Abstract
The purpose of this study was to establish whether UV/ozone (O3) irradiation method can effectively decontaminate hydroxyapatite surfaces, including those modified by the treatment with 30% phosphoric acid solution through morphological and chemical surface analyses (surface roughness, X-ray photoelectron spectroscopy and wettability), and to evaluate the in vitro response of osteoblast-like MC3T3-E1 cells to the modified hydroxyapatite surface decontaminated via this method. The amount of carbon and the contact angle of hydroxyapatite surfaces were significantly decreased by UV/O3 irradiation that lasted for ≥ 5 and ≥ 3 min, respectively (P < 0.01). Additionally, 7-day storage of H3PO4-modified hydroxyapatite surface decontaminated with 5-min irradiation did not affect contact angle values (P > 0.05). MC3T3-E1 cell proliferation, differentiation (as assessed by relative ALP and OCN mRNA levels), and mineralisation were significantly promoted on irradiated surfaces (P < 0.05). These findings show that UV/O3 irradiation for ≥ 5 min significantly decontaminated H3PO4-modified hydroxyapatite surface, improved its wettability, and facilitated osteoblast growth and function.
Keywords: Engineering, Bioengineering, Materials science
1. Introduction
Hydroxyapatite [Ca10(PO4)6(OH)2] (HA) is a candidate bioactive material for bone tissue engineering scaffolds along with β-tricalcium phosphate [β-Ca3(PO4)2] [1, 2]. HA is highly thermodynamically stable at a physiological pH (7.4) but is not bioresorbable [3].
The integration of HA surface with live tissue involves multiple steps including HA solubilisation, establishment of the equilibrium between HA surface and aqueous solutions, protein adsorption, and cell adhesion. According to Dorozhkin [4, 5], HA dissolution occurs in five steps, during which the composition of the surface changes to tricalcium phosphate [Ca3(PO4)2] and dicalcium phosphate dehydrate [CaHPO4] (DCPD). Bertazzo et al. [6] proposed three initial phases in the five steps that occur before the equilibrium is reached between the modified HA surface and biological fluids, with the formation of DCPD on this surface. In our previous study [7], we verified that etching with 30% phosphoric acid of the nanostructured HA surface effectively altered the surface Ca/P ratio and accelerated the initial adhesion, proliferation, and differentiation of osteoblast-like MC3T3-E1 cells. Additionally, bioactive HA surface also enhanced the adhesion and differentiation of RAW 264.7 leukemic macrophage cells, as well as demonstrated favourable properties of a superior surface for bone tissue engineering [8].
The ultraviolet/ozone (UV/O3) irradiation method has been generally used for removal of contaminants from the surfaces of ceramic materials [9]. In that approach, decontamination of ceramic materials occurs via the action of O3 and excited oxygen (O*) atom from atmospheric oxygen (O2) formed by short wavelength UV light irradiation (UV-C: 185 nm and 254 nm). O3 and O* are produced by the following reaction [9]:
| O2 + hν1 (wavelength: 185 nm) → O + O, O + O2 → O3 (1) |
| O3 + hν2 (wavelength: 254 nm) → O* + O2, O* + O2 → O3 (2) |
After Wang et al. demonstrated that UV irradiation induced amphiphilic surfaces of titanium dioxide [10], the UV/O3 irradiation method has been applied to biomaterials such as titanium [11] and zirconium [12]. However, the application of UV/O3 irradiation for the decontamination of HA as a biomaterial has not been reported.
Therefore, the purpose of this study was to examine whether UV/O3 irradiation effectively decontaminates HA surfaces modified by treatment with 30% phosphoric acid solution [7], by morphological and chemical surface analyses. We also evaluated the in vitro response of osteoblast-like cells to the modified HA surface decontaminated with this method.
2. Materials and methods
2.1. Validation of the UV/O3 irradiation method for untreated HA surfaces and surfaces modified by 30% phosphoric acid solution
2.1.1. Sample preparation
HA plates (thickness, 2 mm; width, 10 mm; length, 10 mm) (APP-100; Pentax, Tokyo, Japan) were used in this study. HA plates were either untreated or exposed to 30% phosphoric acid [H3PO4] (lot no. T1949; Sigma-Aldrich Japan, Tokyo, Japan) solution for 10 min at 25 °C, followed by rinsing three times with ultrapure water (MilliQ water: >18 MΩcm) (HAP). The untreated and treated HA samples were irradiated with UV/O3 for 1, 2, 3, 4, 5, 10, 30, 60, or 120 min using a UV/O3 cleaner (ASM401; Asumi, Tokyo, Japan). The apparatus contained low-pressure mercury UV-light that emitted UV-light at 185-nm (1.5 mW/cm2) and 254-nm (15 mW/cm2) wavelengths [13]. The energy of UV-emissions was 647 KJ/mol for 185 nm and 472 KJ/mol for 254 nm. The distance between the UV-source and the sample was 20 mm. Non-irradiated samples were used as controls. Additionally, a modified HA sample irradiated with UV/O3 for 5 min was used to verify the preservation of contact angle during 7 day-storage at room temperature. The surface characteristics of the irradiated samples and controls were evaluated by the following methods. For all samples, each evaluation was conducted in triplicate.
2.1.2. Morphological analysis
Untreated samples and those treated with 30% H3PO4 were exposed to UV/O3 irradiation or kept intact. They were observed for 120 min using a scanning electron microscope (SEM) (VE-8800, Keyence, Japan) and the surface roughness (Ra) was measured using a confocal scanning laser microscope (VK-8500, Keyence, Japan). The Ra value (μm) was defined as the average value of five 100 μm × 100 μm areas.
2.1.3. X-ray photoelectron spectroscopy (XPS) analysis
The samples that had been exposed to UV/O3 irradiation or left intact were mounted individually onto stubs with insulating tape. The surfaces of the samples were chemically analysed using an XPS instrument (AXIS-HS; Kratos, Manchester, UK). The measurements were performed in vacuo (≤ 10−7 Pa) with Al-Kα monochromatic X-rays at a source power of 150 W. The AXIS-HS instrument was equipped with an electron flood gun for charge compensation. Wide- and narrow-scan spectra were acquired at pass energies of 80 eV and 40 eV, respectively. Peak positions were calibrated by referencing a value of 284.6 eV for the peaks corresponding to C-C and C-H in the C 1s spectrum. After smoothing the narrow scans, a straight line (for C 1s, O 1s, Ca 2p, and P 2p) was applied in quantification. The relative sensitivity factors used to calculate the atomic ratios from the peak area ratios were 0.278 for C 1s, 0.780 for O 1s, 1.833 for Ca 2p, and 0.486 for P 2p. Reproducibility was ensured by obtaining five measurements per experimental sample.
2.1.4. Wettability
The wettability of sample surfaces was measured using a contact angle meter (Simage mini; Excimer, Kanagawa, Japan) with the sessile drop method and 3.6 μL ultrapure water. The Young’s equation was used for data analysis.
2.2. Osteoblast-like cells response to the modified HA surface decontaminated by UV/O3 irradiation
2.2.1. Sample preparation
Untreated samples and samples treated with 30% H3PO4 were irradiated with UV/O3 for 5 min or left intact. Their characteristics were evaluated by the following methods. For all samples, each evaluation was conducted in triplicate.
2.2.2. Proliferation of osteoblast-like cells
MC3T3-E1 osteoblast-like cells (derived from mouse calvaria (RIKEN BioResource Center, Tsukuba, Japan)) were cultured in Dulbecco’s modified Eagle’s medium (D-MEM) (lot no. RNBD8499; Sigma-Aldrich Japan) containing 10% foetal bovine serum (lot no. 1324098; Sigma-Aldrich Japan) and 1% penicillin-streptomycin (Sigma- Aldrich Japan) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The medium was changed twice a week.
MC3T3-E1 cells (5 × 103) were seeded onto the 5-min-irradiated and control samples. The cells were incubated at 37 °C in the atmosphere of 95% air and 5% CO2. Cell proliferation was evaluated at 1, 3, and 5 days of culture. Additional cells were seeded on tissue culture plates and incubated as controls over each incubation period.
Cell proliferation was analysed by a Cell Counting Kit-8 assay (Dojindo Laboratory, Kumamoto, Japan), which is a colorimetric test that uses a tetrazolium salt, WST-8, to determine viable cell number. After each incubation period, the growth medium in each well was removed by aspiration and replaced with a mixture of WST-8 and D-MEM in a 1:10 ratio. After 2-h incubation at 37 °C in the atmosphere of 95% air and 5% CO2, a 110-μL aliquot of the mixture that reacted with viable cells was dispensed into each well (n = 5) of a 96-well microplate. The absorbance was read at 450 nm using a microplate reader (Varioskan Flash 2.4; Thermo Fisher Scientific, Waltham, MA, USA).
2.2.3. Differentiation and mineralisation of osteoblast-like cells
MC3T3-E1 cells (10 × 104) were seeded onto the 5-min-irradiated and control samples, as described previously. After a confluent cell monolayer was obtained, minimum essential medium alpha modification (α-MEM: lot no. RNBD3420; Sigma-Aldrich Japan) supplemented with osteoblast-inducer reagent (1% ascorbic acid, 0.2% hydrocortisone, and 2% β-glycerophosphate) replaced D-MEM. Real-time polymerase chain reaction (PCR) analysis of the expression levels of the alkaline phosphatase (ALP) and osteocalcin (OCN) genes was carried out at 7, 14, and 21 days. Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Tokyo, Japan). The total RNA concentration was adjusted to 30 ng/μL using a NanoDrop 1000 system (Thermo Fisher Scientific, Wilmington, DE, USA). One-step PCR was performed from total RNA (30 ng/μL) using a One Step SYBER PrimeScript RT-PCR Kit (TaKaRa, Shiga, Japan) and a Roche LightCycler 1.5 (Roche Diagnostics, Tokyo, Japan). Real-time PCR analysis was performed for ALP, OCN, and GAPDH (internal control). The sequences of the primers used in this analysis were as follows: forward, 5′-AGGGTGGACTACCTCTTAGGTCT-3′ and reverse, 5′-TGGTCAATCCTGCCTCCTTCC-3′ for ALP; forward, 5′-CACAGCAGCTTGGCCCAG-3′ and reverse, 5′-CCTGCTTGGACATGAAGGCTTT-3′ for OCN; and forward, 5′-GCCAGCCTCGTCCCGTAG-3′ and reverse, 5′-CAAATGGCAGCCCTGGTGAC-3′ for GAPDH. The primers were purchased from Sigma.
At 28 days, cell mineralization was evaluated by alizarin red S staining. The growth medium in each well was removed by aspiration and the cells were washed three times with phosphate-buffered saline. After fixation with methanol at 4 °C for 20 min, cells were stained using a calcified nodule staining kit (Cosmo Bio, Tokyo, Japan). Stained samples were quantified using Image J software (National Institutes of Health, Bethesda, MD, USA) as luminance distribution.
2.3. Statistical analysis
The data were analysed by one-way analysis of variance (ANOVA) and post hoc Tukey’s test for multiple comparisons, if appropriate. Pairwise comparisons were performed by the Student’s t-test. Differences were considered significant if P < 0.05.
3. Results
3.1. Validation of the UV/O3 irradiation method
3.1.1. Morphological analysis
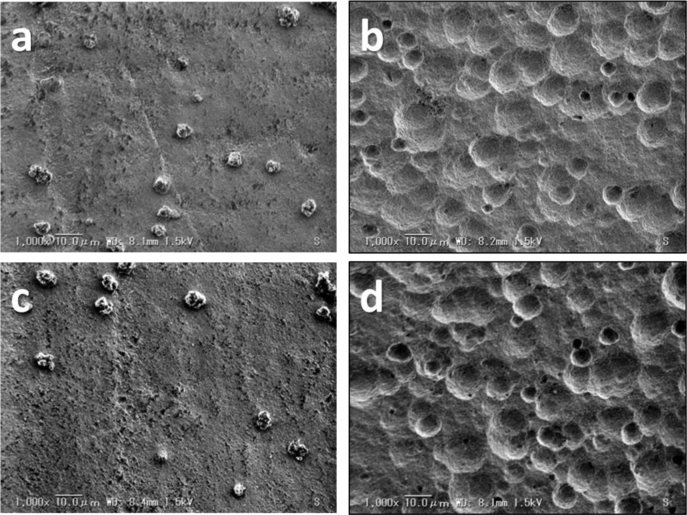
SEM images of the HA samples (untreated or modified by 30% H3PO4) that underwent UV/O3 irradiation at 120 min or were kept intact are shown in Fig. 1. The traces lacking particles appeared at the surface of H3PO4-modified samples. The surface roughness (Ra) values of the samples are given in Table 1. Ra values of samples treated with 30% H3PO4 were significantly larger than those of untreated samples (P < 0.01), whereas UV/O3 irradiation did not have a significant effect.
Fig. 1.
SEM images of untreated HA surface (a) and H3PO4-modified HA surface (b) that did not undergo ultraviolet/ozone (UV/O3) irradiation, and untreated HA surface (c) and H3PO4-modified HA surface (d) exposed to UV/O3 irradiation for 120 min.
Table 1.
Effect of UV/O3 irradiation on surface roughness of untreated and H3PO4-modified HA samples.
| Sample | Irradiation time (min) | Surface roughness Ra (μm) | |
|---|---|---|---|
| Untreated surface | 0 | 0.23 ± 0.02 | a |
| 120 | 0.21 ± 0.03 | a | |
| Modified surface | 0 | 0.91 ± 0.04 | a |
| 120 | 0.86 ± 0.03 | a | |
aP > 0.05 indicates no significant difference between non-irradiated samples and samples irradiated for 120 min.
3.1.2. XPS analysis
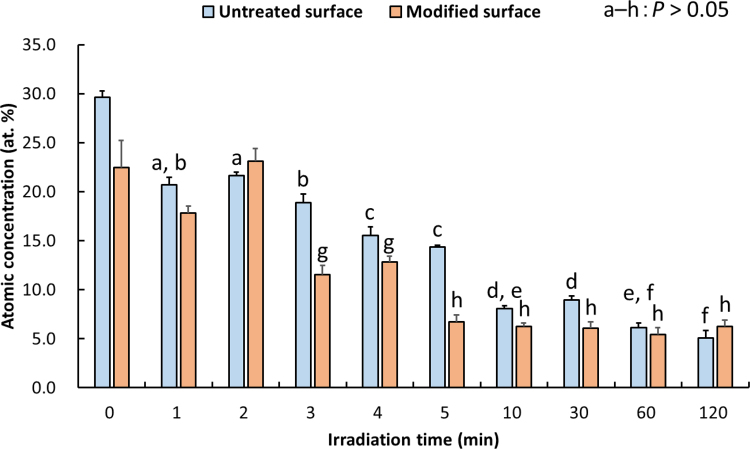
XPS-determined atomic concentrations (at. %) and Ca/P ratios in untreated and H3PO4-modified HA samples that were either kept intact (time 0) or exposed to UV/O3 irradiation of various duration are shown in Table 2. For both types of samples, no significant differences in Ca/P ratios were noted between irradiated and control (irradiation time 0) samples (P > 0.05). The effect of UV/O3 irradiation on at. % values of carbon (C) in untreated and H3PO4-modified HA samples is shown in Fig. 2. The amount of C in untreated samples decreased with the increase of irradiation time (to less than 10%) after irradiation for over 10 min (P < 0.01). The amount of C on H3PO4-modified samples also decreased with the increase of irradiation time, most significantly at ≥ 5 min-irradiation time (P < 0.01).
Table 2.
Effect of UV/O3 irradiation duration on XPS-determined atomic concentration (at. %) and Ca/P ratios of untreated and H3PO4-modified HA samples.
| Sample | Irradiation time | At. % |
Ca/P | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (min) | O 1s | C 1s | Ca 2p | P 2p | |||||||||||||
| Untreated surface |
0 | 39.57 | ± | 3.61 | 30.63 | ± | 2.32 | 17.37 | ± | 0.98 | 11.20 | ± | 0.74 | 1.55 | ± | 0.04 | a |
| 1 | 48.38 | ± | 1.27 | 21.21 | ± | 1.80 | 18.57 | ± | 0.48 | 11.71 | ± | 0.36 | 1.59 | ± | 0.05 | a | |
| 2 | 48.94 | ± | 1.50 | 22.30 | ± | 2.17 | 17.49 | ± | 0.57 | 11.27 | ± | 0.36 | 1.55 | ± | 0.06 | a | |
| 3 | 52.05 | ± | 0.76 | 19.46 | ± | 1.76 | 17.37 | ± | 0.58 | 11.16 | ± | 0.62 | 1.56 | ± | 0.06 | a | |
| 4 | 54.45 | ± | 0.71 | 15.68 | ± | 1.25 | 18.39 | ± | 0.45 | 11.48 | ± | 0.36 | 1.60 | ± | 0.05 | a | |
| 5 | 53.17 | ± | 0.57 | 14.45 | ± | 0.94 | 19.73 | ± | 0.28 | 13.54 | ± | 0.47 | 1.58 | ± | 0.07 | a | |
| 10 | 56.88 | ± | 2.25 | 8.12 | ± | 0.28 | 21.46 | ± | 1.66 | 13.54 | ± | 0.66 | 1.63 | ± | 0.09 | a | |
| 30 | 55.26 | ± | 0.60 | 9.19 | ± | 0.75 | 22.03 | ± | 0.20 | 13.52 | ± | 0.30 | 1.63 | ± | 0.03 | a | |
| 60 | 58.28 | ± | 0.72 | 6.31 | ± | 0.57 | 21.78 | ± | 0.32 | 13.65 | ± | 0.29 | 1.60 | ± | 0.05 | a | |
| 120 | 59.17 | ± | 1.87 | 5.24 | ± | 0.82 | 21.86 | ± | 1.11 | 13.73 | ± | 0.21 | 1.59 | ± | 0.07 | a | |
| Modified surface | 0 | 46.62 | ± | 2.33 | 24.83 | ± | 0.99 | 16.62 | ± | 3.90 | 11.94 | ± | 0.65 | 1.39 | ± | 0.02 | a |
| 1 | 50.71 | ± | 1.23 | 18.59 | ± | 0.29 | 18.05 | ± | 1.25 | 12.65 | ± | 0.27 | 1.43 | ± | 0.03 | a | |
| 2 | 48.93 | ± | 1.27 | 24.65 | ± | 0.72 | 15.26 | ± | 2.35 | 11.16 | ± | 0.40 | 1.37 | ± | 0.02 | a | |
| 3 | 54.79 | ± | 0.96 | 12.46 | ± | 0.40 | 18.96 | ± | 1.57 | 13.79 | ± | 0.30 | 1.37 | ± | 0.03 | a | |
| 4 | 53.27 | ± | 0.66 | 13.51 | ± | 0.43 | 19.41 | ± | 1.15 | 13.81 | ± | 0.28 | 1.41 | ± | 0.04 | a | |
| 5 | 55.83 | ± | 0.53 | 6.95 | ± | 0.36 | 21.78 | ± | 0.80 | 15.46 | ± | 0.57 | 1.41 | ± | 0.05 | a | |
| 10 | 56.51 | ± | 3.23 | 6.74 | ± | 0.26 | 21.80 | ± | 0.75 | 15.07 | ± | 0.36 | 1.45 | ± | 0.04 | a | |
| 30 | 56.83 | ± | 0.73 | 6.93 | ± | 0.44 | 21.53 | ± | 1.24 | 14.72 | ± | 0.36 | 1.46 | ± | 0.04 | a | |
| 60 | 56.81 | ± | 1.02 | 6.21 | ± | 0.40 | 21.78 | ± | 1.33 | 15.20 | ± | 0.25 | 1.43 | ± | 0.04 | a | |
| 120 | 56.94 | ± | 0.71 | 6.82 | ± | 0.48 | 21.67 | ± | 0.96 | 14.57 | ± | 0.24 | 1.49 | ± | 0.03 | a | |
aP > 0.05 indicates no significant difference between samples of untreated and H3PO4 modified surfaces exposed to irradiation of the same duration, respectively.
Fig. 2.
Mean atomic concentration (at. %) values of carbon (C) in untreated and H3PO4-modified HA surfaces irradiated with ultraviolet/ozone (UV/O3). The same letters (a to h) above the values indicate that there were no significant differences between the two types of samples (P > 0.05). The error bars indicate standard deviation.
3.1.3. Wettability
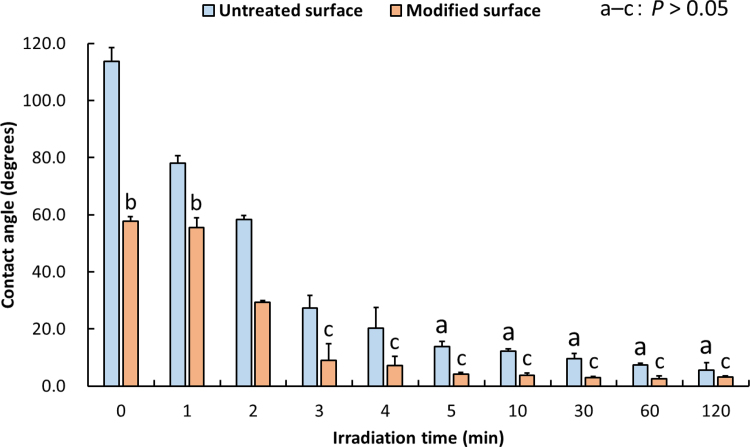
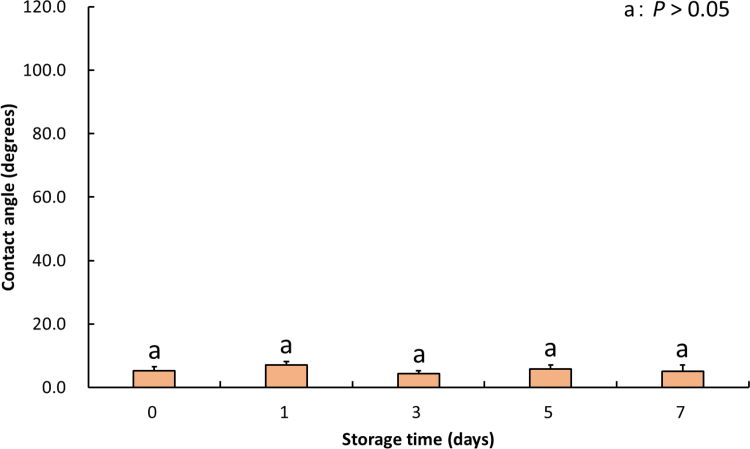
The effect of UV/O3 irradiation on contact angles of untreated and H3PO4-modified HA samples is shown in Fig. 3. The contact angle of untreated samples at 0 min-irradiation time was > 100 degrees (hydrophobic surface). The contact angle decreased with the increase of irradiation time, most significantly at ≥ 5 min-irradiation time (P < 0.01) being < 20 degrees at 5 min-irradiation time (hydrophilic surface). The contact angle of H3PO4-modified samples also decreased with the increase of irradiation time, most significantly at ≥ 3 min-irradiation time (P < 0.01). Furthermore, the contact angle of modified samples irradiated with UV/O3 for 5 min after 7-day storage is shown in Fig. 4. Storage time did not affect the contact angle (P > 0.05).
Fig. 3.
Mean contact angle values in untreated and H3PO4-modified HA surfaces irradiated with ultraviolet/ozone (UV/O3). The same letter (a to c) above the values indicate that there were no significant differences between the two types of samples (P > 0.05). The error bars indicate standard deviation.
Fig. 4.
Mean contact angle values in H3PO4-modified HA surfaces irradiated with UV/O3 for 5 min throughout 7-day storage. No effect of storage duration on the contact angle was observed (P > 0.05). The error bars indicate standard deviation.
3.2. Osteoblast-like cell response to H3PO4 modified HA surface decontaminated by UV/O3 irradiation
3.2.1. Cell proliferation
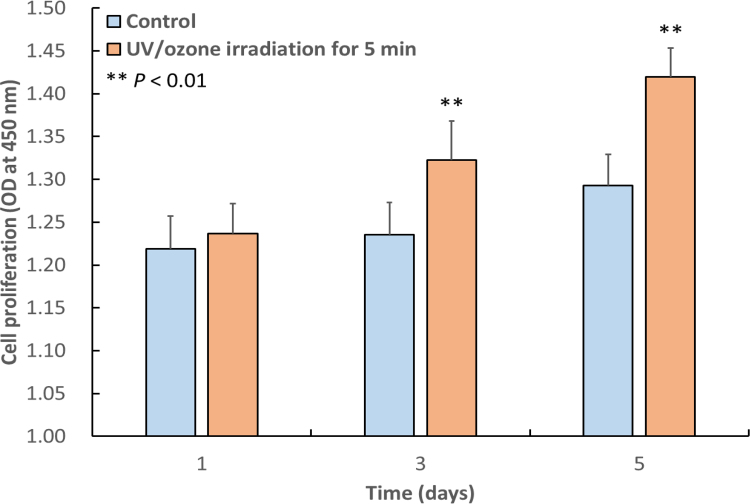
Proliferation of MC3T3-E1 osteoblast-like cells on control H3PO4-modified samples and on those irradiated with UV/O3 for 5 min was monitored for 1, 3, or 5 days (Fig. 5). At 3 and 5 days, proliferation of cells grown on 5 min-irradiated samples were significantly higher than that of cells cultured on control samples (P < 0.01).
Fig. 5.
Mean OD450 values proportional to the extent of proliferation of MC3T3-E1 osteoblast-like cells cultured for 1, 3, or 5 days on control H3PO4-modified HA surfaces and on modified HA surfaces irradiated with UV/O3 for 5 min. At 3 and 5 days, proliferation of cells grown on irradiated surfaces was significantly higher than that of cells cultured on control surfaces (P < 0.01). The error bars indicate standard deviation.
3.2.2. Cell differentiation and mineralisation
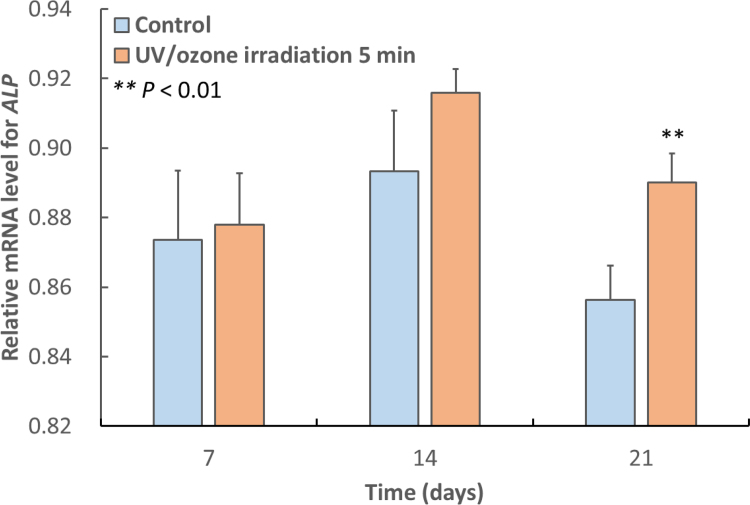
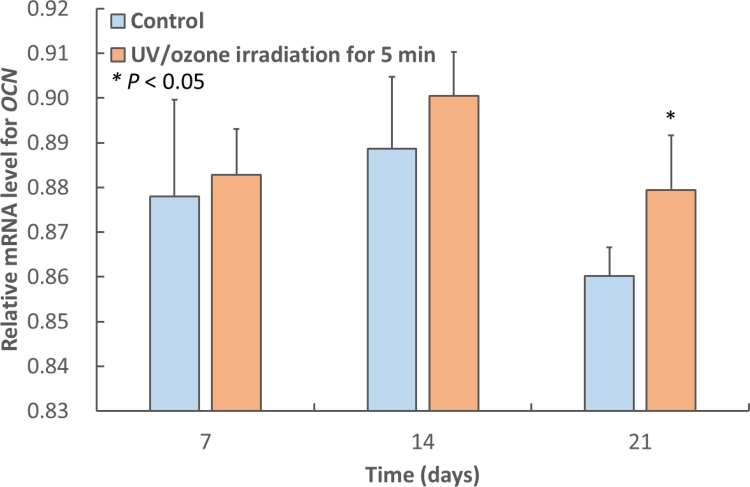
Relative mRNA levels of the ALP and OCN genes were determined in MC3T3-E1 osteoblast-like cells cultured for 7, 14, or 21 days after obtaining a confluent cell monolayer on control H3PO4-modified samples and on those irradiated with UV/O3 (Figs. 6 and 7 ). At days 7 and 14, the expression levels of ALP and OCN in cultures grown on irradiated samples were numerically higher than those in cultures grown on control samples, but the effect did not reach statistical significance (P > 0.05). At 21 days, the expression levels of ALP and OCN in cells grown on irradiated samples were significantly higher than those in cells cultured on control samples (P < 0.01, P < 0.05, respectively).
Fig. 6.
Mean relative ALP mRNA levels in MC3T3-E1 osteoblast-like cells cultured for 7, 14, or 21 days after obtaining a confluent cell monolayer on control H3PO4-modified HA surfaces and on modified HA surfaces irradiated with UV/O3 for 5 min. At 21 days, the expression on the 5 min-irradiated surface was significantly higher than that on the control (P < 0.01). The error bars indicate standard deviation.
Fig. 7.
Mean relative OCN mRNA levels in MC3T3-E1 osteoblast-like cells cultured for 7, 14, or 21 days after obtaining a confluent cell monolayer on control H3PO4-modified HA surfaces and on modified HA surfaces irradiated with UV/O3 for 5 min. At 21 days, the expression on the 5 min-irradiated surface was significantly higher than that on the control (P < 0.01). The error bars indicate standard deviation.
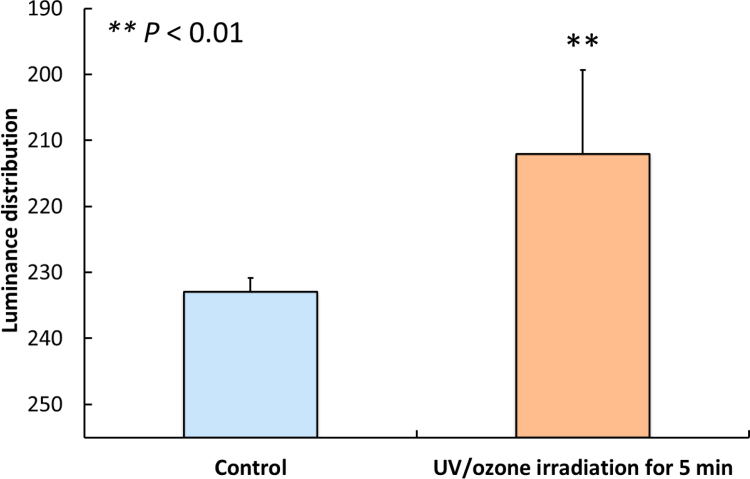
The luminance distribution of alizarin red S staining of cells grown on control and UV/O3-irradiated H3PO4-modified samples at 28 days is shown in Fig. 8. The luminance distribution value in cells cultured on irradiated samples (212.1 ± 12.7) was significantly lower than that in cells grown on control samples (240.0 ± 2.1) (P < 0.01). Because the staining intensity is inversely proportional to the distribution value, mineralisation of cells grown on irradiated samples was significantly higher than that of cells cultured on control samples (P < 0.01).
Fig. 8.
Mean values of luminance distribution of alizarin red S staining in MC3T3-E1 osteoblast-like cells cultured for 28 days on control H3PO4-modified HA surfaces and on modified HA surfaces irradiated with UV/O3 for 5 min. Because the staining intensity is inversely proportional to the distribution value, mineralisation of cells grown on irradiated HA surface was significantly higher than that of cells cultured on control surface. (P < 0.01). The error bars indicate standard deviation.
4. Discussion
HA is considered as a promising material for bone tissue engineering and because its interactions with live cells are a complex, multi-step process, in this study, we examined the effects of UV/O3-irradiation, a recognised decontamination technique, on growth parameters of osteoblast-like MC3T3-E1 cells.
4.1. Validation of the UV/O3 irradiation method
Ra surface roughness values of untreated and H3PO4-modified samples were not significantly affected by UV/O3 irradiation. Furthermore, no significant differences in Ca/P ratios were observed between irradiated and control samples (P > 0.05). Therefore, UV/O3 irradiation did not alter fine morphological and chemical structure of HA surfaces.
The at. % values of C in untreated samples decreased significantly (to less than 10%) when UV/O3 irradiation lasted 10 min or longer compared to that in non-irradiated control samples (30.6 ± 2.3%; P < 0.05; Fig. 2). Similarly, at. % values of C in H3PO4-modified samples decreased significantly upon UV/O3 irradiation for ≥ 5 min (to < 10%) compared to that in non-irradiated control samples (24.8 ± 1.0%; P < 0.05). Thus, UV/O3 irradiation effectively removed the contamination from both untreated and H3PO4-modified HA surfaces.
As H3PO4-modified surface was etched and washed during sample preparation, its at. % of C value was lower than that of untreated surface. The contact angle of untreated samples decreased with the increase of irradiation time, most significantly if irradiation lasted for 5 min or longer (P < 0.01). Similarly, the contact angle of the H3PO4-modified sample decreased with the increase of irradiation time, most significantly at ≥ 3 min-irradiation time (P < 0.01). Notably, the contact angle of H3PO4-modified samples was less than 5 degrees at ≥ 5 min-irradiation time, indicating that the modified surface had become superhydrophilic. In our previous study [7], we found that the modified surface becomes hydrophilic following morphological and chemical modifications. In both the previous and current studies, we used deionized water rather than culture medium for the evaluation of wettability because the latter includes ions that may be absorbed on HA surface. Subsequent UV/O3 irradiation likely removed these contaminants from the surfaces, most of which were hydrophobic in character, resulting in the creation of the superhydrophilic surface. In the present study, no change in wettability of H3PO4-modified surface irradiated with UV/O3 for 5 min was observed over 7-day storage (P > 0.05). Although Noro et al. [14] reported that surface wettability of UV-treated zirconium was maintained after immersion in an aqueous condition, here we showed that the wettability of H3PO4-modified surface irradiated with UV/O3 for 5 min was maintained also after dry storage. These findings demonstrate that UV/O3 irradiated surfaces are less likely to become re-contaminated.
4.2. Osteoblast-like cell response H3PO4-modified HA surface decontaminated by UV/O3 irradiation
Proliferation of MC3T3-E1 osteoblast-like cells on H3PO4-modified HA surface irradiated with UV/O3 was significantly higher than that on control surfaces at 3 and 5 days (P < 0.01). The expression of the ALP and OCN genes in the cells grown on irradiated HA surface was significantly higher than that in cells cultured on control HA surface at 21 days (P < 0.01 and P < 0.05, respectively). Furthermore, at 28 days, alizarin red S staining intensity of cultures grown on irradiated HA surface was significantly higher than that of the cells grown on control HA surface (P < 0.01). These results suggest that decontamination by UV/O3 irradiation promoted cell proliferation and accelerated differentiation and mineralisation.
Ducheyne and Qiu [15] reported that the following events occur at the bioactive ceramics-tissue interface prior to cell attachment and proliferation: (1) dissolution from the ceramics; (2) precipitation from the solution onto ceramics surface; (3) ion exchange and structural rearrangement at the ceramics-tissue interface; (4) interdiffusion from the surface boundary layer into the ceramics; (5) solution-mediated effects on cellular activity; (6) deposition of either the mineral phase or the organic phase; and (7) chemotaxis to the ceramic surface. Furthermore, Bertazzo et al. [6] reported that the equilibrium between modified HA surface and biological fluids leads to protein adsorption and cell adhesion. We suggest that the decontamination of HA surface accelerated the emergence of the equilibrium state as a result of the direct contact at the HAP-tissue interface.
We believe that in our current experiments, dissolution of Ca2+ ions was activated at the interface and the increased amount of Ca2+ ions led to an acceleration of the differentiation and mineralisation of MC3T3-E1 cells. In particular, Wang et al. [16] demonstrated that a calcium phosphate ceramics substrate supported osteoblast growth and bone-related gene expression and that the characteristic gene expression pattern explained the basis of the biocompatibility and bioactivity observed for calcium phosphate ceramics. Additionally, Chang et al. [17] suggested that an elevated concentration of calcium and phosphate is crucial for in vitro mineralisation.
Furthermore, we also suggest that the effects of the combination of acid etching and UV/O3 irradiation on HA surfaces developed in the following steps: (1) surface modification of HA by an acid-etching procedure leading to high solubility because of a reduction in Ca/P ratio; (2) removal of surface contaminants by UV/O3 irradiation; (3) emergence of a more direct surface contact with the culture medium; (4) activation of ion exchange at the interface; (5) earlier attainment of the equilibrium state; and (6) acceleration of the proliferation, differentiation, and mineralisation of MC3T3-E1 cells.
5. Conclusion
In this study, we established a UV/O3 irradiation method for HA surfaces treated with 30% phosphoric acid solution and evaluated in vitro response of MC3T3-E1 cells to the modified HA surface decontaminated by UV/O3 irradiation. UV/O3 irradiation of the modified surface for ≥5 min significantly decontaminated the surface, improved its wettability, and accelerated the proliferation, differentiation, and mineralisation of MC3T3-E1 cells. Therefore, UV/O3 irradiation of HA as a scaffold will likely serve as a clinically beneficial method to provide highly biocompatible HA-containing products to patients.
Declarations
Author contribution statement
Keisuke Yasuda: Performed the experiments; Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper
Yohei Okazaki: Performed the experiments; Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yasuhiko Abe: Conceived and designed the experiments; Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kazuhiro Tsuga: Conceived and designed the experiments.
Funding statement
This study was supported in part by a Grant-in-Aid for Scientific Research (no. 24592915) from the Japan Society for the Promotion of Science (JSPS) and by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (2012-2015).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Hoppe A., Güldal N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Cai S., Xu G.H., Yu X.Z., Zhang W.J., Xiao Z.Y., Yao K.D. Fabrication and biological characteristics of β-tricalcium phosphate porous ceramic scaffolds reinforced with calcium phosphate glass. J. Mater. Sci. Mater. Med. 2009;20:351–358. doi: 10.1007/s10856-008-3591-2. [DOI] [PubMed] [Google Scholar]
- 3.Dorozhkin S.V. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31:1465–1485. doi: 10.1016/j.biomaterials.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 4.Dorozhkin S.V. Surface reactions of apatite dissolution. J. Colloid Interface Sci. 1997;191:489–497. doi: 10.1006/jcis.1997.4942. [DOI] [PubMed] [Google Scholar]
- 5.Dorozhkin S.V. Inorganic chemistry of the dissolution phenomenon: The dissolution mechanism of calcium apatites at the atomic (ionic) level. Comments Inorg. Chem. 1999;20:285–299. [Google Scholar]
- 6.Bertazzo S., Zambuzzi W.F., Campos D.D., Ogeda T.L., Ferreira C.V., Bertran C.A. Hydroxyapatite surface solubility and effect on cell adhesion. Colloids Surf. B Biointerfaces. 2010;78:177–184. doi: 10.1016/j.colsurfb.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Abe Y., Okazaki Y., Hiasa K., Yasuda K., Nogami K., Mizumachi W., Hirata I. Bioactive surface modification of hydroxyapatite. Biomed. Res. Int. 2013;2013:626452. doi: 10.1155/2013/626452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki Y., Abe Y., Yasuda K., Hiasa K., Hirata I. Osteoclast response to bioactive surface modification of hydroxyapatite. Open J. Stomatol. 2014;4:340–344. doi: 10.1155/2013/626452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vig J.R. UV/ozone cleaning of surfaces. J. Vac. Sci. Technol. 1985;3:1027–1034. [Google Scholar]
- 10.Wang R., Hashimoto K., Fujishima A., Chikuni M., Kojima E., Kitamura A., Shimohigoshi M., Watanabe T. Light-induced amphiphilic surfaces. Nature. 1997;388:431–432. [Google Scholar]
- 11.Aita H., Hori N., Takeuchi M., Suzuki T., Yamada M., Anpo M., Ogawa T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials. 2009;30:1015–1025. doi: 10.1016/j.biomaterials.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Att W., Takeuchi M., Suzuki T., Kubo K., Anpo M., Ogawa T. Enhanced osteoblast function on ultraviolet light-treated zirconia. Biomaterials. 2009;30:1273–1280. doi: 10.1016/j.biomaterials.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Oláh A., Hillborg H., Vancso G.J. Hydrophobic recovery of UV/ozone treated poly(dimethylsiloxane): adhesion studies by contact mechanics and mechanism of surface modification. Appl. Surf. Sci. 2005;239:410–423. [Google Scholar]
- 14.Noro A., Kaneko M., Murata I., Yoshinari M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal) J. Biomed. Mater. Res. B Appl. Biomater. 2013;101:355–363. doi: 10.1002/jbm.b.32846. [DOI] [PubMed] [Google Scholar]
- 15.Ducheyne P., Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20:2287–2303. doi: 10.1016/s0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 16.Wang C., Duan Y., Markovic B., Barbara J., Howlett C.R., Zhang X., Zreiqat H. Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different phase compositions. Biomaterials. 2004;25:2507–2514. doi: 10.1016/j.biomaterials.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y.L., Stanford C.M., Keller J.C. Calcium and phosphate supplementation promotes bone cell mineralization: implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000;52:270–278. doi: 10.1002/1097-4636(200011)52:2<270::aid-jbm5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]