Abstract
This meta-analysis was implemented to test the association of a missense mutation, Trp64Arg, in β3-adrenoreceptor-encoding gene (ADRB3) with both hypertension risk and blood pressure (BP) changes. A systematic search of three publicly-available databases was launched to look for articles published as of December 2016. Qualification appraisal and data extraction were independently done by two researchers. Pooled estimates were expressed as odds ratio (OR) or weighted mean difference (WMD), and their 95% confidence intervals (95% CIs). There were separately 21 (3750/4225 patients/controls) and 17 (6100 subjects) individual studies for hypertension risk and BP changes. Integral analyses revealed that Trp64Arg mutation was associated with the significantly increased risk of hypertension, and particularly, the 64Trp/64Arg heterozygote carriers were 1.23-times more likely to develop hypertension compared with the 64Trp/64Trp homozygote carriers (OR = 1.23, 95% CI: 1.02∼1.46, P = 0.021). Publication bias was extremely low for all integral comparisons. In stratified analyses, significance was spotted in populations of Chinese descent, in retrospective studies, in hospital-based studies, in age-matched case-control studies, in studies enrolling patients with mean body mass index < 25 kg/m2 and in studies with total sample size ≥ 240. Heterogeneity was improved for most stratified comparisons. Further in hypertensive patients, the 64Trp/64Arg heterozygote carriers had significantly higher systolic (WMD = 0.87 mmHg, 95% CI: 0.39∼1.35, P < 0.001) and diastolic (WMD = 0.88 mmHg, 95% CI: 0.59∼1.17, P < 0.001) BP than 64Trp/64Trp homozygote carriers. Altogether, ADRB3 gene Trp64Arg mutation was significantly associated with an increased predisposition toward hypertension and elevated systolic/diastolic BP in hypertensive patients, suggesting that Trp64Arg is an important hypertension-susceptibility marker.
Keywords: hypertension, β3-adrenoreceptor, polymorphism, blood pressure, meta-analysis
INTRODUCTION
Obesity in humans is well acknowledged as the most important, independent risk factor for hypertension [1]. Speaking in general terms, obesity is the amassment of excessive fat in adipose tissue [2]. One of the principal components in adipose tissue is β3-adrenoreceptor, which is abundantly expressed in brown adipose tissue, as well as in subcutaneous and abnormal white adipose tissue [3, 4]. An important biological property of β3-adrenoreceptor is to mediate the catecholamine-induced thermogenesis and lipolysis in adipose tissue [5, 6]. Another important property involves the enhanced function of sympathetic nervous system, which represents a chief regulator of blood pressure via altering sodium homeostasis and vascular resistance [7, 8]. Echoing from this interlocked relation, it is intriguing to speculate that high expression of β3-adrenoreceptor in adipose tissue would lead to elevated blood pressure and ultimately to the great risk of hypertension.
There is evidence that β3-adrenoreceptor expression is partly under genetic control [9]. The extensively-assessed bi-allelic polymorphism in the gene encoding for β3-adrenoreceptor (ADRB3, on chromosome 8p11.23) is rs4994, a missense mutation in the 64th position that substitutes a tryptophan with an arginine, termed as Trp64Arg. This substitution can alter the affinity of β3-adrenoreceptor to norepinephrine and its interaction with G-proteins in adipocytes [10]. Since the year 1996, a growing number of epidemiological studies have tested the association of ADRB3 gene Trp64Arg polymorphism with hypertension risk and the changes of relevant intermediate phenotypes, such as blood pressure, body mass index (BMI) and fasting insulin [4, 11–14]. However, the results of these studies are not satisfactory, as positive association cannot be reproduced coherently. As a meta-analysis is widely recognized as a useful and reliable method to address this issue, we set out to meta-analyze the association of ADRB3 gene Trp64Arg polymorphism with both hypertension risk and blood pressure changes using existing publications.
RESULTS
Qualified studies
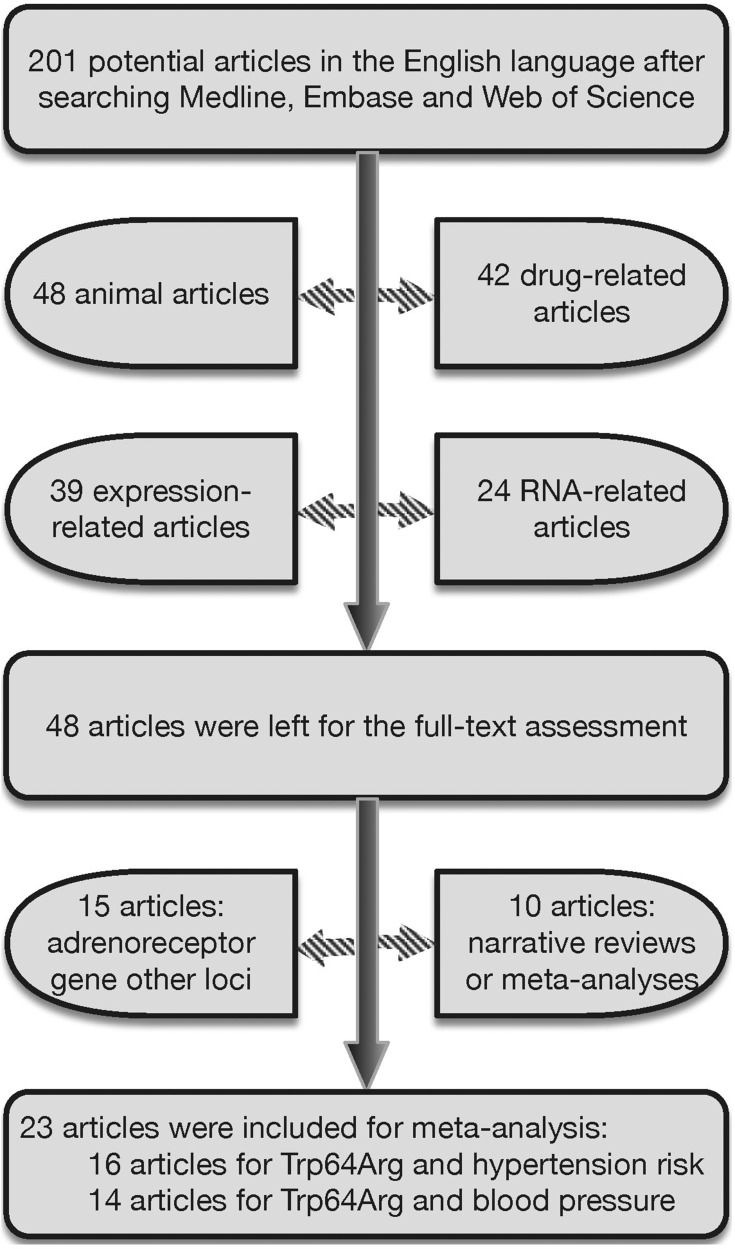
Using the prescribed keywords, a total of 201 publications in the English language were identified from searching three publicly-available databases. Among them, only 23 publications met preset inclusive criteria [4, 10–31]. The selection process of qualified publications is illustrated in Figure 1. There were 16 publications testing the association of ADRB3 gene Trp64Arg polymorphism with hypertension risk [10, 11, 14, 16, 17, 19, 20, 23–31], incorporating 21 individual studies (3750 hypertensive patients and 4225 normotensive controls). Fourteen publications were available for the association of Trp64Arg polymorphism with blood pressure changes [4, 10, 12–15, 17, 18, 21–23, 25, 26, 28], incorporating 17 individual studies (6100 subjects). The baseline characteristics of all qualified studies are present in both Supplementary Table 1 and Table 1.
Figure 1. The selection process of qualified publications for this meta-analysis.
Table 1. The baseline characteristics of observational studies for the changes of blood pressure and related intermediate phenotypes.
| First author | Year | Ethnicity | Design | BP cutoff | Source | Matched | Genotyping | Sample size | Age (years) | Males | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | ||||||||||||
| BMI (kg/m2) | SBP (mmHg) | DBP (mmHg) | Glucose (mmol/L) | TG(mmol/L) | TC (mmol/L) | HDLC (mmol/L) | LDLC (mmol/L) | ||||||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | ||
| Ikegami | 1996 | Japanese | Retrospective | 140/90 | Hospital | n.a. | RFLP | 126 | 97 | n.a. | n.a. | n.a. | n.a. | ||||
| Fujisawa | 1997 | Japanese | Retrospective | 160/95 | Hospital | NO | RFLP | 101 | 73 | 57.7 | 46.2 | 0.475 | 0.712 | ||||
| Baba | 1998 | Japanese | Retrospective | 140/90 | Hospital | YES | RFLP | 37 | 46 | 57.0 | 55.0 | 0.568 | 0.565 | ||||
| Tonolo | 1999 | Caucasian | Retrospective | 160/95 | Hospital | YES | RFLP | 213 | 281 | 62.0 | 61.0 | 0.460 | 0.374 | ||||
| Ringel (Men) | 2000 | Caucasian | Retrospective | n.a. | Hospital | NO | RFLP | 86 | 122 | 61.6 | 57.8 | 1.000 | 1.000 | ||||
| Ringel (Women) | 2000 | Caucasian | Retrospective | n.a. | Hospital | NO | RFLP | 115 | 94 | 64.4 | 59.8 | 0.000 | 0.000 | ||||
| Filigheddu | 2004 | Caucasian | Retrospective | 140/90 | Hospital | NO | RFLP | 526 | 192 | 48.2 | 66.0 | 0.580 | 0.469 | ||||
| Masuo | 2005 | Japanese | Nested | 140/90 | Population | n.a. | TaqMan | 41 | 117 | n.a. | n.a. | n.a. | n.a. | ||||
| Nagano | 2005 | Japanese | Retrospective | 140/90 | Hospital | n.a. | RFLP | 23 | 66 | n.a. | n.a. | 0.551 | 0.551 | ||||
| Kawaguchi | 2006 | Japanese | Nested | 140/90 | Population | n.a. | TaqMan | 27 | 28 | n.a. | n.a. | n.a. | n.a. | ||||
| Hui (Men) | 2007 | Chinese | Retrospective | 160/100 | Hospital | YES | TaqMan | 170 | 182 | 51.0 | 52.0 | 1.000 | 1.000 | ||||
| Hui (Women) | 2007 | Chinese | Retrospective | 160/100 | Hospital | YES | TaqMan | 91 | 89 | 51.1 | 50.4 | 0.000 | 0.000 | ||||
| Mo (Obese) | 2007 | Chinese | Retrospective | 140/90 | Hospital | YES | RFLP | 149 | 149 | 57.4 | 57.8 | 0.597 | 0.456 | ||||
| Mo (Non-obese) | 2007 | Chinese | Retrospective | 140/90 | Hospital | YES | RFLP | 139 | 149 | 58.1 | 57.8 | 0.576 | 0.456 | ||||
| Ruixing (Men) | 2008 | Chinese | Retrospective | 140/90 | Population | NO | RFLP | 287 | 547 | 51.2 | 43.4 | 1.000 | 1.000 | ||||
| Ruixing (Women) | 2008 | Chinese | Retrospective | 140/90 | Population | NO | RFLP | 159 | 676 | 55.2 | 43.8 | 0.000 | 0.000 | ||||
| Mirrakhimov | 2011 | Kyrgyz | Retrospective | n.a. | Population | n.a. | RFLP | 87 | 126 | 50.7 | 50.7 | 0.681 | 0.681 | ||||
| Chou (Normal) | 2012 | Chinese | Nested | 130/85 | Population | n.a. | TaqMan | 22 | 242 | n.a. | n.a. | n.a. | n.a. | ||||
| Chou (Obesity) | 2012 | Chinese | Nested | 130/85 | Population | n.a. | TaqMan | 110 | 129 | n.a. | n.a. | n.a. | n.a. | ||||
| Wang | 2014 | Chinese | Retrospective | 140/90 | Hospital | YES | TaqMan | 1090 | 700 | 51.9 | 51.8 | 0.606 | 0.597 | ||||
| Grygiel-Gorniak | 2015 | Caucasian | Retrospective | 140/90 | Population | NO | TaqMan | 151 | 120 | 59.9 | 58.6 | 0.000 | 0.000 | ||||
| n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| 24.1 | 22.2 | 155 | 122 | 92 | 76 | n.a. | n.a. | n.a. | n.a. | 5.27 | 5.25 | n.a. | n.a. | n.a. | n.a. | ||
| 23.2 | 22.6 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| 30.1 | 26.7 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1.49 | 1.48 | 5.90 | 5.80 | 1.40 | 1.50 | 3.70 | 3.60 | ||
| 27.4 | 27.2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| 29.0 | 27.3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| 26.9 | 25.7 | 157 | 129 | 104 | 80 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| 24.4 | 22.8 | 171.7 | 113.2 | 105.4 | 70.5 | n.a. | n.a. | n.a. | n.a. | 5.30 | 5.09 | 1.44 | 1.39 | n.a. | n.a. | ||
| 24.4 | 22.5 | 177.1 | 112.1 | 70.5 | 68.2 | n.a. | n.a. | n.a. | n.a. | 5.70 | 5.45 | 1.64 | 1.58 | n.a. | n.a. | ||
| 27.3 | 20.2 | 141.9 | 108.5 | 83.4 | 72.1 | 5.30 | 5.28 | 1.87 | 1.22 | n.a. | n.a. | 1.09 | 1.16 | 3.19 | 2.98 | ||
| 21.7 | 20.2 | 141.2 | 108.5 | 82.4 | 72.1 | 5.31 | 5.28 | 1.28 | 1.22 | n.a. | n.a. | 1.17 | 1.16 | 3.01 | 2.98 | ||
| 22.2 | 20.9 | 146.4 | 119.3 | 89.8 | 75.3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| 20.1 | 21.2 | 149.4 | 115.7 | 84.3 | 72.1 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| 26.5 | 24.8 | 141.77 | 115.66 | 90.85 | 75.62 | 5.43 | 5.17 | 2.09 | 1.43 | n.a. | n.a. | 1.16 | 1.40 | 3.42 | 3.48 | ||
| 29.9 | 28.9 | 157.29 | 121.02 | 96.37 | 77.42 | 5.46 | 5.24 | 1.40 | 1.23 | 6.09 | 5.83 | 1.66 | 1.65 | 3.78 | 3.61 | ||
Trp64Arg and hypertension risk: integral analyses
In view of a low frequency of 64Arg/64Arg homozygote and to avoid a fluctuated estimate, the risk prediction of Trp64Arg polymorphism for hypertension was investigated separately under the allelic, heterozygote-genotypic and dominant models.
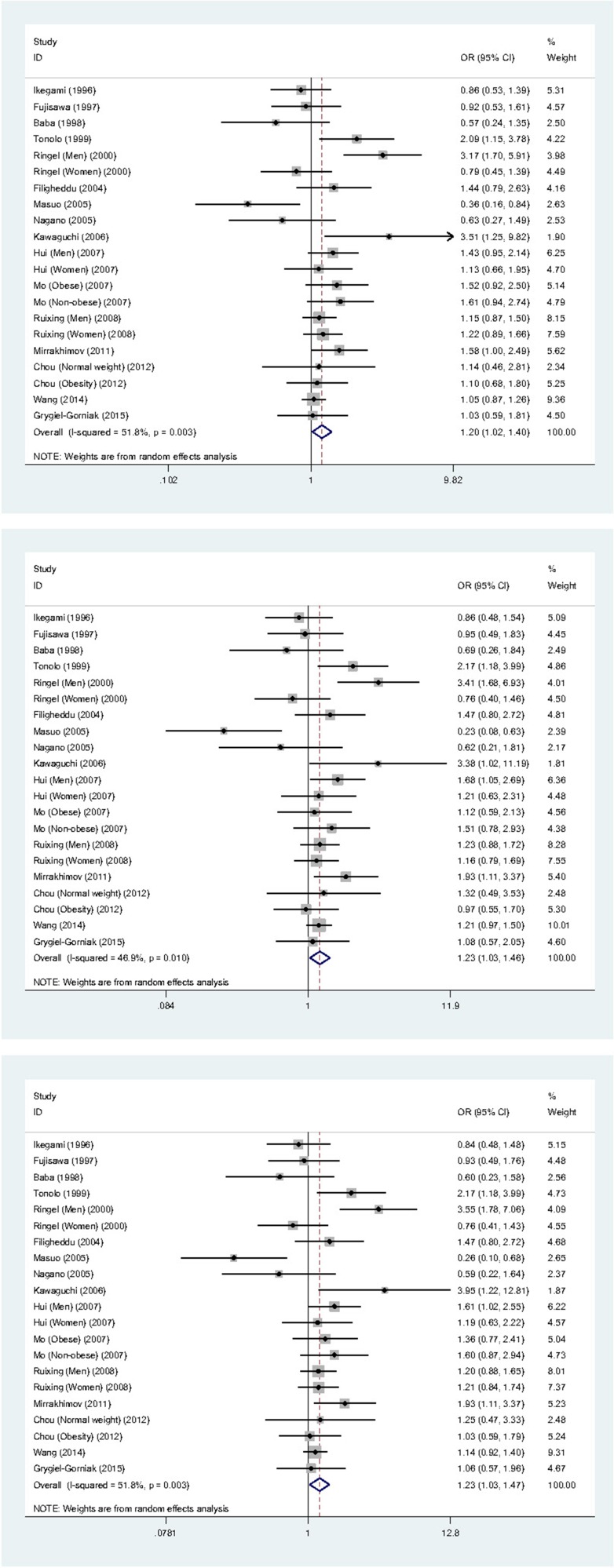
The mean frequency of 64Arg allele was 15.86% in patients and 14.18% in controls, with the statistical power to detect the difference of being 83.74%. Integral analyses of 21 individual studies revealed that the mutation of ADRB3 gene Trp64Arg polymorphism was associated with the significantly increased risk of hypertension under three genetic models mentioned above, in company with moderate or marginally significant heterogeneity as gauged by the I2 statistic (Figure 2). For example, carriers of the 64Trp/64Arg heterozygote were 1.23-times more likely to develop hypertension when compared with those of the 64Trp/64Trp homozygote (OR = 1.23, 95% CI: 1.02 ∼ 1.46, P = 0.021), with moderate heterogeneity (I2 = 46.9%). In addition, the genotype distributions of ADRB3 gene Trp64Arg polymorphism deviated from the Hardy-Weinberg equilibrium in 4 studies, exclusion of these 4 studies generated the very similar effect estimates, with slightly improved heterogeneity.
Figure 2. Forest graphs for the integral association of ADRB3 gene Trp64Arg polymorphism with hypertension risk under the allelic (the upper panel), heterozygote-genotypic (the middle panel) and dominant (the lower panel) models.
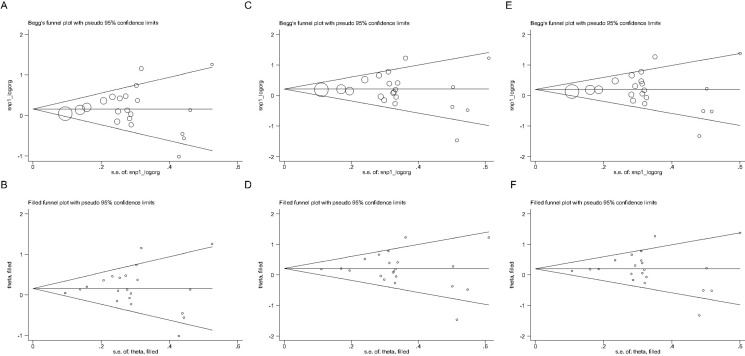
Regarding publication bias, the likelihood of significance was extremely low for the three genetic models mentioned above, as supported by the symmetrical Begg's funnel graphs (Figure 3A, 3C, 3E) and the no-missing-study-reported filled funnel graphs (Figure 3B, 3D, 3F), as well as by the nonsignificant Egger's regression tests for funnel asymmetry (P = 0.705, 0.731 and 0.999 for the allelic, heterozygote-genotypic and dominant models, respectively).
Figure 3.
Begg's and filled funnel graphs for the association of ADRB3 gene Trp64Arg polymorphism with hypertension risk under the allelic (A and B panels), heterozygote-genotypic (C and D panels) and dominant (E and F panels) models.
Trp64Arg and hypertension risk: stratified analyses
Because the heterogeneity in integral analyses was moderate or significant, a string of stratified analyses and meta-regression analyses were implemented to look for the possible reasons in explanation of between-study heterogeneity from other demographic and methodological aspects. In stratified analyses, 21 qualified studies were grouped per ethnicity, study design, source of control populations, genotyping methodology, age-matched condition, BMI in patients and total sample size respectively under the allelic, heterozygote-genotypic and dominant models (Table 2).
Table 2. The risk prediction of ADRB3 gene Trp64Arg mutation for hypertension under three genetic models in stratified analyses.
| Subgroups | Allelic model | Heterozygote-genotypic model | Dominant model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies (N) | OR, 95% CI | P | I2 | OR, 95% CI | P | I2 | OR, 95% CI | P | I2 | |
| Ethnicity | ||||||||||
| Caucasian | 5 | 1.49, 0.92 ∼ 2.41 | 0.103 | 70.1% | 1.54, 0.94 ∼ 2.52 | 0.085 | 65.9% | 1.54, 0.93 ∼ 2.57 | 0.095 | 69.5% |
| Chinese | 9 | 1.17, 1.04 ∼ 1.31 | 0.008 | 0.0% | 1.23, 1.07 ∼ 1.41 | 0.003 | 0.0% | 1.22, 1.07 ∼ 1.39 | 0.003 | 0.0% |
| Japanese | 6 | 0.82, 0.51 ∼ 1.31 | 0.400 | 60.3% | 0.79, 0.45 ∼ 1.37 | 0.402 | 58.7% | 0.79, 0.45 ∼ 1.38 | 0.400 | 62.4% |
| Study design | ||||||||||
| Nested | 4 | 1.08, 0.50 ∼ 2.35 | 0.842 | 74.2% | 0.97, 0.38 ∼ 2.43 | 0.943 | 75.8% | 1.03, 0.41 ∼ 2.59 | 0.948 | 77.1% |
| Retrospective | 17 | 1.22, 1.05 ∼ 1.42 | 0.011 | 45.7% | 1.28, 1.09 ∼ 1.50 | 0.002 | 31.6% | 1.27, 1.08 ∼ 1.50 | 0.004 | 40.9% |
| Source of controls | ||||||||||
| Hospital | 13 | 1.22, 0.98 ∼ 1.50 | 0.072 | 56.5% | 1.27, 1.02 ∼ 1.57 | 0.030 | 42.0% | 1.26, 1.01 ∼ 1.58 | 0.043 | 50.6% |
| Population | 8 | 1.17, 0.91 ∼ 1.50 | 0.234 | 49.8% | 1.16, 0.84 ∼ 1.62 | 0.373 | 58.3% | 1.18, 0.85 ∼ 1.63 | 0.316 | 59.2% |
| Genotyping methodology | ||||||||||
| RFLP | 13 | 1.25, 1.01 ∼ 1.54 | 0.037 | 54.2% | 1.27, 1.02 ∼ 1.59 | 0.034 | 44.4% | 1.28, 1.02 ∼ 1.62 | 0.032 | 51.4% |
| TaqMan | 8 | 1.11, 0.86 ∼ 1.43 | 0.416 | 48.6% | 1.15, 0.84 ∼ 1.58 | 0.382 | 55.8% | 1.14, 0.84 ∼ 1.56 | 0.404 | 56.1% |
| Age-matched condition | ||||||||||
| n.a. | 7 | 1.03, 0.68 ∼ 1.57 | 0.879 | 64.1% | 1.01, 0.60 ∼ 1.71 | 0.968 | 68.0% | 1.03, 0.61 ∼ 1.74 | 0.914 | 69.5% |
| NO | 7 | 1.22, 0.94 ∼ 1.58 | 0.130 | 54.1% | 1.25, 0.95 ∼ 1.65 | 0.115 | 46.3% | 1.25, 0.94 ∼ 1.67 | 0.119 | 52.5% |
| YES | 7 | 1.28, 1.01 ∼ 1.62 | 0.038 | 45.7% | 1.32, 1.10 ∼ 1.58 | 0.003 | 5.8% | 1.33, 1.07 ∼ 1.64 | 0.011 | 25.5% |
| Mean BMI in patients | ||||||||||
| BMI < 25 kg/m2 | 7 | 1.19, 1.02 ∼ 1.39 | 0.029 | 0.0% | 1.24, 1.03 ∼ 1.49 | 0.025 | 0.0% | 1.23, 1.03 ∼ 1.48 | 0.022 | 0.0% |
| BMI ≥ 25 kg/m2 | 14 | 1.22, 0.96 ∼ 1.55 | 0.105 | 63.6% | 1.23, 1.03 ∼ 1.60 | 0.136 | 61.3% | 1.24, 0.95 ∼ 1.63 | 0.118 | 64.4% |
| Total sample size | ||||||||||
| < 240 | 11 | 1.06, 0.76 ∼ 1.48 | 0.725 | 68.5% | 1.07, 0.72 ∼ 1.57 | 0.746 | 67.4% | 1.07, 0.72 ∼ 1.59 | 0.736 | 70.5% |
| ≥ 240 | 10 | 1.20, 1.07 ∼ 1.35 | 0.002 | 0.0% | 1.29, 1.13 ∼ 1.48 | <0.001 | 0.0% | 1.27, 1.11 ∼ 1.44 | <0.001 | 0.0% |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; RFLP, restriction fragment length polymorphism; BMI, body mass index; n.a., not available.
When all studies were grouped by ethnicity, significance was only revealed in populations of Chinese descent (n = 9) for the association of Trp64Arg polymorphism with hypertension risk under all three genetic models, and no evidence of heterogeneity occurred (I2 = 0.0% for all models). By study design, the risk prediction was only significant in retrospective studies (n = 17), with moderate heterogeneity. By source of controls, effect-size estimates were potentiated in hospital-based studies (n = 13) relative to population-based studies (n = 8), especially under the heterozygote-genotypic and dominant models, and there was moderate evidence of heterogeneity. By genotyping methodology, studies using RFLP (restriction fragment length polymorphism) technique reported a significant association of Trp64Arg mutation with hypertension under all three genetic models, and this association was biased by significant heterogeneity. By age-matched condition, the mutation of Trp64Arg polymorphism was significantly associated with the increased risk of hypertension in studies involving age-matched hypertensive patients and normotensive controls (n = 7), and the probability of heterogeneity was considerably improved under the heterozygote-genotypic (I2 = 5.8%) and dominant (I2 = 25.5%) models. By mean BMI in patients at a cutoff value of 25 kg/m2, the risk prediction of Trp64Arg polymorphism for hypertension was significant when analysis was restricted to the studies enrolling patients with mean BMI < 25 kg/m2 (n = 7) under the allelic (OR=1.19, 95% CI: 1.02 ∼ 1.39, P = 0.029), heterozygote-genotypic (OR=1.24, 95% CI: 1.03 ∼ 1.49, P = 0.025) and dominant (OR=1.23, 95% CI: 1.03 ∼ 1.48, P = 0.022), without heterogeneity (all I2 = 0.0%). Finally, grouping studies by total sample size at the medium cutoff value of 240 found a robust association in larger studies (n = 10) between Trp64Arg polymorphism and hypertension risk under the allelic (OR=1.20, 95% CI: 1.07 ∼ 1.35, P = 0.001), heterozygote-genotypic (OR=1.29, 95% CI: 1.13 ∼ 1.48, P < 0.001) and dominant (OR=1.27, 95% CI: 1.11 ∼ 1.44, P < 0.001) models, respectively, and there was no detectable heterogeneity (all I2 = 0.0%).
Further meta-regression analyses, when modeling the baseline (age, gender, BMI, smoking) and clinical (FBG, triglycerides, total cholesterol, HDLC, LDLC) characteristics, failed to detect any significant contributions on the risk prediction of Trp64Arg polymorphism for hypertension under all three genetic models (all P > 0.05) (data not shown).
Trp64Arg and intermediate phenotype changes
Because of the close relation of β3-adrenoreceptor with obesity and lipolysis, besides blood pressure, the levels of other related intermediate phenotypes including BMI, FBG, fasting insulin, total cholesterol and HDLC were also compared across Trp64Arg genotypes. Considering a low frequency of the 64Arg/64Arg homozygote, the 64Trp/64Arg heterozygote is often combined with the 64Arg/64Arg homozygote when assessing the changes of these phenotypes across these genotypes. To avoid deviations from inadequate sample sizes, blood pressure and related intermediate phenotypes were only compared between carriers of the 64Trp/64Trp and 64Trp/64Arg genotypes (Table 3). Integral analyses of 17 individual studies failed to detect any significant changes in blood pressure and related intermediate phenotypes between the 64Trp/64Trp and 64Trp/64Arg genotypes. Further grouping 17 studies by hypertension status found that in hypertensive patients, the 64Trp/64Arg heterozygote carriers had significantly higher SBP (WMD=0.87 mmHg, 95% CI: 0.39 ∼ 1.35, P < 0.001) and DBP (WMD=0.88 mmHg, 95% CI: 0.59 ∼ 1.17, P < 0.001) levels than those with the 64Trp/64Trp homozygote, with no detectable heterogeneity (both I2 = 0.0%). By contrast, BMI was 3.00 kg/m2 higher in normotensive controls possessing the 64Trp/64Arg heterozygote than the 64Trp/64Trp homozygote (WMD=3.00, 95% CI: 0.35 ∼ 5.66, P = 0.026), without heterogeneity (I2 = 0.0%), while it was 0.70 kg/m2 lower in hypertensive patients. As for total cholesterol, normotensive controls with the 64Trp/64Arg heterozygote had a lower level than those with the 64Trp/64Trp homozygote (WMD=−0.39 mmol/L, 95% CI: −0.73 ∼ −0.05, P = 0.026), without heterogeneity (I2 = 0.0%). No significant changes were observed in FBG, fasting insulin and HDLC levels across Trp64Arg genotypes (all P > 0.05).
Table 3. Pooled changes of blood pressure and related intermediate phenotypes between carriers of the 64Trp/64Trp and 64Trp/64Arg genotypes.
| Phenotypes | Groups | Studies (N) | WMD | 95% CI | P | I2 |
|---|---|---|---|---|---|---|
| SBP (mmHg) | All studies | 16 | 1.67 | −0.48 ∼ 3.82 | 0.128 | 80.7% |
| Studies in hypertensives | 6 | 0.87 | 0.39 ∼ 1.35 | < 0.001 | 0.0% | |
| DBP (mmHg) | All studies | 16 | 0.29 | −0.28 ∼ 0.87 | 0.317 | 15.3% |
| Studies in hypertensives | 6 | 0.88 | 0.59 ∼ 1.17 | < 0.001 | 0.0% | |
| BMI (kg/m2) | All studies | 14 | −0.06 | −0.59 ∼ 0.48 | 0.838 | 83.4% |
| Studies in hypertensives | 5 | −0.70 | −1.24 ∼ -0.17 | 0.010 | 42.1% | |
| FBG (mmol/L) | All studies | 6 | 0.01 | −0.06 ∼ 0.07 | 0.835 | 0.0% |
| Studies in hypertensives | 3 | 0.03 | −0.08 ∼ 0.15 | 0.574 | 12.0% | |
| Fasting insulin (pmol/L) | All studies | 11 | −0.94 | −7.00 ∼ 5.12 | 0.762 | 71.1% |
| Studies in hypertensives | 3 | −2.07 | −11.54 ∼ 7.39 | 0.668 | 0.0% | |
| Total cholesterol (mmol/L) | All studies | 12 | −0.03 | −0.12 ∼ 0.05 | 0.433 | 49.9% |
| Studies in hypertensives | 4 | 0.00 | −0.04 ∼ 0.04 | 0.891 | 2.8% | |
| HDLC (mmol/L) | All studies | 9 | −0.01 | −0.06 ∼ 0.03 | 0.580 | 64.9% |
| Studies in hypertensives | 4 | −0.01 | −0.08 ∼ 0.07 | 0.879 | 50.7% |
Abbreviations: WMD, weighted mean difference; 95% CI, 95% confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; FBG, fasting blood glucose; HDLC, high-density lipoprotein cholesterol.
DISCUSSION
To the best of our knowledge, this is to date the first meta-analysis to test the association of ADRB3 gene Trp64Arg polymorphism with both hypertension risk and blood pressure changes. Most notably, the mutation of Trp64Arg polymorphism was significantly associated not only with an increased predisposition toward hypertension, but also with elevated SBP and DBP in hypertensive patients, suggesting that Trp64Arg polymorphism might represent an important genetic marker in the development of hypertension.
Human β3-adrenoreceptor is a member of the family of 7-transmembrance G-protein-coupled receptors. It is widely recognized that the β3-adrenoreceptor is indirectly involved in the etiology of hypertension through reducing lipolysis and activating the sympathetic nervous system [25, 32]. Numerous studies have suggested that hypertension is partly under genetic control, and therefore to look for candidate genetic markers responsible for hypertension predisposition and/or blood pressure changes is justifiable [33, 34]. Given the biological importance of β3-adrenoreceptor, it would be tempting to speculate that certain genetic alterations of its coding gene, ADRB3, might play a critical role in the regulation of blood pressure and ultimately predispose to the occurrence of hypertension.
The genomic sequence of ADRB3 gene is covered with many polymorphic loci, and a missense mutation, Trp64Arg, in its first exon has been widely evaluated in susceptibility to hypertension and other complications in populations from different regions of the world [20, 24, 35]. Some studies have reported the increased risk of hypertension associated with the 64Arg allele [10, 17, 26], whereas others have found an apparently conflicting association [25, 31]. This controversy could be attributed to the differences in genetic background, study design, source of study subjects, sample size and so on [36]. So, attempts to account for these differences have justified the necessity of undertaking a systematic meta-analysis, just as the present study did.
Our integral analyses demonstrated that the mutation of Trp64Arg polymorphism was associated with the significantly increased risk of hypertension, and this association was further substantiated in age-matched case-control studies and in larger studies, irrespective of three genetic models under investigation, highlighting the robustness of our meta-analytical findings. However, in many cases, complex diseases like hypertension are complicated by genetic heterogeneity [37]. As demonstrated by our ethnicity-stratified analyses, there was no exception for ADRB3 gene Trp64Arg polymorphism either. The risk prediction of this polymorphism for hypertension was spotted in populations of Chinese descent, whereas the Trp64Arg mutation seemed to act as a protective factor against the occurrence of hypertension in Japanese, albeit nonsignificantly. The possible reasons for such divergence could be due to differences in statistical power, genetic background, environmental exposure or dietary habit. Hence, the presence of genetic heterogeneity might undermine the replication of association between ADRB3 gene and hypertension. In addition, our findings also suggested that the differences in study design, genotyping methodology, age-matched condition, obesity and total sample size might be the potential sources of between-study heterogeneity. Altogether, our findings not only confirmed the susceptible role of ADRB3 gene Trp64Arg polymorphism in the development of hypertension, but also aid in explaining the conflicting patterns of the association seen in prior studies.
Another important finding of this meta-analysis was the significant changes in blood pressure across ADRB3 gene Trp64Arg genotypes. In fact, the heterozygous carriers of Trp64Arg polymorphism were found to have significantly higher SBP and DBP than those with the 64Trp/64Trp genotype in hypertensive patients, which can serve as an apt explanation for the risk-conferring association of the 64Arg allele with hypertension, as discussed above. It is generally believed that the sympathetic nervous system represents a major regulator of blood pressure through alterations in sodium homeostasis and vascular resistance [38, 39]. So, we speculate that the mutation of Trp64Arg polymorphism might impact on the function of β3-adrenoreceptor, which can further activate sympathetic nervous system, regulate blood pressure and ultimately precipitate the development of hypertension.
Study limitations
This meta-analysis is based on observational data from genetic association studies, and the potential causality cannot be handled. Moreover, only the English publications were meta-analyzed, and doing so might raise a possible selection bias. However, as manifested by the Begg's funnel graph, the filled funnel graph and the Egger's regression asymmetry test, publication bias was unlikely to be a perplexing issue. In addition, only one missense mutation was analyzed in the present study, and it is highly encouraged to incorporate more polymorphisms in close linkage disequilibrium with Trp64Arg polymorphism in ADRB3 gene and other adrenoreceptor genes, such as ADRB1 and ADRB2 genes, pending sufficient available publications to investigate their joint impact on blood pressure and hypertension risk. Also, because β3-adrenoreceptor is a principal component of adipose tissue, we only stratified the studies according to mean BMI, an indication of general obesity, and did not assess another important index of abdominal obesity, waist-to-hip ratio due to insufficient eligible studies. Furthermore, this meta-analysis was limited by inadequate sample size, especially in some subgroups. We concede that replication of our findings in other well-designed studies with a larger sample size will add additional evidence to our existing knowledge on a contributing role of ADRB3 gene to the etiology of hypertension.
MATERIALS AND METHODS
Search information
To be as comprehensive as possible, a systematic search of three publicly-available electronic databases, Medline, Embase (Excerpta Medica Database) and Web of Science, was launched to look for articles published as of December 14, 2016. The prescribed MeSH key terms incorporated “β3-adrenoreceptor” or “β3-adrenergic receptor” or “beta3-adrenoreceptor” or “beta3-adrenergic receptor” or “β3-adrenoreceptor” or “ADRB3” or “3-BAR” or “beta3-AR” in the Title/Abstract, annexed with “hypertension” or “hypertensive” or “blood pressure” in the Title and “genetic” or “polymorphism” or “SNP” or “variant” or “mutation” or “genotype” or “allele” in the Title/Abstract. Search results were primarily confined to the English-only articles involving human beings, irrespective of races or ethnicities or study designs. Search scope was further expanded to include reference lists of the keywords-indexed results to avoid possible omissions. Search process was done by two researchers - Hualing Yang and Dongmiao Cai, and the final publication list was based on congregated results of two researchers by removing duplications with the EndNote citation manager software version X8 (Thomson Reuters).
Inclusive criteria
Inclusive criteria were determined by all listed authors of this study, from the following three respects: (i) study design: either retrospective or nested; (ii) genetic data: the genotype or allele distributions of ADRB3 gene Trp64Arg polymorphism between hypertensive patients and normotensive controls or the mean and standard deviation of systolic blood pressure (SBP) and diastolic blood pressure (DBP) in mmHg, and when available other related intermediate phenotypes, including BMI, fasting blood glucose (FBG), fasting insulin, total cholesterol and HDLC across Trp64Arg genotypes; (iii) the genotypes of Trp64Arg polymorphism distinguished by validated methods. Two researchers - Hualing Yang and Dongmiao Cai, independently filtrated eligible articles from all keywords- and references-indexed search results per the inclusive criteria formulated above, and they also fixed all discrepancies with consensus.
Data extraction
A data-extraction table in Excel version 2016 was delineated by all listed authors, and extraction process was implemented by the same two researchers - Hualing Yang and Dongmiao Cai. The extracted data of interest contained the first author's family name, year of publication, country of samples collected, race or ethnicity of study subjects, study design, source of control populations, age-matched condition between patients and controls, genotyping methodology, sample size, age, gender, BMI, smoking, SBP, DBP, FBG, triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol, the genotype or allele distributions of Trp64Arg polymorphism between patients and controls, and the mean and standard deviation of SBP, DBP, BMI, FBG, fasting insulin, total cholesterol and HDLC levels across Trp64Arg genotypes, where existing. Two independently-extracted tables were compared for divergence, and any disagreement was resolved through discussion.
Statistical analyses
The risk prediction of ADRB3 gene Trp64Arg polymorphism for hypertension was signified as odds ratio (OR) and its 95% confidence interval (95% CI), and the changes of blood pressure and other related intermediate phenotypes were signified as weighted mean difference (WMD) and its 95% CI. Individual effect-size estimates were combined under random-effects model adopting the method developed by DerSimonian R and Laird N [40]. Statistical heterogeneity of individual studies was expressed as the I2 statistic, a per cent number that can be interpreted as low (I2 ≤ 25%), moderate (I2 > 25% and I2 < 50%) or high (I2 ≥ 50%) [41]. Other sources of heterogeneity from demographic and methodological aspects were probed by stratified analyses and meta-regression analyses. Publication bias was gauged by the Begg's funnel graph, the filled funnel graph and the Egger's regression asymmetry test [42]. The probability for the Egger's test was significant at 10% or less. In the presence of missing studies, an unbiased estimate was derived by the trim-and-fill method. Data were statistically analyzed by the Meta-analysis module [43] in the Stata/SE software version 14.1 for the Windows. Study power was estimated by the “sampsi” order in the Stata/SE software.
CONCLUSIONS
The collective findings of this meta-analysis demonstrated that the mutation of Trp64Arg polymorphism was significantly associated not only with an increased predisposition toward hypertension, but also with elevated SBP and DBP in hypertensive patients, suggesting that Trp64Arg polymorphism might represent an important genetic marker in the development of hypertension. These findings will potentially further our understanding for the contributing role of ADRB3 gene Trp64Arg polymorphism in blood pressure regulation and in the pathogenesis of hypertension.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Footnotes
Authors’ contributions
H.Y., Z.W. conceived and designed the experiments; H.Y., D.C. performed the experiments; H.Y., Q.Z. analyzed the data; D.W., Q.W. contributed materials/analytical tools; H.Y., Z.W. wrote and revised the manuscript. All authors reviewed and approved the manuscript prior to submission.
CONFLICTS OF INTEREST
None.
FUNDING
None.
REFERENCES
- 1.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabia B, Andrade S, Carreira MC, Casanueva FF, Crujeiras AB. A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes Rev. 2016;17:361–376. doi: 10.1111/obr.12377. [DOI] [PubMed] [Google Scholar]
- 3.Deiuliis JA, Liu LF, Belury MA, Rim JS, Shin S, Lee K. Beta(3)-adrenergic signaling acutely down regulates adipose triglyceride lipase in brown adipocytes. Lipids. 2010;45:479–489. doi: 10.1007/s11745-010-3422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erhardt E, Czako M, Csernus K, Molnar D, Kosztolanyi G. The frequency of Trp64Arg polymorphism of the beta3-adrenergic receptor gene in healthy and obese Hungarian children and its association with cardiovascular risk factors. Eur J Clin Nutr. 2005;59:955–959. doi: 10.1038/sj.ejcn.1602164. [DOI] [PubMed] [Google Scholar]
- 5.Philipson LH. beta-Agonists and metabolism. J Allergy Clin Immunol. 2002;110:S313–317. doi: 10.1067/mai.2002.129702. [DOI] [PubMed] [Google Scholar]
- 6.Mund RA, Frishman WH. Brown adipose tissue thermogenesis: beta3-adrenoreceptors as a potential target for the treatment of obesity in humans. Cardiol Rev. 2013;21:265–269. doi: 10.1097/CRD.0b013e31829cabff. [DOI] [PubMed] [Google Scholar]
- 7.Dessy C, Balligand JL. Beta3-adrenergic receptors in cardiac and vascular tissues emerging concepts and therapeutic perspectives. Adv Pharmacol. 2010;59:135–163. doi: 10.1016/S1054-3589(10)59005-7. [DOI] [PubMed] [Google Scholar]
- 8.Gasparetti AL, Alvarez-Rojas F, de Araujo EP, Hirata AE, Saad MJ, Velloso LA. beta3-Adrenergic-dependent and -independent mechanisms participate in cold-induced modulation of insulin signal transduction in brown adipose tissue of rats. Pflugers Arch. 2005;449:537–546. doi: 10.1007/s00424-004-1359-1. [DOI] [PubMed] [Google Scholar]
- 9.Campagna F, Montali A, Baroni MG, Maria AT, Ricci G, Antonini R, Verna R, Arca M. Common variants in the lipoprotein lipase gene, but not those in the insulin receptor substrate-1, the beta3-adrenergic receptor, and the intestinal fatty acid binding protein-2 genes, influence the lipid phenotypic expression in familial combined hyperlipidemia. Metabolism. 2002;51:1298–1305. doi: 10.1053/meta.2002.35197. [DOI] [PubMed] [Google Scholar]
- 10.Mirrakhimov AE, Kerimkulova AS, Lunegova OS, Moldokeeva CB, Zalesskaya YV, Abilova SS, Sovhozova NA, Aldashev AA, Mirrakhimov EM. An association between TRP64ARG polymorphism of the B3 adrenoreceptor gene and some metabolic disturbances. Cardiovasc Diabetol. 2011;10:89. doi: 10.1186/1475-2840-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikegami H, Yamato E, Fujisawa T, Hamada Y, Fujioka Y, Rakugi H, Higaki J, Murakami H, Shimamoto K, Ogihara T. Analysis of candidate genes for insulin resistance in essential hypertension. Hypertens Res. 1996;19:S31–34. doi: 10.1291/hypres.19.supplementi_s31. [DOI] [PubMed] [Google Scholar]
- 12.Buettner R, Schaffler A, Arndt H, Rogler G, Nusser J, Zietz B, Enger I, Hugl S, Cuk A, Scholmerich J, Palitzsch KD. The Trp64Arg polymorphism of the beta 3-adrenergic receptor gene is not associated with obesity or type 2 diabetes mellitus in a large population-based Caucasian cohort. J Clin Endocrinol Metab. 1998;83:2892–2897. doi: 10.1210/jcem.83.8.5004. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto Y, Ohishi M, Yuan M, Tatara Y, Kato N, Takeya Y, Onishi M, Maekawa Y, Kamide K, Rakugi H. beta-Adrenergic receptor gene polymorphism is a genetic risk factor for cardiovascular disease: a cohort study with hypertensive patients. Hypertens Res. 2011;34:573–577. doi: 10.1038/hr.2010.281. [DOI] [PubMed] [Google Scholar]
- 14.Grygiel-Gorniak B, Kaczmarek E, Mosor M, Przyslawski J, Nowak J. Association of PPAR-gamma2 and beta3-AR Polymorphisms With Postmenopausal Hypertension. J Clin Hypertens (Greenwich) 2015;17:549–556. doi: 10.1111/jch.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan M, Ohishi M, Ito N, Sugimoto K, Takagi T, Terai M, Katsuya T, Rakugi H, Wu Z, Ogihara T. Genetic influences of beta-adrenoceptor polymorphisms on arterial functional changes and cardiac remodeling in hypertensive patients. Hypertens Res. 2006;29:875–881. doi: 10.1291/hypres.29.875. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Zhang B, Li M, Li C, Liu J, Liu Y, Wang Z, Zhou J, Wen S. Association between single-nucleotide polymorphisms in six hypertensive candidate genes and hypertension among northern Han Chinese individuals. Hypertens Res. 2014;37:1068–1074. doi: 10.1038/hr.2014.124. [DOI] [PubMed] [Google Scholar]
- 17.Tonolo G, Melis MG, Secchi G, Atzeni MM, Angius MF, Carboni A, Ciccarese M, Malavasi A, Maioli M. Association of Trp64Arg beta 3-adrenergic-receptor gene polymorphism with essential hypertension in the Sardinian population. J Hypertens. 1999;17:33–38. doi: 10.1097/00004872-199917010-00006. [DOI] [PubMed] [Google Scholar]
- 18.Strazzullo P, Iacone R, Siani A, Cappuccio FP, Russo O, Barba G, Barbato A, D'Elia L, Trevisan M, Farinaro E. Relationship of the Trp64Arg polymorphism of the beta3-adrenoceptor gene to central adiposity and high blood pressure: interaction with age. Cross-sectional and longitudinal findings of the Olivetti Prospective Heart Study. J Hypertens. 2001;19:399–406. doi: 10.1097/00004872-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Ruixing Y, Jinzhen W, Shangling P, Weixiong L, Dezhai Y, Yuming C. Sex differences in environmental and genetic factors for hypertension. Am J Med. 2008;121:811–819. doi: 10.1016/j.amjmed.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Ringel J, Kreutz R, Distler A, Sharma AM. The Trp64Arg polymorphism of the beta3-adrenergic receptor gene is associated with hypertension in men with type 2 diabetes mellitus. Am J Hypertens. 2000;13:1027–1031. doi: 10.1016/s0895-7061(00)00290-9. [DOI] [PubMed] [Google Scholar]
- 21.Pamies-Andreu E, Garcia-Lozano R, Palmero-Palmero C, Garcia-Morillo S, Alonso-Arcas A, Stiefel P, Carneado de la Fuente J, Villar J. Genetic variation in the beta-3-adrenergic receptor in essential hypertension. Life Sci. 2000;67:391–397. doi: 10.1016/s0024-3205(00)00634-2. [DOI] [PubMed] [Google Scholar]
- 22.Oizumi T, Daimon M, Saitoh T, Kameda W, Yamaguchi H, Ohnuma H, Igarashi M, Eguchi H, Manaka H, Tominaga M, Kato T. Genotype Arg/Arg, but not Trp/Arg, of the Trp64Arg polymorphism of the beta(3)-adrenergic receptor is associated with type 2 diabetes and obesity in a large Japanese sample. Diabetes Care. 2001;24:1579–1583. doi: 10.2337/diacare.24.9.1579. [DOI] [PubMed] [Google Scholar]
- 23.Nagano T, Matsuda Y, Tanioka T, Yoshioka T, Hiroi T, Yoshikawa K, Okabe K, Osaka K, Nagamine I, Takasaka Y. No association of the Trp 64 Arg mutation of the beta3-adrenergic receptor gene with obesity, type 2 diabetes mellitus, hyperlipidemia, and hypertension in Japanese patients with schizophrenia. J Med Invest. 2005;52:57–64. doi: 10.2152/jmi.52.57. [DOI] [PubMed] [Google Scholar]
- 24.Mo W, Zhang GG, Yang TL, Dai XP, Li HH, Zeng H, Liu J, Tan YM, Zhou HH, Liu ZQ. The genetic polymorphisms of beta3-adrenergic receptor (AR) Trp64Arg and beta2-AR Gln27Glu are associated with obesity in Chinese male hypertensive patients. Clin Chem Lab Med. 2007;45:493–498. doi: 10.1515/CCLM.2007.089. [DOI] [PubMed] [Google Scholar]
- 25.Masuo K, Katsuya T, Fu Y, Rakugi H, Ogihara T, Tuck ML. Beta2- and beta3-adrenergic receptor polymorphisms are related to the onset of weight gain and blood pressure elevation over 5 years. Circulation. 2005;111:3429–3434. doi: 10.1161/CIRCULATIONAHA.104.519652. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi H, Masuo K, Katsuya T, Sugimoto K, Rakugi H, Ogihara T, Tuck ML. beta2- and beta3-Adrenoceptor polymorphisms relate to subsequent weight gain and blood pressure elevation in obese normotensive individuals. Hypertens Res. 2006;29:951–959. doi: 10.1291/hypres.29.951. [DOI] [PubMed] [Google Scholar]
- 27.Hui P, Nakayama T, Morita A, Sato N, Hishiki M, Saito K, Yoshikawa Y, Tamura M, Sato I, Takahashi T, Soma M, Izumi Y, Ozawa Y, et al. Common single nucleotide polymorphisms in Japanese patients with essential hypertension: aldehyde dehydrogenase 2 gene as a risk factor independent of alcohol consumption. Hypertens Res. 2007;30:585–592. doi: 10.1291/hypres.30.585. [DOI] [PubMed] [Google Scholar]
- 28.Fujisawa T, Ikegami H, Yamato E, Hamada Y, Kamide K, Rakugi H, Higaki J, Murakami H, Shimamoto K, Ogihara T. Trp64Arg mutation of beta3-adrenergic receptor in essential hypertension: insulin resistance and the adrenergic system. Am J Hypertens. 1997;10:101–105. doi: 10.1016/s0895-7061(96)00297-x. [DOI] [PubMed] [Google Scholar]
- 29.Filigheddu F, Reid JE, Troffa C, PinnaParpaglia P, Argiolas G, Testa A, Skolnick M, Glorioso N. Genetic polymorphisms of the beta-adrenergic system: association with essential hypertension and response to beta-blockade. Pharmacogenomics J. 2004;4:154–160. doi: 10.1038/sj.tpj.6500247. [DOI] [PubMed] [Google Scholar]
- 30.Chou YC, Tsai CN, Lee YS, Pei JS. Association of adrenergic receptor gene polymorphisms with adolescent obesity in Taiwan. Pediatr Int. 2012;54:111–116. doi: 10.1111/j.1442-200X.2011.03516.x. [DOI] [PubMed] [Google Scholar]
- 31.Baba T, Nakajima S, Yajima Y. Beta3-adrenergic receptor gene polymorphism is not associated with hypertension in NIDDM patients without nephropathy. Horm Metab Res. 1998;30:629–632. doi: 10.1055/s-2007-978947. [DOI] [PubMed] [Google Scholar]
- 32.Sheng LJ, Ruan CC, Ma Y, Chen DR, Kong LR, Zhu DL, Gao PJ. Beta3 adrenergic receptor is involved in vascular injury in deoxycorticosterone acetate-salt hypertensive mice. FEBS Lett. 2016;590:769–778. doi: 10.1002/1873-3468.12107. [DOI] [PubMed] [Google Scholar]
- 33.Niu W, Wu S, Zhang Y, Li W, Ji K, Gao P, Zhu D. Validation of genetic association in apelin-AGTRL1 system with hypertension in a larger Han Chinese population. J Hypertens. 2010;28:1854–1861. doi: 10.1097/HJH.0b013e32833b1fad. [DOI] [PubMed] [Google Scholar]
- 34.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res. 2014;115:189–202. doi: 10.1161/CIRCRESAHA.115.303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zafarmand MH, van der Schouw YT, Grobbee DE, de Leeuw PW, Bots ML. T64A polymorphism in beta3-adrenergic receptor gene (ADRB3) and coronary heart disease: a case-cohort study and meta-analysis. J Intern Med. 2008;263:79–89. doi: 10.1111/j.1365-2796.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- 36.Kong H, Li X, Zhang S, Guo S, Niu W. The beta1-adrenoreceptor gene Arg389Gly and Ser49Gly polymorphisms and hypertension: a meta-analysis. Mol Biol Rep. 2013;40:4047–4053. doi: 10.1007/s11033-012-2482-2. [DOI] [PubMed] [Google Scholar]
- 37.Kato N. Ethnic differences in genetic predisposition to hypertension. Hypertens Res. 2012;35:574–581. doi: 10.1038/hr.2012.44. [DOI] [PubMed] [Google Scholar]
- 38.Padmanabhan S, Caulfield M, Dominiczak AF. Genetic and molecular aspects of hypertension. Circ Res. 2015;116:937–959. doi: 10.1161/CIRCRESAHA.116.303647. [DOI] [PubMed] [Google Scholar]
- 39.Kelly TN, He J. Genomic epidemiology of blood pressure salt sensitivity. J Hypertens. 2012;30:861–873. doi: 10.1097/HJH.0b013e3283524949. [DOI] [PubMed] [Google Scholar]
- 40.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mavridis D, Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid Based Ment Health. 2014;17:30. doi: 10.1136/eb-2013-101699. [DOI] [PubMed] [Google Scholar]
- 43.Palmer T, Sterne J. Meta-Analysis in Stata: An Updated Collection from the Stata Journal. 2. College Station, TX: Stata Press; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.