Abstract
The effects of electrospray ionization (ESI) solvent and source temperature on the relative abundance of the preferred solution-phase (N-protonated; i.e. amine) versus preferred gas-phase (O-protonated; i.e., acid) isomers of p-aminobenzoic acid (PABA) were investigated. When PABA was electrosprayed from protic solvents (i.e., methanol/water), the infrared multiple photon dissociation (IRMPD) spectrum recorded was consistent with that for O-protonation, according to both calculations and previous studies. When aprotic solvent (i.e., acetonitrile) was used, a different spectrum was recorded and was assigned to the N-protonated isomer. As the amine is the preferred protonation site in solution, this suggests that an isomerization takes place under certain conditions. Photodissociation at the diagnostic band for the O-protonated isomer (NH2 stretching mode) was used to quantify the relative contributions of each isomer to ion signal as a function of ESI conditions. For mixtures of methanol and acetonitrile, the relative contribution of the O-protonated gas-phase structure increased as a function of methanol content. Yet, substituting methanol for water resulted in a marked decrease of isomerization to the O-protonated structure. The source temperature (i.e., temperature of a heated desolvation capillary) was found to play a key role in determining the extent of isomerization, with higher temperatures yielding increased presence of gas-phase structures. These results are consistent with a protic bridge mechanism, in which the ESI droplet temperatures, dependent on endothermic desolvation and radiative heating from the capillary, may determine the isomerization yield.
Keywords: Electrospray, infrared ion spectroscopy, p-aminobenzoic acid, proton isomers
Graphical Abstract

1 Introduction
The determination of preferred (de)protonation sites in molecules with multiple acidic/basic sites is an important fundamental goal of gas-phase structural studies.[1–6] Furthermore, competing gas-phase (de)protonation sites can have important analytical implications. For instance, if relative abundances of the (de)protonation sites are dependent on experimental conditions, so too might be the observed spectra from tandem mass spectrometry,[7] ion mobility,[8, 9] or infrared spectroscopy.[10–12] This is especially important in establishing spectral libraries for the identification of analytes, where inter-lab reproducibility is critical.
Ion mobility spectrometry (IMS)-mass spectrometry (MS) [13, 14] experiments for anilines indicated that ring and amine protonation were, in some cases, competitive.[1, 15] Other IMS-MS experiments have focused on solvent effects on the relative abundance of protonation-site isomers, for example, of p-aminobenzoic acid (PABA).[9] Infrared multiple photon dissociation (IRMPD) spectroscopy [16, 17] experiments have also been implemented to investigate the phenomenon of competing protonation sites. For instance, solvent-dependent spectra attributed to (de)protonation isomers have been recorded for p-hydroxybenzoic acid,[12] PABA,[10] and p-coumaric acid.[11] Notably, IMS-MS has recently been combined with IRMPD spectroscopy to investigate the solvent-dependence of observed protonation isomers of benzocaine.[8]
Specifically, the deprotonation of p-hydroxybenzoic acid as a function of solvent conditions has received considerable attention in the literature. Deprotonation of the carboxylic acid, as assigned by IRMPD spectroscopy, of p-hydroxybenzoic acid was demonstrated to be preferred when electrosprayed from protic solution, whereas deprotonation of the phenol is preferentially observed from aprotic solution.[12] However, these structural assignments were found to be contradictory to previous analyses from CID and hydrogen-deuterium exchange experiments,[18] which also raised the important question whether the gas-phase measurements represented differences in solution-phase conformations or whether a gas-phase isomerization took place. A more systematic study,[19] with complementary solution-phase NMR spectroscopy, confirmed both that the structural assignments made by IRMPD spectroscopy were correct [12] and that the solvent system had no effect on which structure (i.e., deprotonation of the carboxylic acid) was favored in solution.[18] Furthermore, the (apparently) contradictory gas-phase results could be rationalized via isomerization during the electrospray process (or in the gas phase) from the solution-phase protonation site to the more stable gas-phase protonation site. It was hypothesized that one route to isomerization was likely the disruption of gas-phase dimers and this hypothesis was supported by the fact that isomerization generally increased with analyte concentration.[19]
For the case of protonation, a related model compound, p-aminobenzoic acid (PABA), has been investigated. There are two competitive protonation sites in this molecule: the amine and the carbonyl oxygen (see figure 1). It has been reported that the amine is the preferred protonation site (N-protonated) in solution; whereas carbonyl protonation (O-protonated) is energetically preferred in the gas phase according to theory.[10]
Figure 1.
Illustration of the path from solution-phase protonation at the amine to gas-phase protonation, which can be observed at either the amine or the (thermodynamically preferred) carboxylic acid. The isomerization from the N-protonated structure to the O-protonated structure could hypothetically proceed via a solvent bridge or a proton-bound dimer.
Nonetheless, either structure might be observed in the gas phase, depending on whether the solution-phase structure is maintained or isomerization to the thermodynamically-preferred gas-phase structure occurs. It has been confirmed by differential mobility spectroscopy (DMS) that two proton isomers can be formed and that their relative abundances depend on the percentages of protic versus aprotic solvent used for electrospray.[9] In an IRMPD spectroscopy study, the spectrum of the O-protonated isomer was reported and the presence of N-protonation was inferred by selectively “burning away” the O-protonated contribution to the ion signal.[10] Furthermore, it has been shown that the degree of ion solvation can shift the observed protonation site in PABA and its methyl ester.[20] Under desolvated or partially solvated conditions, the O-protonated structure dominates; conversely, for the non-covalent cluster PABAH+ (H2O)6 the N-protonated signal becomes considerable. This suggests that there may be a brief window during the desolvation process in the source in which there are sufficient protic solvent molecules to facilitate proton migration, but few enough solvent molecules for the gas-phase structure to be preferred (i.e., a period of time in which the isomerization reaction is thermodynamically favored and kinetically feasible). In a more recent study, it has been found that hydrogen-deuterium exchange (HDX) by D2O can also lead to isomerization from one structure to the other, which further supports a gas-phase protic-solvent-based mechanism for the interconversion of the N-protonated to the O-protonated structure of PABA.[21]
Summarizing the cases of p-hydroxybenzoic acid and p-aminobenzoic acid, there are two hypothetical pathways for (de)protonation isomerization: (protic) solvent bridges facilitating the shuttling of protons or dissociation of gas-phase dimers. These pathways are summarized in figure 1 for the case of p-aminobenzoic acid. Figure 1 also shows that either protonation isomer may be observed in the gas phase, depending on whether proton migration occurs.
Whereas the deprotonation of p-hydroxybenzoic acid has been investigated in quite some detail, investigations into the protonation sites of PABA has primarily been limited to the differences observed between protic and aprotic solvents. We report herein results for more extensive coverage of solvent conditions. This includes some seeming discrepancies with previous reports which, upon further investigation, could be attributed to differences in additional experimental parameters, notably the source temperature. A discussion on potential mechanistic and analytical implications of these findings are included.
2 Experimental methods
2.1 Sample preparation
Para-aminobenzoic acid (PABA), with a stated purity of ≥99%, was purchased from Sigma-Aldrich (St. Louis, MO, USA). Solutions were made to either ~10−4 M for most experiments, or 10−6 M for the concentration experiment. Solvent system was an important variable in these experiments, thus they are given for each data set, reported as percentages by volume (vol. %). In all cases, solvents were HPLC grade and 1% formic acid was added for improved electrospray stability and ionization efficiency.
2.2 Instrumentation
All infrared multiple photon dissociation (IRMPD) spectra were measured on a custom quadrupole mass filter – quadrupole ion trap – time of flight (QMF-QIT-TOF) mass spectrometer, which has been previously described.[22] Since that report, the source region has been modified (see figure 2) and the infrared light source upgraded; the instrument will be briefly described herein.
Figure 2.
The current source of the instrument used in this work, including (A) an encapsulated electrospray ionization unit, (B) a heated metal capillary, and (C) an electrodynamic ion funnel.
Ions were generated using a custom built encapsulated electrospray ionization (ESI) source. A high voltage potential was applied to the analyte solution prior to being sprayed from a fused silica emitter (50 μm ID/360 μm OD), which is ground to a tip. A room temperature nitrogen sheath gas was applied to help guide the ions created from the ESI plume into a 7.09” long, heated metal desolvation capillary with a 0.02″ ID. The temperature of the capillary was maintained by a temperature controller (CN 740, Omega Engineering, Stamford, CT, USA), equipped with a cartridge heater (60 W, Watlow, St. Louis, MO, USA) and thermocouple (K type, Omega Engineering). Upon exiting the capillary, the ions were focused using an ion funnel,[23] consisting of 50 brass ring electrodes and equipped with a jet disruptor for the dispersion of neutral species. The pressure in the encapsulated spray unit is approximately atmospheric pressure and the pressure in the ion funnel is approximately 1.3 Torr. The ions were then accumulated in the hexapole of a commercial ion source (Analytica, Branford, CT), prior to being guided to the QIT (Jordan TOF Products, Grass Valley, CA) via a mass-selective QMF (Ardara Technologies, Ardara, PA). Since collision-induced dissociation (CID) can occur during injection into the QIT, the rf amplitude of the QIT was briefly increased near the beginning of the trapping period, and prior to irradiation, to eject fragment ions due to CID (see SI for further details). Once the precursor ions were isolated in the QIT, they were irradiated by an optical parametric oscillator/amplifer (OPO/A) (LaserVision, Bellevue, WA), pumped by a Surelite III unseeded 1064 nm Nd:YAG laser (Continuum, San Jose CA), which was focused by a ø1″ CaF2 plano-convex lens (250 mm focal length) through a ø1″ BaF2 window and into the QIT. The timescale of irradiation was determined by a mechanical shutter (model VS25S20, Unblitz, Rochester, NY), controlled by either a delay generator or LabVIEW programming. Following irradiation, the remaining precursor and photodissociation products were extracted into the TOF drift tube for detection. Voltages, timings, and data acquisition were controlled by custom LabView (National Instruments, Austin, TX) software.
2.3 Experimental details
2.3.1 Infrared ion spectroscopy
Infrared multiple photon dissociation (IRMPD) spectra were obtained by measuring photodissociation yield after a single OPO pulse as a function of irradiation wavelength. The OPO wavelength calibration was verified at the beginning and end of each IRMPD spectrum measurement by comparison with a known spectrum, namely protonated tryptophan, which had previously been measured in our group [24] using a continuous wave (cw) OPO (OS4000, LINOS Photonics, Munich, Germany) with the wavelength monitored by an external wavemeter (WA-1000, EXFO, Quebec, Canada), see Supporting Information (SI) and figures S2 and S3 for further details. For the sake of convenience for isomer quantitation (see below), the photodissociation yield is defined here on a percentage scale, given by equation 1,
2.3.2 Relative isomer quantitation
The photodissociation yield was measured as a function of irradiation time, with total trapping time held constant for the protonation isomer obtained from protic solvent at the diagnostic NH2 stretching mode (~3440 cm−1). From this, it was determined that maximum photofragmentation yield (~95–98%) was achieved by 1000 ms (i.e., equivalent to 10 OPO shots at 10 Hz). To account for any potential day-to-day fluctuations in, for instance, laser alignment or power, 1500 ms irradiation time was used for all isomer-burning experiments. Again, the photodissociation yield is reported using equation 1, and is taken to be representative of the relative proportion of the O-protonated isomer produced and analyzed. Relative quantitation experiment data points represent the mean of triplicate measurements, with error bars showing the standard deviation.
2.4 Computational details
Low energy conformers for each proton isomer (based on the study from Kass and colleagues) [10] were submitted to Gaussian 09 [25] for energy optimization and frequency calculations by density functional theory calculations at the B3LYP level of theory with the cc-pVTZ basis set. Structures, vibrations, and spectra were visualized with Avogadro [26] and/or Gabedit [27] visualization software. Vibrational spectra were scaled by 0.960 [28] and were convoluted using a 15 cm−1 full-width-at-half-maximum Gaussian for better comparison with experimental spectra.
3 Results and discussion
3.1 IRMPD spectroscopy and relative quantitation
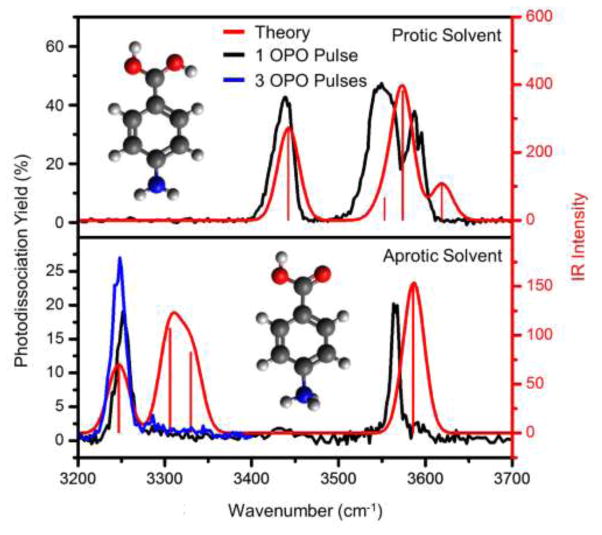
IRMPD spectra for PABA sprayed from 70/30 methanol/water (top) and acetonitrile (bottom) are shown in figure 3. Examples of IRMPD mass spectra are presented in figure S4. When sprayed from protic solvent, relatively high photodissociation yields (~47 % at maximum) are obtained with a single OPO pulse and peaks are observed at ~3590, 3550, and 3440 cm−1. However, when sprayed from aprotic solvent, a very different IRMPD spectrum is obtained. In this case, relatively weak photodissociation is observed (~20% maximum) and peaks are observed at ~3565 and 3250 cm−1. This provides clear evidence that at least two different structures can be formed, depending on the ESI solvent. By comparison to theoretical spectra (red traces in figure 3), the structure of PABA sprayed from protic solvent (top) can be confirmed as protonation at the carboxylic acid. The match between the spectrum obtained from aprotic solvent (bottom) and the amine-protonated theoretical spectrum is not as convincing, as the band predicted at ~3310 cm−1 is not well produced. Yet, there is a small amount of photodissociation, evidenced as a shoulder on the high-energy side of the peak at ~3250 cm−1, which may represent absorption of these modes in this region. However, even at 3 OPO pulses (see figure 3 and figure S5), this feature remains very low in intensity.
Figure 3.
Single OPO-shot IRMPD spectra (black) of PABA sprayed from protic solvent (top) and aprotic solvent (bottom) compared to theoretical infrared spectra (red) for O-protonated (top) and N-protonated (bottom) PABA. In the lower spectrum, an additional measurement at 3 OPO pulses is presented (blue).
A potential third candidate structure is ring protonation, which has previously been calculated to be even less energetically favorable in the gas phase than N-protonation.[29] It is also worth noting that the absence of the NH2 stretches and presence of NH3+ stretches all but rules out any structure other than the N-protonated isomer for the structure obtained from spraying from aprotic solvent.
The spectral assignments are summarized in table 1. Note that a previous study reported peak positions at 3605, 3565, and 3455 cm−1 for (O-protonated) [PABA + H]+ sprayed from an aqueous methanol solution.[10] As discussed in section 2.3.1 the spectrum of tryptophan ([Trp + H]+) was measured and compared to previous spectra obtained using a cw OPO (equipped with a wavemeter), before and after each of the IRMPD spectra used herein to assign protonation site. The overlapping spectra for the pulsed and cw OPO measurements confirm the correct wavelength calibration of these measurements. See SI section 2 for additional details.
Table 1.
Experimental and theoretical IR peak positions
| Vibrational Mode | Band Position/cm−1 B3LYP/cc-PVTZ | Band Position/cm−1 Experimental |
|---|---|---|
| O-Protonated PABA | ||
| OH+ Stretch | 3619 | 3590 |
| OH+ Stretch | 3574 | 3550 |
| Asymmetric NH2 stretch | 3553 | |
| Symmetric NH2 stretch | 3442 | 3440 |
| N-Protonated PABA | ||
| OH Stretch | 3586 | 3565 |
| NH3+ stretches | 3330, 3306, 3247 | 3250 |
The lower photodissociation yield for the N-protonated isomer indicates that the measurement of this spectrum is more challenging; in fact, the N-protonated structure could not be photodissociated at all in the earlier work by Kass.[10] Calculations by Kass and coworkers suggest that the rate determining step for dissociation of the O-protonated ion (by water loss) has a barrier of 44.2 kcal/mol (as calculated with B3LYP) and that fragmentation from the N-protonation structure requires ~20 kcal/mol more than fragmentation from the O-protonated structure.[10]
The lack of a clear feature at ~3310 cm−1 in our N-protonated spectrum may arise due to a shift out of resonance with the laser irradiation upon photon absorption, thus impeding photodissociation. This rationale is based on the IRMPD mechanism. When an infrared photon is absorbed, the internal energy of the molecule increases, and the mode is redshifted due to cross-anharmonicities. Under this rationalization, the band at 3250 cm−1 is observed because at higher internal energies, the higher energy NH3+ mode may shift into resonance with the irradiation energy. In fact, previous studies have shown that the IRMPD yield can be vastly amplified in these conditions.[30, 31] Conversely, the expected band at ~3310 cm−1 might not be observed, since there is not a nearby higher energy mode to shift into resonance with it at higher internal energies.
As an aside, it also appears as if the bottom spectrum in figure 3 shows some evidence for O-protonated structures, based on the weak feature at ~3440 cm−1, suggesting that not all solution-phase structures are retained.
Relative quantitation of ion species can be obtained by selectively photodissociating that species from a larger population using isomer-specific IR wavelengths and, if populations are interconvertible, kinetics of dissociation at those wavelengths.[32]
By introducing multiple OPO pulses into the QIT during trapping, a maximum yield of ~97% can be achieved for the O-protonated structure with the laser parked at the diagnostic NH2 band (~3440 cm−1). The photodissociation yield as a function of irradiation time is shown in figure 4. It should be noted that by ~1 s (i.e., 10 OPO pulses) maximum photodissociation was achieved; however, a longer irradiation time of 1.5 s was implemented for all further relative quantitation experiments to account for any potential day-to-day experimental fluctuations in, for instance, laser power.
Figure 4.
Photodissociation yield at 3440 cm−1 of PABA sprayed from protic solvent (70/30 methanol/water, black squares), and from protic/aprotic mixed solvent (20/80 methanol/acetonitrile, red circles) as a comparison, as a function of time, with the OPO operating at 10 Hz. The arrow represents the time point used for all additional relative quantitation experiments.
In the context of the system under investigation here, caveats for quantitation include incomplete OPO beam overlap with the ion cloud, which would make 100% depletion impossible, and the potential for isomerization upon irradiation, which would shift the structure out of resonance and limit the photodissociation yield. It should be noted that either of these two mechanisms would decrease photodissociation yield and, thus, underestimate the relative amounts of the O-protonated structure. Since ~97% photodissociation yield can be achieved, it is unlikely that either of these two mechanisms are significantly shifting the qualitative results observed.
3.2 Effects of solution conditions
As described in figure 1, two pathways can be conceived to proceed from the solution-phase amine-protonated structure to the thermodynamically preferred gas-phase O-protonated structure. If the dimerization pathway is predominantly at play, one would expect much higher relative proportions of the gas-phase structure when higher-concentration ESI solutions are used. If, on the other hand, the protic solvent bridge mechanism is responsible for the observed isomerization reaction, analyte concentration would be expected to be much less important and the protic nature of the solvent would be expected to cause the most pronounced effect. These two possibilities were thus tested and the results are presented in the top half of figure 5.
Figure 5.
Top: Photodissociation yield (%) at 3440 cm−1 as a function of volume % acetonitrile and methanol for PABA at ~10−4 M (black squares) and 10−6 M (red x’s). Bottom: Photodissociation yield for ~10−4 M PABA sprayed from a 3-solvent system consisting of 20% acetonitrile and 80% protic solvent, where the protic solvent contribution is varied between pure methanol (left) and pure water (right).
For mixtures of varying volume percent of methanol and acetonitrile, there is a clear trend towards higher contributions of the O-protonated structure to the observed ion signal as the percentage of methanol is increased. However, when comparing ~10−4 M (black squares) and 10−6 M (red x’s) analyte concentrations, little difference is observed. In the case of p-hydroxybenzoic acid, in which dimerization is hypothesized to play an important role, a 100:10 phenoxide:carboxylate ratio was observed at 10−3 M concentrations, whereas a 100:45 ratio was observed at 10−5 M.[19] Thus, a pronounced difference, which was not observed in figure 5, would also be expected if dimerization was critically important to the isomerization pathway in the present study. Taken together, these results suggest that only the protic bridge pathway is consistent with the observed results.
Given that water is a highly protic solvent, it might be expected to produce the same—or greater—degree of isomerization as methanol. However, when a three-solvent system, in which the aprotic proportion of 20% acetonitrile was held constant and the 80% protic solvent contribution was systematically varied between pure water and pure methanol, this prediction did not hold true (see figure 5, lower panel). In fact, the photodissociation yield for the acetonitrile/water solution was more similar to that for the aprotic case than for the acetonitrile/methanol case. Table 2 contrasts some of the solvent mixture results of the present study with previous studies [10] [9]. For methanol/water (70/30 in the present study and 75/25 in [9]), the results are remarkably similar between all studies, showing >90% O-protonated PABA in all cases. For the acetonitrile/water (50/50) mixture, conversely, the present study only confirms ~14% O-protonated structure, as opposed to 70% or more for the previous studies. This discrepancy strongly suggests that additional variables (beyond the (a)protic nature of the solvents) must be important in terms of affecting isomerization.
Table 2.
Percentage of O-protonated PABA for methanol/water and acetonitrile/water solutions from three studies, including the one presented herein.
3.3 Effects of capillary temperature
An exploratory preliminary investigation of various source conditions led us to the finding that the source temperature—investigated herein by changing the temperature of the desolvation capillary—had large, reproducible effects on the observed proportion of O-protonated isomers contributing to the ion signal for both 50/50 acetonitrile/methanol (red triangles) and acetonitrile/water (black circles), as shown in figure 6. In acetonitrile/water, as the temperature is increased from 50 °C to 150 °C, the share of O-protonation is observed to increase from ~3% to ~45%, which is much closer to the previously reported observations (i.e., 70% & 78%). Thus, it is likely that at sufficiently high temperatures, our results would fall in line with the previously published results; however, due to the materials used in the encapsulated spray unit, higher temperatures are not currently experimentally possible. The results for methanol/acetonitrile show a similar trend, in that the proportion of O-protonated isomer is enhanced at higher capillary temperatures, yet, strikingly, the O-protonated percentage is also consistently higher in the methanol/acetonitrile results.
Figure 6.

Photodissociation yield (%) at 3440 cm−1 as a function of capillary temperature for PABA sprayed from 50/50 acetonitrile/water (black circles) and 50/50 acetonitrile/methanol (red triangles).
Based on computations and chemical intuition, it stands to reason that smaller analyte-solvent clusters (of perhaps 6 or so solvent molecules) play a key role in terms of thermodynamically and kinetically allowing proton transfer.[20, 21] It follows that efficient proton transfer would occur under conditions where these clusters can exist for sufficiently long timescales. Conversely, if these critical clusters were quickly destroyed, this could impede proton transfer, and thus kinetically trap the solution-phase structure.
A significant difference between water and methanol is found in the higher enthalpy of vaporization for water, which effectively means that more energy must be absorbed in water-rich systems in order to reach critical cluster sizes. In general, thermal heating from the metal capillary plays a vital role in the desolvation process, but collisional heating (due to electric field acceleration) downstream in the ion funnel, skimmer, and hexapole are probably also important, particularly in the latter stages of desolvation. Given the higher vapor pressure for methanol, one would expect that in the ESI source analytes from methanol droplets would reach critical cluster size more quickly than analytes from water droplets. In terms of promoting proton transfer, the critical cluster size would ideally be formed inside the metal capillary, as the droplet evaporation rate would be slow, in equilibrium with the thermal heating from the surroundings. Conversely, the proton transfer could be hindered under energetic collisional activation, where a large number of solvent molecules are detached in quick succession. As there is a distribution of sizes of ESI droplets, it is probable that some analyte ions (originating from smaller droplets) form the critical cluster size inside the metal capillary, and therefore have a higher chance of isomerization; other analyte ions (originating from larger droplets) are only desolvated in the critical cluster regime via energetic collisions in the ion funnel and skimmer regions, and thus have a lower chance of isomerization. This scenario could in principle explain the consistent difference between water and methanol, given that water droplets exhibit less desolvation in the capillary. In addition, it could rationalize the temperature-dependent measurements, where higher capillary temperatures lead to enhanced desolvation in the capillary, and thus a higher isomerization yield.
Now, we consider the heating mechanisms occurring within the capillary. The endothermic solvent evaporation from the droplets is counteracted by collisions with ambient gas molecules in the capillary, which can be understood as convective heat transfer from the metal capillary to the droplets, and the absorption by the droplets of (primarily IR) photons emitted from the metal capillary (as a blackbody emitter), which can be understood as radiative heat transfer. The relative contributions of convective and radiative heat transfers in different air pressure regimes, and as a function of temperature, have been studied in recent publications.[33, 34] Based on these findings, both convective and radiative heating play a role in the pressures present in the metal capillary, with convective heating being more important at pressures above 0.1 atm (i.e., 76 Torr), but radiative heating being dominant at pressures below 0.1 atm. While convective heating is unlikely to differ much between different solvent systems, the inherent IR absorbances for each solvent are different, thus possibly giving rise to differences in blackbody absorption and hence droplet heating.
The absorbed spectral radiance for each solvent was approximated from their IR absorption spectra (based on literature spectra [35–37] of liquids), and calculated blackbody radiation emission curves (see SI section 5). The published IR absorption spectra for these solvents are plotted in figure S6, and the absorbed spectral powers are shown in figure S7. A complicating factor is the limited IR spectral range available for acetonitrile (i.e., 700–4000 cm−1), as opposed the full spectral range for water and methanol (i.e., 2–4000 cm−1). Thus for the sake of comparison, the absorbed radiance for water and methanol are hence given for the limited and full ranges, as depicted in figure S8.
When only considering the limited IR range (i.e., 700–4000 cm−1), it is striking that the absorbed radiance for acetonitrile is considerably lower than that of the protic solvents. This is reflected by the intense and broad absorption features for methanol and water in figure S6. In the absence of absorption spectra for acetonitrile below 700 cm−1, it is presently unclear whether its absorbed radiance for the total IR range is also significantly lower (than for the protic solvents). Nonetheless, given the prominent role of hydrogen-bonding in low-frequency modes of protic liquids,[38] as well as band broadening effects, it seems reasonable to hypothesize that protic solvents like water and methanol absorb blackbody radiation more efficiently (at these particular temperatures) than acetonitrile. Assuming this hypothesis is correct, this might in fact explain some of our experimental results more convincingly. In all cases where significant kinetic trapping of the solution-phase structure was observed, mixtures of acetonitrile were involved, whereas almost no trapping was observed for methanol/water. The gradual increase in kinetic trapping as a function of acetonitrile content in the methanol/acetonitrile results would thus be ascribed to reduced desolvation in the metal capillary due to lower radiative heating (with increasing acetonitrile content). In that sense, the role of acetonitrile in trapping solution-phase structures would not exclusively be due to its aprotic nature, but also due to its radiative absorption characteristics. When considering the entire IR range, the highest absorbed radiance is expected for water, making the water versus methanol trend in figure 5 (inset) less clear cut at first sight. Nonetheless, the higher vaporization enthalpy of water also implies that more energy must be absorbed to desolvate the analyte-solvent clusters than in the case of methanol and acetonitrile, suggesting that some of the desolvation occurs via collisional activation (as discussed above).
It is not surprising that differences in kinetically trapped structures might be observed between different ESI sources and source conditions, in light of results from spectroscopic investigations of ESI droplet temperatures using “thermometer molecules”; those studies have shown that ESI droplets tend to decrease in temperature,[39] but given sufficiently heated sources, can even rise in temperature.[40] This suggests that sources must be individually characterized to realistically determine droplet temperatures—a feat not readily implemented for most working sources, due to the spectroscopic requirements. However, based on the results observed in the experiments reported herein, it can be concluded that our source is likely relatively “cold”, due to the absence of a heating gas.
4 Conclusions
It was shown here that two distinct ion populations—differing in protonation site—can be formed by electrospray ionization of PABA. The effects of solvent (acetonitrile versus methanol versus water) were systematically determined and, when available, compared to previously published results. Furthermore, the role of source temperature on the proton migration isomerization was investigated by varying the temperature applied to the heated metal desolvation capillary of our source. Our source appears to be relatively “cold” and whilst cold sources may be beneficial for probing many fundamental questions, running a “hotter” source may be superior when attempting to enhance inter-instrument reproducibility (such as, in the case of spectral libraries), though this could lead to additional challenges (e.g., increasing other rearrangement/dissociation reactions).
Based on these results, several conclusions can be drawn. In certain cases (i.e., when the preferred protonation site in solution and the gas phase are different and there is a kinetic barrier to isomerization) competing protonation isomers can be obtained from electrospray ionization. In the case of PABA, where protonation can be observed at the amine (energetically preferred in solution) or the carboxylic acid (energetically preferred in the gas phase), several general trends can be noted:
Analyte concentration does not have a strong influence on the extent of isomerization observed.
Isomerization is hindered when only aprotic solvent (acetonitrile) is used.
Protic solvent (methanol/water) produces mainly isomerized (i.e., O-protonated) PABA.
For mixtures of acetonitrile/protic solvent, methanol tends to produce greater isomerization than water at a given capillary temperature.
For mixtures of acetonitrile/protic solvent, the isomerization reaction strongly depends on source temperature and increases with both source temperature and percentage of protic solvent.
Taken together, this suggests that electrospray conditions should be tightly controlled and carefully reported when comparing results from different experiments and/or instruments (e.g., for spectral library comparisons) any time multiple (de)protonation sites are available in a molecule.
Supplementary Material
Acknowledgments
This paper is a tribute to Professor Jose M. Riveros’ distinguished career and research interests in fundamental ion chemistry and ion structure. The project was financially supported by the United States National Science Foundation (NSF) under grant number CHE-1403262 and the United States National Institutes of Health (NIH) under grant number R01GM110077. A.P. acknowledges fellowships from the University of Florida Graduate School and the NSF Graduate Research Fellowship Program (grant no. DGE-1315138).
Footnotes
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karpas Z, Berant Z, Stimac RM. An ion mobility spectrometry/mass spectrometry (IMS/MS) study of the site of protonation in anilines. Struct Chem. 1990;1:201–204. [Google Scholar]
- 2.Graton J, Berthelot M, Gal J-F, Girard S, Laurence C, Lebreton J, Le Questel J-Y, Maria P-C, Nauš P. Site of Protonation of Nicotine and Nornicotine in the Gas Phase: Pyridine or Pyrrolidine Nitrogen? J Am Chem Soc. 2002;124:10552–10562. doi: 10.1021/ja017770a. [DOI] [PubMed] [Google Scholar]
- 3.Dopfer O, Solcà N, Lemaire J, Maitre P, Crestoni M-E, Fornarini S. Protonation Sites of Isolated Fluorobenzene Revealed by IR Spectroscopy in the Fingerprint Range. J Phys Chem A. 2005;109:7881–7887. doi: 10.1021/jp052907v. [DOI] [PubMed] [Google Scholar]
- 4.Wu R, McMahon TB. An Investigation of Protonation Sites and Conformations of Protonated Amino Acids by IRMPD Spectroscopy. ChemPhysChem. 2008;9:2826–2835. doi: 10.1002/cphc.200800543. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen VQ, Tureček F. Protonation Sites in Pyrimidine and Pyrimidinamines in the Gas Phase. J Am Chem Soc. 1997;119:2280–2290. [Google Scholar]
- 6.Bokatzian SS, Stover ML, Plummer CE, Dixon DA, Cassady CJ. An Experimental and Computational Investigation into the Gas-Phase Acidities of Tyrosine and Phenylalanine: Three Structures for Deprotonated Tyrosine. J Phys Chem B. 2014;118:12630–12643. doi: 10.1021/jp510037c. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Aubry A, Bolgar MS, Gu H, Olah TV, Arnold M, Jemal M. Effect of mobile phase pH, aqueous-organic ratio, and buffer concentration on electrospray ionization tandem mass spectrometric fragmentation patterns: implications in liquid chromatography/tandem mass spectrometric bioanalysis. Rapid Commun Mass Spectrom. 2010;24:3221–3229. doi: 10.1002/rcm.4748. [DOI] [PubMed] [Google Scholar]
- 8.Warnke S, Seo J, Boschmans J, Sobott F, Scrivens JH, Bleiholder C, Bowers MT, Gewinner S, Schöllkopf W, Pagel K, von Helden G. Protomers of Benzocaine: Solvent and Permittivity Dependence. J Am Chem Soc. 2015;137:4236–4242. doi: 10.1021/jacs.5b01338. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JL, Le Blanc JCY, Schneider BB. Probing Electrospray Ionization Dynamics Using Differential Mobility Spectrometry: The Curious Case of 4-Aminobenzoic Acid. Anal Chem. 2012;84:7857–7864. doi: 10.1021/ac301529w. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt J, Meyer MM, Spector I, Kass SR. Infrared Multiphoton Dissociation Spectroscopy Study of Protonated p-Aminobenzoic Acid: Does Electrospray Ionization Afford the Amino- or Carboxy-Protonated Ion? J Phys Chem A. 2011;115:7625–7632. doi: 10.1021/jp203829z. [DOI] [PubMed] [Google Scholar]
- 11.Almasian M, Grzetic J, van Maurik J, Steill JD, Berden G, Ingemann S, Buma WJ, Oomens J. Non-Equilibrium Isomer Distribution of the Gas-Phase Photoactive Yellow Protein Chromophore. J Phys Chem Lett. 2012;3:2259–2263. doi: 10.1021/jz300780t. [DOI] [PubMed] [Google Scholar]
- 12.Steill JD, Oomens J. Gas-Phase Deprotonation of p-Hydroxybenzoic Acid Investigated by IR Spectroscopy: Solution-Phase Structure Is Retained upon ESI. J Am Chem Soc. 2009;131:13570–13571. doi: 10.1021/ja903877v. [DOI] [PubMed] [Google Scholar]
- 13.Creaser CS, Griffiths JR, Bramwell CJ, Noreen S, Hill CA, Thomas CLP. Ion mobility spectrometry: a review. Part 1. Structural analysis by mobility measurement. Analyst. 2004;129:984–994. [Google Scholar]
- 14.Kolakowski BM, Mester Z. Review of applications of high-field asymmetric waveform ion mobility spectrometry (FAIMS) and differential mobility spectrometry (DMS) Analyst. 2007;132:842–864. doi: 10.1039/b706039d. [DOI] [PubMed] [Google Scholar]
- 15.Lalli PM, Iglesias BA, Toma HE, de Sa GF, Daroda RJ, Silva Filho JC, Szulejko JE, Araki K, Eberlin MN. Protomers: formation, separation and characterization via travelling wave ion mobility mass spectrometry. J Mass Spectrom. 2012;47:712–719. doi: 10.1002/jms.2999. [DOI] [PubMed] [Google Scholar]
- 16.Polfer NC. Infrared multiple photon dissociation spectroscopy of trapped ions. Chem Soc Rev. 2011;40:2211–2221. doi: 10.1039/c0cs00171f. [DOI] [PubMed] [Google Scholar]
- 17.Eyler JR. Infrared multiple photon dissociation spectroscopy of ions in Penning traps. Mass Spectrom Rev. 2009;28:448–467. doi: 10.1002/mas.20217. [DOI] [PubMed] [Google Scholar]
- 18.Tian Z, Kass SR. Does Electrospray Ionization Produce Gas-Phase or Liquid-Phase Structures? J Am Chem Soc. 2008;130:10842–10843. doi: 10.1021/ja802088u. [DOI] [PubMed] [Google Scholar]
- 19.Schröder D, Budĕšínský M, Roithová J. Deprotonation of p-Hydroxybenzoic Acid: Does Electrospray Ionization Sample Solution or Gas-Phase Structures? J Am Chem Soc. 2012;134:15897–15905. doi: 10.1021/ja3060589. [DOI] [PubMed] [Google Scholar]
- 20.Chang TM, Prell JS, Warrick ER, Williams ER. Where’s the Charge? Protonation Sites in Gaseous Ions Change with Hydration. J Am Chem Soc. 2012;134:15805–15813. doi: 10.1021/ja304929h. [DOI] [PubMed] [Google Scholar]
- 21.Campbell JL, Yang AM-C, Melo LR, Hopkins WS. Studying Gas-Phase Interconversion of Tautomers Using Differential Mobility Spectrometry. J Am Soc Mass Spectrom. 2016;27:1277–1284. doi: 10.1007/s13361-016-1392-2. [DOI] [PubMed] [Google Scholar]
- 22.Gulyuz K, Stedwell CN, Wang D, Polfer NC. Hybrid quadrupole mass filter quadrupole ion trap time-of-flight-mass spectrometer for infrared multiple photon dissociation spectroscopy of mass-selected ions. Rev Sci Instrum. 2011;82:054101. doi: 10.1063/1.3585982. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RT, Tolmachev AV, Page JS, Tang K, Smith RD. The ion funnel: Theory, implementations, and applications. Mass Spectrom Rev. 2010;29:294–312. doi: 10.1002/mas.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mino WK, Gulyuz K, Wang D, Stedwell CN, Polfer NC. Gas-Phase Structure and Dissociation Chemistry of Protonated Tryptophan Elucidated by Infrared Multiple-Photon Dissociation Spectroscopy. J Phys Chem Lett. 2011;2:299–304. [Google Scholar]
- 25.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision A.2. Gaussian, Inc; Wallingford, CT, USA: 2009. [Google Scholar]
- 26.Hanwell M, Curtis D, Lonie D, Vandermeersch T, Zurek E, Hutchison G. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. Journal of Cheminformatics. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allouche A-R. Gabedit—A graphical user interface for computational chemistry softwares. J Comput Chem. 2011;32:174–182. doi: 10.1002/jcc.21600. [DOI] [PubMed] [Google Scholar]
- 28.Lanucara F, Chiavarino B, Scuderi D, Maitre P, Fornarini S, Crestoni ME. Kinetic control in the CID-induced elimination of H3PO4 from phosphorylated serine probed using IRMPD spectroscopy. Chem Commun. 2014;50:3845–3848. doi: 10.1039/c4cc00877d. [DOI] [PubMed] [Google Scholar]
- 29.Tian Z, Kass SR. Gas-Phase versus Liquid-Phase Structures by Electrospray Ionization Mass Spectrometry. Angew Chem Int Edit. 2009;48:1321–1323. doi: 10.1002/anie.200805392. [DOI] [PubMed] [Google Scholar]
- 30.Oomens J, Sartakov BG, Meijer G, von Helden G. Gas-phase infrared multiple photon dissociation spectroscopy of mass-selected molecular ions. Int J Mass Spectrom. 2006;254:1–19. [Google Scholar]
- 31.Parneix P, Basire M, Calvo F. Accurate Modeling of Infrared Multiple Photon Dissociation Spectra: The Dynamical Role of Anharmonicities. The Journal of Physical Chemistry A. 2013;117:3954–3959. doi: 10.1021/jp402459f. [DOI] [PubMed] [Google Scholar]
- 32.Prell JS, Chang TM, Biles JA, Berden G, Oomens J, Williams ER. Isomer Population Analysis of Gaseous Ions From Infrared Multiple Photon Dissociation Kinetics. J Phys Chem A. 2011;115:2745–2751. doi: 10.1021/jp2004166. [DOI] [PubMed] [Google Scholar]
- 33.Saidi M, Abardeh RH. Air Pressure Dependence of Natural-Convection Heat Transfer. Proceedings of The World Congress on Engineering 2010, IAENG; London, UK. 2010; pp. 1444–1447. [Google Scholar]
- 34.Hosseini R, Saidi M. Experimental Study of Air Pressure Effects on Natural Convection From a Horizontal Cylinder. Heat Transfer Engineering. 2012;33:878–884. [Google Scholar]
- 35.Bertie JE, Lan Z. Infrared Intensities of Liquids XX: The Intensity of the OH Stretching Band of Liquid Water Revisited, and the Best Current Values of the Optical Constants of H2O(l) at 25°C between 15,000 and 1 cm-1. Appl Spectrosc. 1996;50:1047–1057. [Google Scholar]
- 36.Bertie JE, Lan Z. Liquid Water Acetonitrile Mixtures at 25 °C: The Hydrogen-Bonded Structure Studied through Infrared Absolute Integrated Absorption Intensities. J Phys Chem B. 1997;101:4111–4119. [Google Scholar]
- 37.Bertie JE, Zhang SL, Eysel HH, Baluja S, Ahmed MK. Infrared Intensities of Liquids XI: Infrared Refractive Indices from 8000 to 2 cm 1, Absolute Integrated Intensities, and Dipole Moment Derivatives of Methanol at 25°C. Appl Spectrosc. 1993;47:1100–1114. [Google Scholar]
- 38.Chen W, Sharma M, Resta R, Galli G, Car R. Role of dipolar correlations in the infrared spectra of water and ice. Phys Rev B. 2008;77:245114. [Google Scholar]
- 39.Gibson SC, Feigerle CS, Cook KD. Fluorometric Measurement and Modeling of Droplet Temperature Changes in an Electrospray Plume. Anal Chem. 2014;86:464–472. doi: 10.1021/ac402364g. [DOI] [PubMed] [Google Scholar]
- 40.Soleilhac A, Dagany X, Dugourd P, Girod M, Antoine R. Correlating Droplet Size with Temperature Changes in Electrospray Source by Optical Methods. Anal Chem. 2015;87:8210–8217. doi: 10.1021/acs.analchem.5b00976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.