance of extracapillary proliferation. Clin J Am Soc Nephrol. 2011;6:2806–13. doi: 10.2215/CJN.02890311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi SF, Wang SX, Jiang L, Lv JC, Liu LJ, Chen YQ, Zhu SN, Liu G, Zou WZ, Zhang H, Wang HY. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–84. doi: 10.2215/cjn.11521210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Hashimura Y, Kaito H, Sako M, Iijima K, Yoshikawa N. Validity of the Oxford classification of IgA nephropathy in children. Pediatr Nephrol. 2012;27:783–92. doi: 10.1007/s00467-011-2061-0. [DOI] [PubMed] [Google Scholar]
- 13.Edstrom Halling S, Soderberg MP, Berg UB. Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification) Nephrol Dial Transplant. 2012;27:715–22. doi: 10.1093/ndt/gfr339. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka H, Ohara M, Shibui K, Sato M, Suzuki T, Amemiya N, Watanabe Y, Honda K, Mochizuki T, Nitta K. Overweight and obesity accelerate the progression of IgA nephropathy: prognostic utility of a combination of BMI and histopathological parameters. Clin Exp Nephrol. 2012;16:706–12. doi: 10.1007/s10157-012-0613-7. [DOI] [PubMed] [Google Scholar]
- 15.Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, Wang C, Li S, Zhang J, Zhang J, Liu L, Shi S, Wang S, et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol. 2013;24:2118–25. doi: 10.1681/asn.2012101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rafalska A, Franczuk J, Franczuk P, Augustyniak-Bartosik H, Krajewska M. Stratifying risk for progression in IgA nephropathy: how to predict the future? Pol Arch Med Wewn. 2014;124:365–72. doi: 10.20452/pamw.2341. [DOI] [PubMed] [Google Scholar]
- 17.Lee MJ, Kim SJ, Oh HJ, Ko KI, Koo HM, Kim CH, Doh FM, Yoo TH, Kang SW, Choi KH, Lim BJ, Jeong HJ, Han SH. Clinical implication of crescentic lesions in immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29:356–64. doi: 10.1093/ndt/gft398. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko Y, Yoshita K, Kono E, Ito Y, Imai N, Yamamoto S, Goto S, Narita I. Extracapillary proliferation and arteriolar hyalinosis are associated with long-term kidney survival in IgA nephropathy. Clin Exp Nephrol. 2016;2:569–77. doi: 10.1007/s10157-015-1185-0. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura T, Joh K, Okonogi H, Koike K, Utsunomiya Y, Miyazaki Y, Matsushima M, Yoshimura M, Horikoshi S, Suzuki Y, Furusu A, Yasuda T, Shirai S, et al. A histologic classification of IgA nephropathy for predicting long-term prognosis: emphasis on end-stage renal disease. J Nephrol. 2013;26:350–7. doi: 10.5301/jn.5000151. [DOI] [PubMed] [Google Scholar]
- 20.Le W, Zeng CH, Liu Z, Liu D, Yang Q, Lin RX, Xia ZK, Fan ZM, Zhu G, Wu Y, Xu H, Zhai Y, Ding Y, et al. Validation of the Oxford classification of IgA nephropathy for pediatric patients from China. BMC Nephrol. 2012;13:158. doi: 10.1186/1471-2369-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, Wang R, Lv Z, Chen J, Tian J, Chen N, Pan X, Fu P, et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis. 2012;60:812–20. doi: 10.1053/j.ajkd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Bazzi C, Rizza V, Raimondi S, Casellato D, Napodano P, D'Amico G. In crescentic IgA nephropathy, fractional excretion of IgG in combination with nephron loss is the best predictor of progression and responsiveness to immunosuppression. Clin J Am Soc Nephrol. 2009;4:929–35. doi: 10.2215/CJN.05711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Liu CH, Gao B, Xu DL. Clinical-pathologic significance of CD163 positive macrophage in IgA nephropathy patients with crescents. Int J Clin Exp Med. 2015;8:9299–305. [PMC free article] [PubMed] [Google Scholar]
- 24.Chan SK, Lo HL, Ho YW, Tam CH, Tang WCA, Lam CKD, Wong SHS. Outcome of IgAN with or without crescent formationda restrospective kidney survival analysis. Hong Kong J Nephrol. 2015;17:S40–S1. [Google Scholar]
- 25.Zhang X, Xie J, Wang W, Pan X, Guo S, Shen P, Zhang W, Chen N. Crescents in IGA nephropathy: a clinical-pathological study. Nephrol Dial Transplant. 2013;28:i178. [Google Scholar]
- 26.Stangou M, Bantis C, Skoularopoulou M, Korelidou L, Kouloukouriotou D, Scina M, Labropoulou IT, Kouri NM, Papagianni A, Efstratiadis G. Th1, Th2 and Treg/T17 cytokines in two types of proliferative glomerulonephritis. Indian J Nephrol. 2016;26:159–66. doi: 10.4103/0971-4065.159303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefan G, Stancu S, Zugravu A, Ismail G, Mandache E, Mircescu G. The significance of extra capillary proliferation in IGA nephropathy. Nephrol Dial Transplant. 2016;31:i370–i1. [Google Scholar]
- 28.Lv J, Shi S, Xu D, Zhang H, Troyanov S, Cattran DC, Wang H. Evaluation of the Oxford Classification of IgA nephropathy: a systematic review and meta-analysis. Am J Kidney Dis. 2013;62:891–9. doi: 10.1053/j.ajkd.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Abe T, Kida H, Yoshimura M, Yokoyama H, Koshino Y, Tomosugi N, Hattori N. Participation of extracapillary lesions (ECL) in progression of IgA nephropathy. Clin Nephrol. 1986;25:37–41. [PubMed] [Google Scholar]
- 30.Nicholls K, Walker RG, Dowling JP, Kincaid-Smith P. Malignantamp IgA nephropathy. Am J Kidney Dis. 1985;5:42–6. doi: 10.1016/s0272-6386(85)80134-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Shi S, Xie J, Lv J, Wang W, Pan X, Ren H, Shen P, Zhang H, Chen N. Moderate crescent formation does not correlate with IgA nephropathy progression. Hong Kong J Nephrol. 2015;17:S44–S5. [Google Scholar]
- 32.Zhang W, Zhou Q, Hong L, Yang Q, Chen W, Yu X. Clinical outcomes of IgA nephropathy patients with different proportions of crescents. Hong Kong J Nephrol. 2015;17:S35. doi: 10.1097/MD.0000000000006190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka S, Ninomiya T, Katafuchi R, Masutani K, Nagata M, Tsuchimoto A, Hirakata H, Kitazono T, Tsuruya K. The effect of renin-angiotensin system blockade on the incidence of end-stage renal disease in IgA nephropathy. Clin Exp Nephrol. 2016;20:689–98. doi: 10.1007/s10157-015-1195-y. [DOI] [PubMed] [Google Scholar]
- 34.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


