Abstract
Adenomatous polyposis coli (APC) promoter hypermethylation has been frequently observed in colorectal cancer (CRC). The association between APC promoter methylation and clinicopathological significance in CRC is under investigation. We performed a meta-analysis to quantitatively evaluate the significance of APC methylation in CRC. The study included a total of 24 articles and 2025 CRC patients. The frequency of APC promoter hypermethylation was significantly higher in colorectal adenoma than in normal colorectal tissue, OR was 5.76, 95% CI, 2.45-13.56; p<0.0001, I2=0%. APC promoter more frequently hypermethylated in CRC stage I compared to normal colorectal tissue, OR was 13.42, 95% CI, 3.66-49.20; p<0.0001, I2=31%. The risk of incidence of CRC was significantly correlated to APC promoter hypermethylation, pooled OR was 9.80, 95%CI, 6.07-15.81; p<0.00001, I2=43%. APC methylation was not associated with grade, stage of CRC as well as tumor location, patients’ gender, and smoking behavior. The results indicate that APC promoter hypermethylation is an early event in carcinogenesis of CRC, could be a valuable diagnostic marker for early-stage CRC. APC methylation is not significantly associated with overall survival in patients with CRC. APC is a potential drug target for development of personalized treatment.
Keywords: adenomatous polyposis coli, APC, methylation, biomarker, adenoma
INTRODUCTION
Colorectal cancer (CRC) is one of the most common types of cancer worldwide and results from the accumulation of genetic and epigenetic alterations in colonic mucosa cells, which ultimately leads to colorectal adenoma, advanced to invasive and metastatic CRC. Unfortunately, the prognosis of CRC in late stages is still poor and the search of novel diagnostic and prognostic biomarkers is highly desired to prevent CRC-related mortality. During last decade, epigenetic alterations have been reported to play an important role in many cancers initiation, progression, and metastasis [1, 2]. DNA methylation within CpG island in promoter region of genes is associated with the loss of gene expression and is observed in many types of cancers including CRC. Adenomatous polyposis coli (APC), a suppressor gene, is located at chromosomal band 5q21-q22 and consists of 15 exons. APC was discovered by genetic linkage analysis in familial adenomatous polyposis (FAP) and was reported by Kinzler [3], Nishisho [4], Joslyn [5] and Groden [6]. Recently APC is thought of as a negative regulator in Wnt/beta-catenin signaling pathway. Loss of APC function leads to the destabilization and degradation of beta-catenin, and the nuclear accumulation of beta-catenin results in the activation of T-cell factor/LEF target gene and initiates tumorgenesis [7, 8]. APC along with several other inactivated genes plays a prognostic indicatory role in squamous cell and adenocarcinoma of esophagus, bladder and lung cancers [9]. In the past two decades, APC promoter hypermethylation was frequently observed in sporadic and familial CRC. However, the association between clinicopathological significance and APC methylation was under investigated. The present article aims to summarize the most recent findings concerning the use of epigenetic (mainly related to DNA methylation) biomarkers for CRC diagnosis, progression, and response to treatment.
RESULTS
Identification of relevant studies
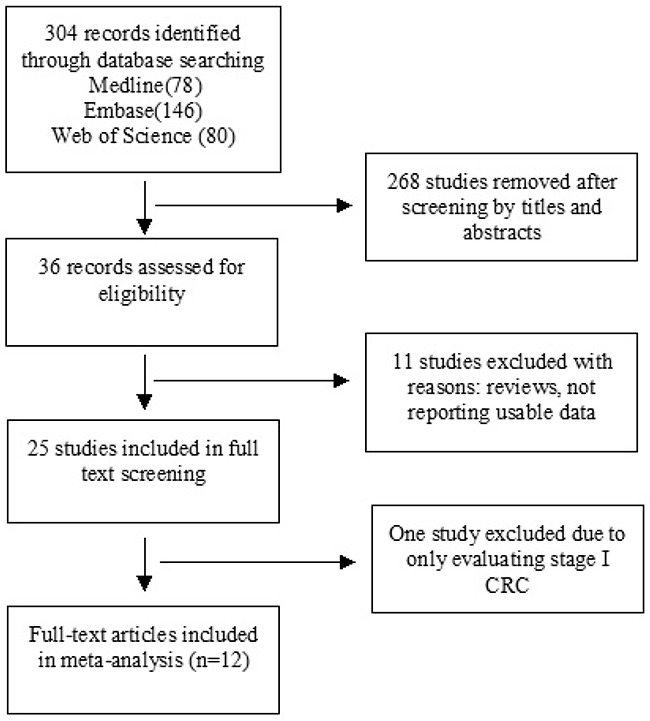
36 publications were identified by the search method as described above. Eleven of those were excluded due to laboratory studies, non-original articles (review), or studies irrelevant to the current analysis. Eventually, there were 24 studies included in the final meta-analysis as shown in Figure 1.
Figure 1. Schematic flow diagram for selection of included studies.
Study characteristics
24 studies published from 2004 to 2015 were eligible for meta-analysis. A total of 1396 samples including CRC, colorectal adenoma and normal control tissues from Greece, Iran, Sweden, Vietnam, China, South Korea, Japan, UK, Kashmir, Czech Republic, Australia, Netherland, Germany, Norway, and USA were included in the analysis. Their basic characteristics are summarized in Table 1.
Table 1. Main characteristics of included studies.
| Author | Year | Country | Methods | Histology | Tumor location | Stage (TNM) | Grade | Smoking status | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT | Ade | CRC | Proximal | Distal | I+II | III+IV | L | H | + | - | ||||
| Michailidi [33] | 2015 | Greece | MSP | 12/14 | - | 18/61 | - | - | - | - | - | - | - | - |
| Samaei [34] | 2014 | Iran | MSP | 0/125 | - | 44/125 | 29/36 | 18/50 | 24/56 | 22/69 | 34/100 | 10/25 | - | - |
| Dimberg [35] | 2013 | Sweden/Vietnam | MSP | 66/101 | - | 50/101 | - | - | - | - | - | - | - | - |
| Pack [36] | 2013 | Korea | MSP | 2/10 | 6/10 | - | - | - | - | - | - | - | - | - |
| Qiu [37] | 2014 | China | MSP | 1/10 | 45/67 | 44/70 | 21/35 | 23/35 | - | - | - | - | - | - |
| Gay [38] | 2012 | UK | Pyrosequencing | - | - | - | 22/59 | 48/112 | 22/87 | 14/69 | 61/141 | 6/25 | - | - |
| Kang [39] | 2012 | Korea | Q-MSP | 2/14 | - | 52/100 | - | - | 28/52 | 24/48 | - | - | 12/20 | 40/80 |
| Leong [40] | 2011 | UK | Q-MSP | 1/19 | 22/51 | - | - | |||||||
| Naghibalhossaini [41] | 2011 | Iran | MSP | - | - | - | 30/30 | 73/80 | 66/71 | 26/28 | 48/49 | 42/48 | 53/56 | 50/54 |
| Sameer [42] | 2011 | Kashmir | MSP | - | - | - | - | - | - | - | 9/38 | 38/48 | 33/55 | 14/31 |
| Vasovcak [43] | 2011 | Czech Republic | MS-MLPA | - | - | - | 26/34 | 45/60 | 38/56 | 30/44 | - | - | - | - |
| Belshaw [44] | 2010 | UK | Q-MSP | 2/8 | - | 2/5 | - | - | - | - | - | - | - | - |
| Kim [45] | 2010 | Korea | Pyrosequencing | - | - | - | 9/68 | 36/217 | 28/169 | 24/116 | 40/253 | 5/32 | - | - |
| Kamiyama [46] | 2009 | Japan | Q-MSP | - | - | - | 6/20 | 10/25 | - | - | - | - | - | - |
| Derks [47] | 2006 | European/USA | MSP | 3/18 | 17/34 | 10/18 | - | - | - | - | - | - | ||
| Iacopetta [27] | 2006 | Australia | Q-MSP | - | - | - | 24/90 | 33/106 | - | - | 41/122 | 6/28 | - | - |
| Brandes [48] | 2005 | Netherlands | MSP | - | - | - | - | - | 19/30 | 6/14 | - | - | - | - |
| Chen [49] | 2005 | Germany | MSP | 0/14 | - | 17/34 | - | - | - | - | - | - | - | - |
| Ebert [50] | 2005 | Germany | MSP | 0/21 | 10/47 | - | - | - | - | - | - | - | - | |
| Kim [51] | 2005 | Korea | MSP | 6/40 | 7/36 | - | - | - | - | - | - | - | - | |
| Bai [52] | 2004 | China | MSP | 1/34 | 28/47 | - | - | - | - | - | - | - | - | |
| Lee [53] | 2004 | Korea | MSP | 3/24 | 34/95 | 76/149 | 29/56 | 47/93 | - | - | - | - | - | - |
| Lind [54] | 2004 | Norway | MSP | - | - | - | 7/18 | 13/37 | 13/31 | 8/26 | 16/44 | 4/11 | - | - |
| Xu [55] | 2004 | China | MSP | 0/6 | 2/8 | 5/65 | - | - | - | - | - | - | - | - |
MSP: methylation-specific PCR; MS-MLPA: methylation specific multiplex ligation-dependent probe amplification, NCT: normal control tissue; Ade: adenoma; CRC: colorectal carcinoma; L: low grade; H: high grade.
The correlation of APC hypermethylation with clinicopathological features
1. The inactivation of APC through promoter hypermethylation in adenoma and CRC.
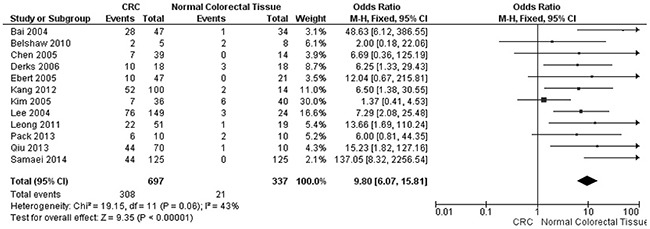
APC promoter hypermethylation was an early event in carcinogenesis. The frequency of APC promoter hypermethylation was significantly increased in adenoma than in normal colorectal tissues, OR was 5.76, 95%CI, 2.45-13.56; p<0.0001, I2=0% (Figure 2). APC promoter was more frequently hypermethylated in CRC stage I than normal colorectal tissue, OR was 13.42, 95% CI, 3.66-49.20; p<0.0001, I2=31% (Figure 3). The risk of incidence of CRC was significantly correlated to APC promoter hypermethylation, pooled OR was 9.80, 95%CI, 6.07-15.81; p<0.00001, I2=43% (Figure 4).
Figure 2. Forest plot for APC methylation in adenoma and normal colorectal tissue.
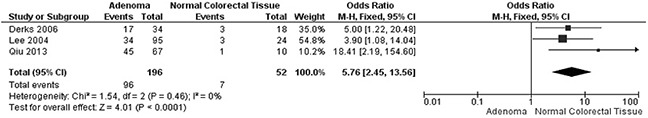
Figure 3. Forest plot for APC methylation in CRC stage I and normal colorectal tissue.
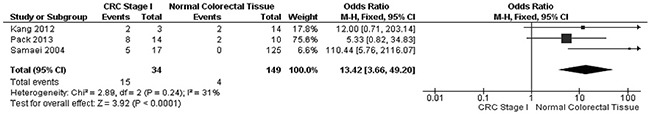
Figure 4. Forest plot for APC methylation in CRC and normal colorectal tissue.
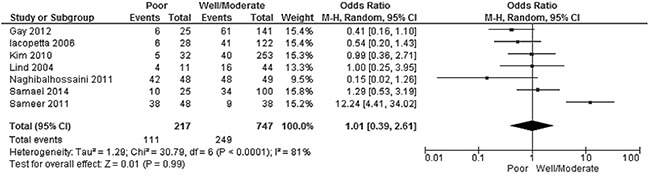
2. APC promoter hypermethylation was not associated with grade and stage of CRC.
The frequency of APC promoter hypermethylation was similar between low and high grade of CRC, pooled OR was 1.01, 95%CI, 039-2.61; p=0.99, I2=81% (Figure 5). There was no difference when comparing the frequency of APC promoter hypermethylation between I/II stage and III/IV stage of CRC, pooled OR was 0.85, 95%CI, 0.63-1.15; p=0.29, I2=0% (Figure 6).
Figure 5. Forest plot for APC methylation in stage III/IV and stage I/II of CRC.
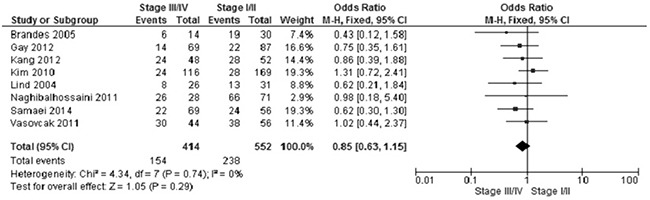
Figure 6. Forest plot for APC methylation in high grade and low grade of CRC.
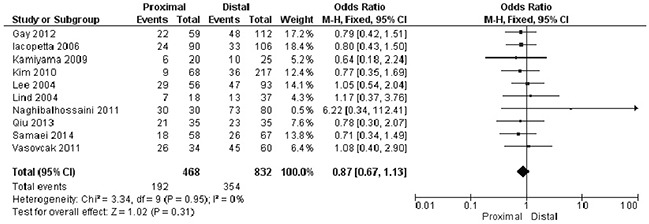
3. There was no statistically significant association between APC methylation status and other clinical parameters, including tumor location, gender and smoking status of CRC patients.
Proximal versus distal: OR was 0.87, 95%CI, 0.67-1.13, p=0.31, I2=0% (Figure 7).
Figure 7. Forest plot for the correlation of APC hypermethylation and location of CRC.
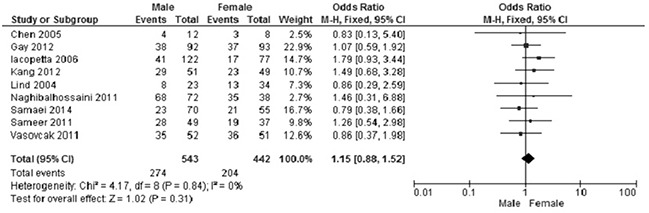
Male versus female: OR was 1.55, 95%CI, 0.88-1.52, p=0.31, I2=0% (Figure 8).
Figure 8. The frequency of APC hypermethylation was similar in male and female CRC patients.
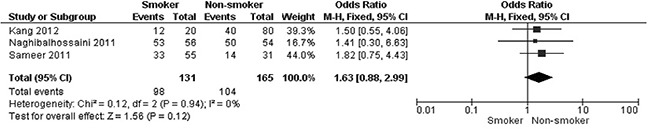
Smoker versus non-smoker: OR was 1.63, 95%CI, 0.88-2.99, p=0.12, I2=0% (Figure 9).
Figure 9. Plot for the relationship of APC hypermethylation and smoking status of patients with CRC.
4. Overall survival was analyzed by selecting Colorectal Adenocarcinoma (TCGA, Nature 2012) [10] and gene APC via cBioPortal for provisional data. The survival curve was plotted on 236 cases (methylation HM27) which included 72 cases with APC hypermethylation (methylation beta-value was more than 0.3) and 164 cases with APC low methylation (methylation beta-value was more than 0.3). T-test p-value was 0.5798, indicating APC methylation was not significantly associated with overall survival in patients with CRC (Figure 10).
Figure 10. Plot for the overall survival of CRC patient with different APC promoter methylation status.
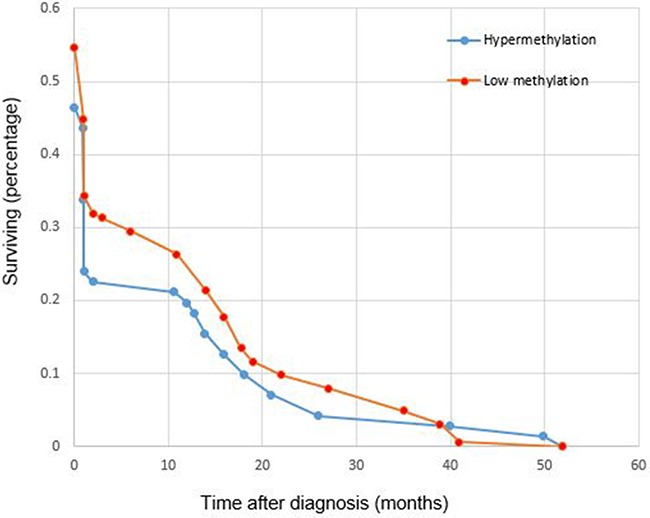
Blue circles represent cases with APC hypermethylation, red circles represent cases with APC low methylation. Test p-value 0.5798.
Sensitivity analyses and publication bias
To minimize the effect of confounders, a sensitivity analysis, in which one study was removed at a time, was conducted to assess the result stability. The pooled ORs were not significantly changed, indicating the stability of our analyses. The funnel plots demonstrates no obvious asymmetry (Figure 11A-11H), suggesting the absence of publication biases in the meta-analysis of APC hypermethylation and clinicopathological features.
Figure 11. Funnel plot for publication bias.
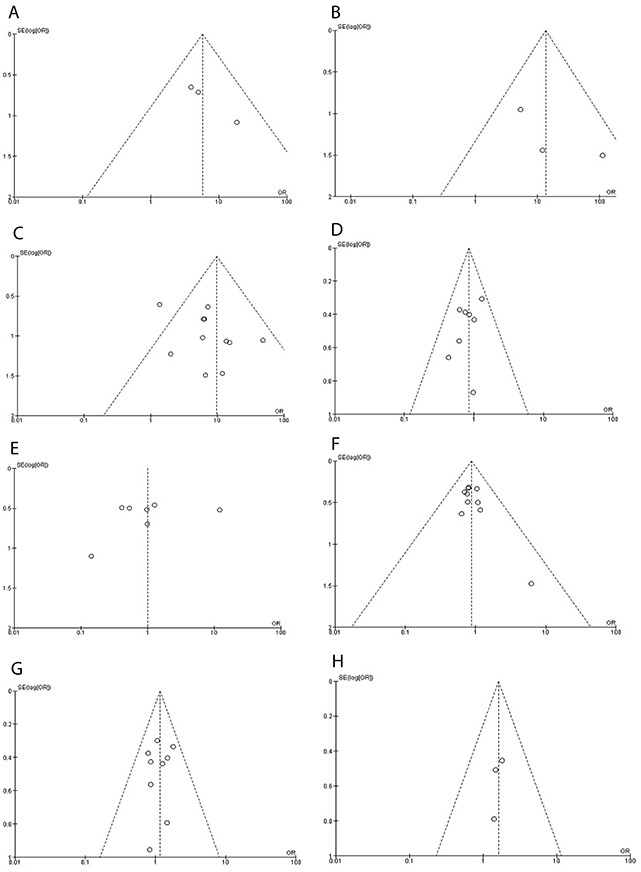
A. APC methylation in adenoma and normal colorectal tissue. B. APC methylation in CRC stage I and normal colorectal tissue. C. APC methylation in CRC and normal colorectal tissue. D. APC methylation in stage III/IV and stage I/II of CRC. E. APC methylation in high grade and low grade of CRC. F. The correlation of APC hypermethylation and location of CRC. G. APC hypermethylation in male and female CRC patients. H. The relationship of APC hypermethylation and smoking status of patients with CRC. S.E.: standard error; area of the circle represents the weight of individual study.
DISCUSSION
CRC is thought to develop from adenomatous polyps following the accumulation of mutations which includes the activation of oncogenes and the inactivation of tumor suppressor genes [11–15]. Tumor suppressor genes associated with CRC include APC, p53, BRAF and DCC [10, 15], and the loss of APC has been observed in approximately 70-80% of CRC [16–19], but the association between APC promoter methylation and clinicopathological significance in CRC is unclear. The current study systematically reviewed all published evidence before July 2016 and synthesized data from three studies and 196 adenoma samples using a meta-analysis. Our result indicated that the APC promoter is 5.76 times more frequently hypermethylated in adenoma than in normal colorectal tissue. In addition, the frequency of APC hypermethylation in CRC stage I was 13.42 times higher than in normal colorectal tissue. Patients with early-stage CRC could expect a long survival with surgery alone, but about 50% stage III and 25% stage II will relapse and need adjuvant chemotherapy [20]. Therefore, APC hypermethylation as a valuable early diagnostic marker could contribute to making the decision whether or not to accomplish chemotherapy. Ding et al published a meta-analysis of the association between APC promoter methylation and colorectal cancer, in which the APC promoter hypermethylation has not been analyzed in early-stage CRC.
We pooled 12 studies and included 762 CRC samples and 343 normal colorectal tissues, and the data demonstrated the frequency of APC hypermethylation in CRC was 9.8 times higher than in normal colorectal tissues; the heterogeneity was 43%. We removed three studies that caused higher heterogeneity (the heterogeneity was 83%, OR was 4.35, 95% CI was 1.56-12.12). One of the three studies (Xu et al.) reported low frequency of APC methylation in CRC (7.7%) compared to other studies (APC methylation rate arranged from 17.9% to 62.8%), two studies (Dimberg at al. and Michailidi et al.) reported high rate of APC methylation (65.3% and 85.7%) in normal colorectal tissues compared to other studies (APC methylation rate arranged 0-25%). The frequency of APC hypermethylation was similar between CRC and adenoma (data not shown), this result is consistent with previous study [21]. Our results suggest that APC promoter hypermethylation is an early event during colorectal carcinogenesis. Previous evidence suggest that APC methylation is not a “second hit” in two hit model of APC mutation in tumor [22]. This explains why APC promoter hypermethylation was an early event during the development of CRC. As the changes in APC promoter hypermethylation are reversible, demethylation with drug could delay carcinogenesis and progression of CRC. Previous studies showed that adenoma formation in APC min/+ was inhibited by 5-aza-deoxycitidine, a demethylation agent [23]. In addition, Eads et al demonstrated that the expression of full-length Dnmt3b1 enhanced the number of colon tumors in APC min/+ mice by approximately twofold and increased the average size of colonic microadenomas [24, 25]. Taken together, APC is a potential drug target for the development of personalized therapy in patients with CRC; further investigation is required in future.
Among the included studies, the frequency of APC methylation have varied greatly from 17.9% to 62.8%. This phenomenon maybe due to ethnic differences, different PCR primers used in the detection, as well as cancer heterogeneity. According to recent classification system, CRC is classified into two major groups: 1) hypermutated cancers with either microsatellite instability due to defective mismatch repair or ultramutated cancers with DNA polymerase epsilon proofreading mutations; 2) non-hypermutated, microsatellite stable cancers with a high frequency of DNA somatic copy number alterations, which showed common mutations in APC, TP53, KRAS, SMAD4 and PIK3CA. APC methylation is present more often in the first group of tumors with microsatellite-stable compared to the second group with microsatellite instability [26–29]. In addition, the APC methylation was inversely associated with TIMP3, TP53 and BRAF methylation [27]. Previous publications reported that APC was less frequently mutated alone, more commonly mutated with KRAS, TP53, PIK3CA and SMAD [22, 27], suggesting that the APC mutation occurs early in carcinogenesis, the alterations of other genes were involved during the transition from adenoma to carcinoma. APC methylation combined with the mutation of other genes could be a valuable biomarker for diagnosis and prognosis of CRC. Further study is necessary to substantiate this issue.
We pooled seven studies and included 964 samples and analyzed the relationship of APC promoter methylation with the grade of CRC; the power was 0.89, which indicated that APC promoter hypermethylation is not associated with grade. Furthermore, present analysis showed that APC promoter hypermethylation is not correlated with stages of CRC, since the power of the study is small, further study with a larger number of samples is need to confirm this relationship.
Consistent results were shown in sensitivity analyses, indicating the stability of our analyses. All funnel plots did not show any obvious asymmetry, suggesting there is no publication bias in the meta-analysis. This study has several potential limitations. First, selection bias may exist since only publications in English and Chinese were included in the present study, which could affect the accuracy of results in certain extent. Caution should be taken when our findings are interpreted. Second, the possibility of information, selection biases and unidentified confounders could not be completely excluded because all of the included studies were observational. Third, our results showed that there is no significant correlation between APC methylation and gender, smoking behavior of CRC patients as well as tumor locations; since the power of the study is small, further evaluation with a larger number of samples is required in future.
In summary, our meta-analysis indicates that APC promoter hypermethylation is an early event of carcinogenesis of CRC, and APC methylation combined with the mutation of other genes could be a valuable biomarker for diagnosis and prognosis of CRC. APC promoter methylation is not significantly associated with overall survival in patients with CRC. APC is a potential drug target for development of personalized therapy.
METHODS
Search strategy
We performed this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [30]. We searched the database of Medline, Web of science, and Embase up to July, 2016 without language limitations. The following items were used for searching: APC, adenomatous polyposis coli, methylation, neoplasm, tumor, colorectal carcinoma, and CRC. A manual search using references from retrieved articles was performed for additional pertinent studies. We chose the most complete study to avoid duplication when the same populations were reported in several publications.
Study selection
Studies were included if they met the following inclusion criteria: 1) investigation APC methylation status and clinicopathological significance in CRC. 2) case-control, and cohort studies published as original studies. 3) studies that provided sufficient data to calculate ORs and 95% confidence interval (CI).
Exclusion criteria were: 1) lack of sufficient data on APC methylation and clinicopathological features in CRC, 2) reviews, case report, conference abstract and expert opinion and letters, 3) all publications regarding in vitro studies.
Quality assessment
The quality of each study was individually evaluated by each investigator utilizing Newcastle-Ottawa quality assessment scale [31]. All observational studies were considered moderate to high quality, with median Newcastle-Ottawa quality assessment scale of 7 (range, 6-9) (data not shown).
Data extraction
A standardized data extraction form was used. Eligible studies were reviewed and the following data were extracted: (1) first author's name, (2) year of study, (3) study location, (4) methylation detect methods, (5) sample size, (5) tumor location, cancer TMN stages and grade (6) gender and smoking status of participants.
Survival analysis with TCGA data
Overall survival was analyzed by selecting Colorectal Adenocarcinoma (TCGA, Nature 2012) and gene APC via cBioPortal for provisional data. APC methylation and overall survival data were downloaded. Hypermethylation and Low methylation were sorted out according to methylation beta-value. If the methylation beta-value was more than 0.3, the case was considered as hypermethylation, if the methylation beta-value was less than 0.3, the case was considered as low methylation. The overall survival was plotted on 236 cases (methylation HM27) which included 72 cases with APC hypermethylation (methylation beta-value was more than 0.3) and 164 cases with APC low methylation (methylation beta-value was less than 0.3) with Excel 2013.
Statistical analysis
Review Manage 5.3 from the Cochrane Collaboration was used for data analysis. Odds Ratio with 95% confidence intervals (CIs). This statistic was complemented with the I2 statistic, which quantifies the proportion of the cumulative variation across studies that is due to heterogeneity rather than chance. When heterogeneity was not an issue (I2 values <50%), a fixed effect model was used to calculate parameters. When there was substantial heterogeneity (I2 values ≥50%), a random-effects model was used to pool data and attempt to identify potential sources of heterogeneity based on subgroup analyses. Two sided statistical tests and p-value were used.
Evaluation for publication bias
The presence of publication bias was assessed by funnel plots of logarithm of odds ratios versus their standard errors [32].
Acknowledgments
This work is supported by the Key research and development program of Shandong Province, 2016GSF201018 (TL). The funding institution does not have any roles in the study design, data collection, or analysis.
Footnotes
Author contributions
TL, HW, YZ, and YC contributed substantially to the study and design, collection of data, and analysis of data. YZ, XW, and XZ contributed substantially to the acquisition, analysis, interpretation of data and performed the statistical analysis. TL and SD have been involved in the drafting and revision of the article. SD has full access to all data and the final responsibility for the decision to submit the article for publication. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Carmona FJ, Esteller M. Epigenomics of human colon cancer. Mutat Res. 2010;693:53–60. doi: 10.1016/j.mrfmmm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Venkatachalam R, Ligtenberg MJ, Hoogerbrugge N, de Bruijn DR, Kuiper RP, Geurts van Kessel A. The epigenetics of (hereditary) colorectal cancer. Cancer Genet Cytogenet. 2010;203:1–6. doi: 10.1016/j.cancergencyto.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finniear R, Markham A, Groffen J, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 4.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 5.Joslyn G, Carlson M, Thliveris A, Albertsen H, Gelbert L, Samowitz W, Groden J, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, et al. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 6.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 7.Cole AM, Myant K, Reed KR, Ridgway RA, Athineos D, Van den Brink GR, Muncan V, Clevers H, Clarke AR, Sicinski P, Sansom OJ. Cyclin D2-cyclin-dependent kinase 4/6 is required for efficient proliferation and tumorigenesis following Apc loss. Cancer Res. 2010;70:8149–8158. doi: 10.1158/0008-5472.CAN-10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panarelli NC, Vaughn CP, Samowitz WS, Yantiss RK. Sporadic microsatellite instability-high colon cancers rarely display immunohistochemical evidence of Wnt signaling activation. Am J Surg Pathol. 2015;39:313–317. doi: 10.1097/PAS.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 9.Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, Ames S, Glockner S, Piantadosi S, Gabrielson E, Pridham G, Pelosky K, Belinsky SA, Yang SC, et al. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med. 2008;358:1118–1128. doi: 10.1056/NEJMoa0706550. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton SR. The molecular genetics of colorectal neoplasia. Gastroenterology. 1993;105:3–7. doi: 10.1016/0016-5085(93)90003-u. [DOI] [PubMed] [Google Scholar]
- 12.Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 13.Tahara E. Genetic alterations in human gastrointestinal cancers. The application to molecular diagnosis. Cancer. 1995;75:1410–1417. doi: 10.1002/1097-0142(19950315)75:6+<1410::aid-cncr2820751504>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 15.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 16.Jen J, Powell SM, Papadopoulos N, Smith KJ, Hamilton SR, Vogelstein B, Kinzler KW. Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 1994;54:5523–5526. [PubMed] [Google Scholar]
- 17.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 18.Levy DB, Smith KJ, Beazer-Barclay Y, Hamilton SR, Vogelstein B, Kinzler KW. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 1994;54:5953–5958. [PubMed] [Google Scholar]
- 19.Smith AJ, Stern HS, Penner M, Hay K, Mitri A, Bapat BV, Gallinger S. Somatic APC and K-ras codon 12 mutations in aberrant crypt foci from human colons. Cancer Res. 1994;54:5527–5530. [PubMed] [Google Scholar]
- 20.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Z, Jiang T, Piao Y, Han T, Han Y, Xie X. Meta-analysis of the association between APC promoter methylation and colorectal cancer. Onco Targets Ther. 2015;8:211–222. doi: 10.2147/OTT.S75827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schell MJ, Yang M, Teer JK, Lo FY, Madan A, Coppola D, Monteiro AN, Nebozhyn MV, Yue B, Loboda A, Bien-Willner GA, Greenawalt DM, Yeatman TJ. A multigene mutation classification of 468 colorectal cancers reveals a prognostic role for APC. Nat Commun. 2016;7:11743. doi: 10.1038/ncomms11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eads CA, Nickel AE, Laird PW. Complete genetic suppression of polyp formation and reduction of CpG-island hypermethylation in Apc(Min/+) Dnmt1-hypomorphic mice. Cancer Res. 2002;62:1296–1299. [PubMed] [Google Scholar]
- 24.Eads CA, Lord RV, Kurumboor SK, Wickramasinghe K, Skinner ML, Long TI, Peters JH, DeMeester TR, Danenberg KD, Danenberg PV, Laird PW, Skinner KA. Fields of aberrant CpG island hypermethylation in Barrett's esophagus and associated adenocarcinoma. Cancer Res. 2000;60:5021–5026. [PubMed] [Google Scholar]
- 25.Linhart HG, Lin H, Yamada Y, Moran E, Steine EJ, Gokhale S, Lo G, Cantu E, Ehrich M, He T, Meissner A, Jaenisch R. Dnmt3b promotes tumorigenesis in vivo by gene-specific de novo methylation and transcriptional silencing. Genes Dev. 2007;21:3110–3122. doi: 10.1101/gad.1594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derks S, Postma C, Carvalho B, van den Bosch SM, Moerkerk PT, Herman JG, Weijenberg MP, de Bruine AP, Meijer GA, van Engeland M. Integrated analysis of chromosomal, microsatellite and epigenetic instability in colorectal cancer identifies specific associations between promoter methylation of pivotal tumour suppressor and DNA repair genes and specific chromosomal alterations. Carcinogenesis. 2008;29:434–439. doi: 10.1093/carcin/bgm270. [DOI] [PubMed] [Google Scholar]
- 27.Iacopetta B, Grieu F, Li W, Ruszkiewicz A, Caruso M, Moore J, Watanabe G, Kawakami K. APC gene methylation is inversely correlated with features of the CpG island methylator phenotype in colorectal cancer. Int J Cancer. 2006;119:2272–2278. doi: 10.1002/ijc.22237. [DOI] [PubMed] [Google Scholar]
- 28.Thorstensen L, Lind GE, Lovig T, Diep CB, Meling GI, Rognum TO, Lothe RA. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia. 2005;7:99–108. doi: 10.1593/neo.04448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469:125–134. doi: 10.1007/s00428-016-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 32.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 33.Michailidi C, Theocharis S, Tsourouflis G, Pletsa V, Kouraklis G, Patsouris E, Papavassiliou AG, Troungos C. Expression and promoter methylation status of hMLH1, MGMT, APC, and CDH1 genes in patients with colon adenocarcinoma. Exp Biol Med (Maywood) 2015;240:1599–1605. doi: 10.1177/1535370215583800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samaei NM, Yazdani Y, Alizadeh-Navaei R, Azadeh H, Farazmandfar T. Promoter methylation analysis of WNT/beta-catenin pathway regulators and its association with expression of DNMT1 enzyme in colorectal cancer. J Biomed Sci. 2014;21:73. doi: 10.1186/s12929-014-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimberg J, Hong TT, Skarstedt M, Lofgren S, Zar N, Matussek A. Analysis of APC and IGFBP7 promoter gene methylation in Swedish and Vietnamese colorectal cancer patients. Oncol Lett. 2013;5:25–30. doi: 10.3892/ol.2012.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pack SC, Kim HR, Lim SW, Kim HY, Ko JY, Lee KS, Hwang D, Park SI, Kang H, Park SW, Hong GY, Hwang SM, Shin MG, Lee S. Usefulness of plasma epigenetic changes of five major genes involved in the pathogenesis of colorectal cancer. Int J Colorectal Dis. 2013;28:139–147. doi: 10.1007/s00384-012-1566-8. [DOI] [PubMed] [Google Scholar]
- 37.Qiu Y, Fu X, Zhang W, Xu Y, Xiao L, Chen X, Shi L, Zhou X, Xia G, Peng Y, Deng M. Prevalence and molecular characterisation of the sessile serrated adenoma in a subset of the Chinese population. J Clin Pathol. 2014;67:491–498. doi: 10.1136/jclinpath-2013-202092. [DOI] [PubMed] [Google Scholar]
- 38.Gay LJ, Mitrou PN, Keen J, Bowman R, Naguib A, Cooke J, Kuhnle GG, Burns PA, Luben R, Lentjes M, Khaw KT, Ball RY, Ibrahim AE, Arends MJ. Dietary, lifestyle and clinicopathological factors associated with APC mutations and promoter methylation in colorectal cancers from the EPIC-Norfolk study. J Pathol. 2012;228:405–415. doi: 10.1002/path.4085. [DOI] [PubMed] [Google Scholar]
- 39.Kang HJ, Kim EJ, Kim BG, You CH, Lee SY, Kim DI, Hong YS. Quantitative analysis of cancer-associated gene methylation connected to risk factors in Korean colorectal cancer patients. J Prev Med Public Health. 2012;45:251–258. doi: 10.3961/jpmph.2012.45.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leong KJ, Wei W, Tannahill LA, Caldwell GM, Jones CE, Morton DG, Matthews GM, Bach SP. Methylation profiling of rectal cancer identifies novel markers of early-stage disease. Br J Surg. 2011;98:724–734. doi: 10.1002/bjs.7422. [DOI] [PubMed] [Google Scholar]
- 41.Naghibalhossaini F, Hosseini HM, Mokarram P, Zamani M. High frequency of genes' promoter methylation, but lack of BRAF V600E mutation among Iranian colorectal cancer patients. Pathol Oncol Res. 2011;17:819–825. doi: 10.1007/s12253-011-9388-5. [DOI] [PubMed] [Google Scholar]
- 42.Syed Sameer A, Shah ZA, Abdullah S, Chowdri NA, Siddiqi MA. Analysis of molecular aberrations of Wnt pathway gladiators in colorectal cancer in the Kashmiri population. Hum Genomics. 2011;5:441–452. doi: 10.1186/1479-7364-5-5-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasovcak P, Pavlikova K, Sedlacek Z, Skapa P, Kouda M, Hoch J, Krepelova A. Molecular genetic analysis of 103 sporadic colorectal tumours in Czech patients. PLoS One. 2011;6:e24114. doi: 10.1371/journal.pone.0024114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belshaw NJ, Pal N, Tapp HS, Dainty JR, Lewis MP, Williams MR, Lund EK, Johnson IT. Patterns of DNA methylation in individual colonic crypts reveal aging and cancer-related field defects in the morphologically normal mucosa. Carcinogenesis. 2010;31:1158–1163. doi: 10.1093/carcin/bgq077. [DOI] [PubMed] [Google Scholar]
- 45.Kim JC, Choi JS, Roh SA, Cho DH, Kim TW, Kim YS. Promoter methylation of specific genes is associated with the phenotype and progression of colorectal adenocarcinomas. Ann Surg Oncol. 2010;17:1767–1776. doi: 10.1245/s10434-009-0901-y. [DOI] [PubMed] [Google Scholar]
- 46.Kamiyama H, Noda H, Takata O, Suzuki K, Kawamura Y, Konishi F. Promoter hypermethylation of tumor-related genes in peritoneal lavage and the prognosis of patients with colorectal cancer. J Surg Oncol. 2009;100:69–74. doi: 10.1002/jso.21291. [DOI] [PubMed] [Google Scholar]
- 47.Derks S, Postma C, Moerkerk PT, van den Bosch SM, Carvalho B, Hermsen MA, Giaretti W, Herman JG, Weijenberg MP, de Bruine AP, Meijer GA, van Engeland M. Promoter methylation precedes chromosomal alterations in colorectal cancer development. Cell Oncol. 2006;28:247–257. doi: 10.1155/2006/846251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandes JC, van Engeland M, Wouters KA, Weijenberg MP, Herman JG. CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis. 2005;26:1152–1156. doi: 10.1093/carcin/bgi058. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Rocken C, Lofton-Day C, Schulz HU, Muller O, Kutzner N, Malfertheiner P, Ebert MP. Molecular analysis of APC promoter methylation and protein expression in colorectal cancer metastasis. Carcinogenesis. 2005;26:37–43. doi: 10.1093/carcin/bgh280. [DOI] [PubMed] [Google Scholar]
- 50.Ebert MP, Mooney SH, Tonnes-Priddy L, Lograsso J, Hoffmann J, Chen J, Rocken C, Schulz HU, Malfertheiner P, Lofton-Day C. Hypermethylation of the TPEF/HPP1 gene in primary and metastatic colorectal cancers. Neoplasia. 2005;7:771–778. doi: 10.1593/neo.05235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HC, Roh SA, Ga IH, Kim JS, Yu CS, Kim JC. CpG island methylation as an early event during adenoma progression in carcinogenesis of sporadic colorectal cancer. J Gastroenterol Hepatol. 2005;20:1920–1926. doi: 10.1111/j.1440-1746.2005.03943.x. [DOI] [PubMed] [Google Scholar]
- 52.Bai AH, Tong JH, To KF, Chan MW, Man EP, Lo KW, Lee JF, Sung JJ, Leung WK. Promoter hypermethylation of tumor-related genes in the progression of colorectal neoplasia. Int J Cancer. 2004;112:846–853. doi: 10.1002/ijc.20485. [DOI] [PubMed] [Google Scholar]
- 53.Lee S, Hwang KS, Lee HJ, Kim JS, Kang GH. Aberrant CpG island hypermethylation of multiple genes in colorectal neoplasia. Lab Invest. 2004;84:884–893. doi: 10.1038/labinvest.3700108. [DOI] [PubMed] [Google Scholar]
- 54.Lind GE, Thorstensen L, Lovig T, Meling GI, Hamelin R, Rognum TO, Esteller M, Lothe RA. A CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell lines. Mol Cancer. 2004;3:28. doi: 10.1186/1476-4598-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu XL, Yu J, Zhang HY, Sun MH, Gu J, Du X, Shi DR, Wang P, Yang ZH, Zhu JD. Methylation profile of the promoter CpG islands of 31 genes that may contribute to colorectal carcinogenesis. World J Gastroenterol. 2004;10:3441–3454. doi: 10.3748/wjg.v10.i23.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]


