Abstract
Legionella pneumophila is a Gram-negative, flagellated bacterium that survives in phagocytes and causes Legionnaires’ disease. Upon infection of mammalian macrophages, cytosolic flagellin triggers the activation of Naip/NLRC4 inflammasome, which culminates in pyroptosis and restriction of bacterial replication. Although NLRC4 and caspase-1 participate in the same inflammasome, Nlrc4-/- mice and their macrophages are more permissive to L. pneumophila replication compared with Casp1/11-/-. This feature supports the existence of a pathway that is NLRC4-dependent and caspase-1/11-independent. Here, we demonstrate that caspase-8 is recruited to the Naip5/NLRC4/ASC inflammasome in response to flagellin-positive bacteria. Accordingly, caspase-8 is activated in Casp1/11-/- macrophages in a process dependent on flagellin, Naip5, NLRC4 and ASC. Silencing caspase-8 in Casp1/11-/- cells culminated in macrophages that were as susceptible as Nlrc4-/- for the restriction of L. pneumophila replication. Accordingly, macrophages and mice deficient in Asc/Casp1/11-/- were more susceptible than Casp1/11-/- and as susceptible as Nlrc4-/- for the restriction of infection. Mechanistically, we found that caspase-8 activation triggers gasdermin-D-independent pore formation and cell death. Interestingly, caspase-8 is recruited to the Naip5/NLRC4/ASC inflammasome in wild-type macrophages, but it is only activated when caspase-1 or gasdermin-D is inhibited. Our data suggest that caspase-8 activation in the Naip5/NLRC4/ASC inflammasome enable induction of cell death when caspase-1 or gasdermin-D is suppressed.
Author summary
Legionella pneumophila is the causative agent of Legionnaires’ disease, an atypical pneumophila that affects people worldwide. Besides the clinical importance, L. pneumophila is a very useful model of pathogenic bacteria for investigation of the interactions of innate immune cells with bacterial pathogens. Studies using L. pneumophila demonstrated that Naip5 and NLRC4 activate caspase-1 and this inflammasome is activated by bacterial flagellin. However, macrophages and mice deficient in NLRC4 are more susceptible for L. pneumophila replication than those deficient in caspase-1, indicating that the flagellin/Naip5/NLRC4 inflammasome triggers responses that are independent on caspase-1. Here, we used L. pneumophila to investigate this novel pathway and found that caspase-8 interacts with NLRC4 in a process that is dependent on ASC and independent of caspase-1 and caspase-11. Although caspase-8 is recruited to the Naip5/NLRC4/ASC inflammasome, it is only activated when caspase-1 or gasdermin-D is inhibited. Our data suggest that caspase-8 activation in the Naip5/NLRC4/ASC inflammasome may favor host responses during infections against pathogens that inhibit components of the pyroptotic cell death including caspase-1 and gasdermin-D.
Introduction
Legionella pneumophila is the causative agent of Legionnaires’ disease. It was identified for the first time in 1976, after an atypical pneumonia affected the participants of the American Legion Convention in Philadelphia, United States [1]. After isolation, L. pneumophila were characterized as Gram-negative, flagellated, intracellular facultative bacteria [2,3]. The species of Legionella were found mainly in freshwater and soil environments, including lakes and irrigation systems [4]. Infection of humans occurs upon inhalation of water droplets derived from these environments containing Legionella [5]. After inhalation, L. pneumophila can subvert the normal vesicle traffic within alveolar macrophages and form LCV (Legionella-containing vacuoles), a process that is mediated by the injection of hundreds of bacterial effectors through a type IV secretion system called Dot/Icm [6–9]. During its evolution, L. pneumophila were selected based on their replication in protozoa but not in humans, which are accidental hosts [10]. Consequently, L. pneumophila can be recognized by many innate immune receptors in mammalian cells, including proteins from the family of the nucleotide-binding domain and leucine-rich repeat-containing proteins (NLRs). These characteristics make L. pneumophila an excellent model for the study of innate immunity, including intracellular signaling pathways and inflammasomes.
The major inflammasome that leads to the restriction of Legionella replication in macrophages is Naip5/NLRC4. This pathway was discovered in mouse cells upon observations that macrophages from the A/J mouse strain, but not cells from other mice strains, are susceptible to L. pneumophila replication [11]. The resistance was mapped to the Lgn1 locus, which encodes several copies of Naip genes, including Naip5 (Birc1e), which is the gene responsible for resistance [12–16]. Successful lines of investigation culminated in the demonstration that Naip5 recognizes bacterial flagellin and interacts with NLRC4 for caspase-1 activation and the restriction of bacterial replication [17–20]. This platform was named the Naip5/NLRC4 inflammasome and triggers pore formation and pyroptosis, which has been considered one of the most important mechanisms for the restriction of intracellular pathogen replication via inflammasomes [21–24]. Host cell death via pyroptosis eliminates intracellular parasite replication and traps intracellular microbes in pyroptotic cells, facilitating microbial destruction by additional phagocytes [23,25–29]. Pyroptosis occurs concomitantly with the secretion of inflammatory cytokines such as IL-1β and IL-18, a process that requires the adaptor molecule ASC and the formation of NLRC4/ASC puncta [20,30]. ASC also functions as an adaptor protein for other inflammasomes, including AIM2 and NLRP3, which triggers the processing of caspase-1 and caspase-8 [21,31–34]. Of note, Naip5/NLRC4 appears to be the only inflammasome required for the restriction of L. pneumophila replication. Macrophages that are deficient in NLRP3 or AIM2 can efficiently restrict L. pneumophila replication [20,21,23,35]. However, the participation of ASC in the resistance of L. pneumophila infection is controversial. In murine macrophages, ASC is dispensable for the induction of pyroptosis and the restriction of bacterial replication [20,21]. By contrast, experiments performed with human monocytes indicate that ASC silencing leads to an increase in bacterial replication [36,37]. Thus, the role of ASC in the restriction of L. pneumophila replication is still unclear.
We have previously demonstrated the existence of a pathway that is dependent on flagellin and NLRC4 but independent of caspase-1 [38]. Here, we used macrophages and Casp1/11-/- mice to systematically assess this pathway. By searching for additional components that operate in the NLRC4 inflammasome independently of caspase-1/11, we found that caspase-8 interacts with NLRC4 in a process that is dependent on ASC. This pathway effectively accounts for resistance to infection in macrophages and in vivo when caspase-1 is absent. In wild-type cells, caspase-8 is recruited to the Naip5/NLRC4/ASC/caspase-1 inflammasome, but is not activated. Caspase-8 activation in this platform only occurs when caspase-1 or gasdermin-D is inhibited, suggesting that this pathway may be important when pyroptosis is inhibited.
Results
Restriction of L. pneumophila replication in BMDMs is flagellin/NLRC4-dependent, ASC-independent and partially caspase-1/11-dependent
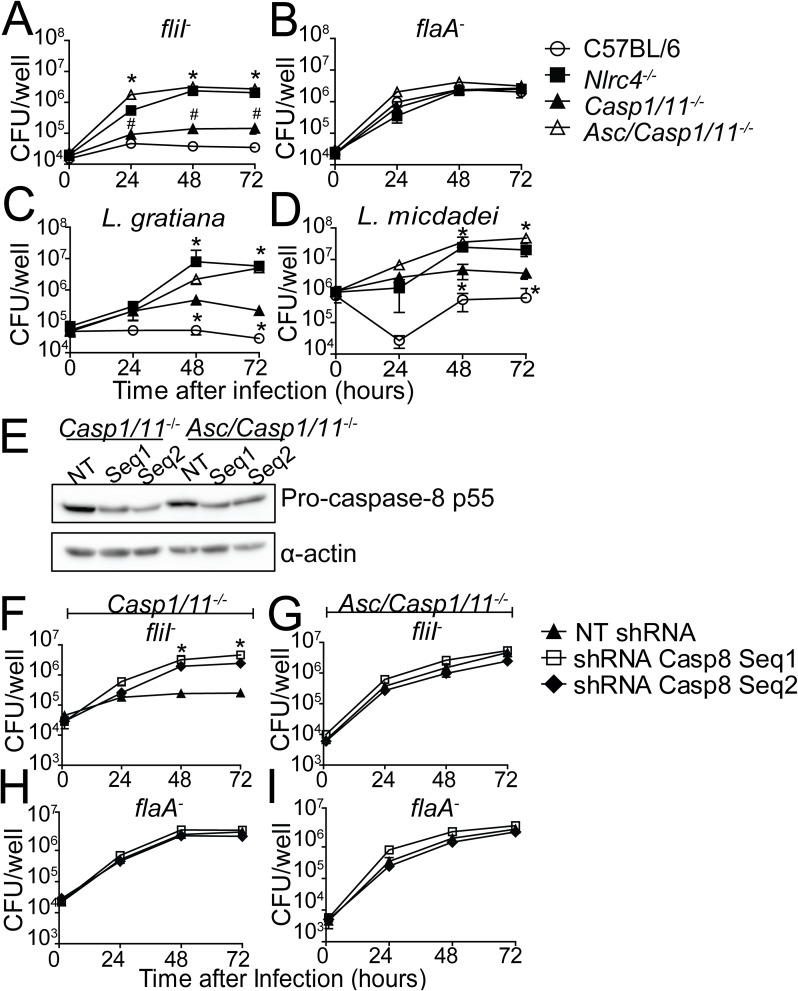
We have previously demonstrated that activation of the flagellin/NLRC4 inflammasome triggers caspase-1-dependent and independent responses to restrict Legionella replication in macrophages and in mouse lungs [38]. However, the caspase-1-independent mechanisms underlying this pathway are unknown. To further characterize this pathway, we performed growth curves using high and very low multiplicity of infections (MOIs) in bone marrow-derived macrophages (BMDMs). Macrophages were infected with wild-type L. pneumophila in the JR32 background (WT Lp) and the isogenic mutants flaA- and fliI-. FliI is an ATPase that is required for the secretion of flagellin through the flagellar apparatus [39]. Consequently, fliI- mutants express flagellin but are non-motile and non-flagellated, making them an appropriate control for flaA- mutants for investigations related to the role of flagellin. We found that BMDMs from C57BL/6 and Asc-/- mice fully restrict the replication of WT Lp and fliI- bacteria at low and high MOIs. In contrast, Nlrc4-/- cells are permissive and Casp1/11-/- cells are partially restrictive (S1 Fig). Bacterial mutants for flagellin bypass NLRC4-mediated growth restriction and replicate in all macrophages as previously described [17–19,40]. These data support previous reports showing that ASC is not required for the restriction of L. pneumophila replication in the presence of caspase-1/11 [20,21]. In addition, these data further support our previous assertion that flagellin triggers an uncharacterized pathway that is dependent on NLRC4 and independent of caspase-1 and caspase-11 [38]. We decided to use BMDMs from Casp1/11-/- mice to further investigate this NLRC4-dependent and caspase-1/11-independent pathway.
Flagellated L. pneumophila triggers NLRC4 puncta that associate with caspase-8 in a process that is ASC-dependent
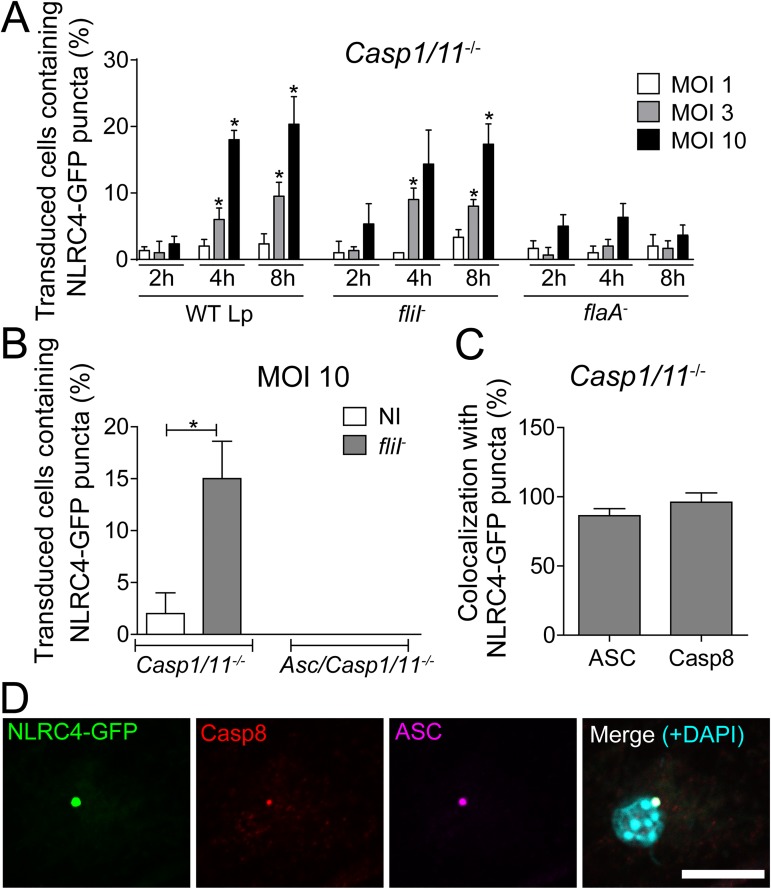
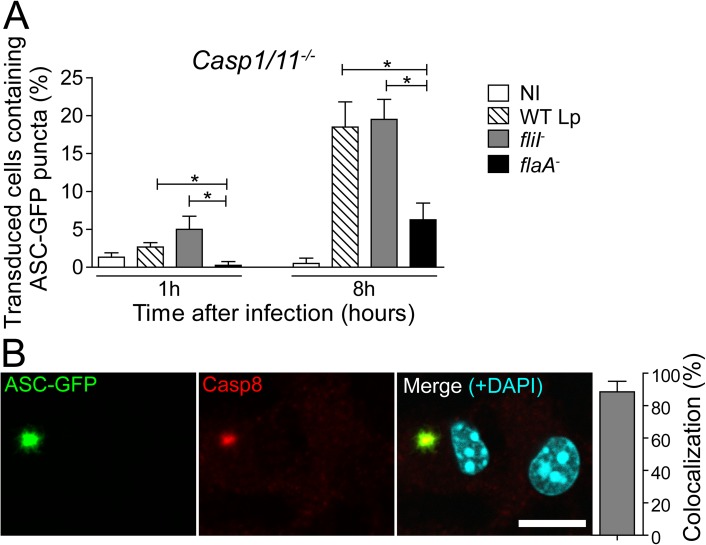
The transduction of BMDMs with a retrovirus encoding NLRC4 fused to GFP allows the visualization of NLRC4 puncta in the cytoplasm of macrophages infected with flagellated bacteria [30]. Here, we used this retroviral system to investigate the formation of the NLRC4 inflammasome in the absence of caspase-1/11. BMDMs from Casp1/11-/- mice were transduced with NLRC4-GFP and infected with WT Lp, fliI- and flaA- at different MOIs and time points. We found that WT Lp and fliI- triggered the formation of NLRC4 puncta in the absence of caspase-1/11 (Fig 1A). The formation of NLRC4 puncta was influenced by the MOI and significantly diminished in response to flaA- bacteria (Fig 1A). Next, we evaluated the requirement of ASC for the formation of NLRC4 puncta in the absence of caspase-1/11. We constructed a mouse that was deficient in ASC and caspase-1/11 and found that whereas the formation of NLRC4 puncta occurred in the absence of caspase-1/11, ASC was essential for formation of the NLRC4 puncta (Fig 1B). These data are in agreement with previous findings indicating that ASC is critical for the nucleation of several inflammasomes, including AIM2, NLRP3 and NLRC4 [30,32,33,41–46]. Our results confirm that ASC is essential to NLRC4 puncta formation formed in the absence of caspase-1/11. Next, we used this NLRC4-GFP system to identify additional components of the NLRC4 inflammasome that operates in the absence of caspase-1/11. Non-inflammatory caspases have been previously shown to participate in the assembly of inflammasomes and to interact with ASC, including caspase-3, caspase-7 and caspase-8 [32–34,37,44,46–51]. Thus, we transduced Casp1/11-/- macrophages with a retrovirus encoding NLRC4-GFP and evaluated the colocalization of NLRC4 with these caspases. In this experiment, we used the pan-caspase inhibitor Z-VAD to block caspase activation and to visualize puncta formation. We did not detect significant numbers of NLRC4 or ASC puncta containing caspase-3 and caspase-7 (S2 Fig). In contrast, caspase-8 and ASC was present in more than 90% of the NLRC4 puncta (Fig 1C and S2 Fig). These data are in agreement with our findings indicating that ASC is required for NLRC4 puncta formation, accordingly, endogenous ASC colocalizes with NLRC4 and caspase-8 in the same puncta (Fig 1D). To evaluate the participation of caspase-8 in the NLRC4 inflammasome, we transduced BMDMs from Casp1/11-/- mice with a retrovirus encoding ASC fused to GFP (ASC-GFP) and analyzed ASC puncta colocalization with caspase-8. We found that ASC puncta formed readily after the infection and that this process occurred in response to WT Lp and fliI- but not flaA- (Fig 2A). After 8 hours of infection, the formation of ASC puncta was partially dependent on flagellin (Fig 2A). We stained caspase-8 in macrophages transduced with retrovirus encoding ASC-GFP and found that caspase-8 colocalized with ASC puncta in response to infection with flagellated bacteria (Fig 2B). Collectively, these results indicate that flagellin triggers the assembly of an inflammasome composed of NLRC4 and ASC, which colocalizes with caspase-8.
Fig 1. Legionella triggers the formation of NLRC4/caspase-8 puncta in a process that is dependent on ASC and flagellin and independent of caspase-1/11.
Bone marrow-derived macrophages (BMDMs) obtained from Casp1/11-/- and Asc/Casp1/11-/- mice were transduced with retrovirus encoding NLRC4-GFP. Cells were infected with wild-type L. pneumophila (WT Lp), motility-deficient mutants expressing flagellin (fliI-) or with flagellin-deficient mutants (flaA-) at a MOI of 1, 3 or 10. (A) After 2, 4 and 8 hours of infection, the cells were fixed, and the percentage of transduced cells containing NLRC4-GFP puncta were determined using an epifluorescence microscope. *, P<0.05 compared with BMDMs infected with flaA-, Student’s t test. (B-D) Transduced cells were infected with fliI- (MOI 10) and fixed after 8 hours of infection. (B) The percentages of transduced Casp1/11-/- and Asc/Casp1/11-/- cells containing NLRC4-GFP puncta were determined. (C) The percentages of NLRC4-GFP puncta that colocalized with ASC and caspase-8 were determined. (D) Representative images of a transduced BMDM infected with fliI- at a MOI of 10. The cultures were stained with anti-caspase-8 (red) and anti-ASC (purple). The cell nuclei were stained with DAPI (cyan); NLRC4-GFP is shown in green. The images show the colocalization of NLRC4-GFP, ASC and caspase-8 in Casp1/11-/- BMDMs. The images were acquired by multiphoton microscopy using a 63x oil immersion objective and analyzed using ImageJ Software. Scale bar, 10μm. Data show the average ± SD of triplicate wells. NI, uninfected. Data are presented for one representative experiment of five (A) and two (B-D) experiments with similar results.
Fig 2. ASC puncta are induced in response to flagellated bacteria and colocalize with caspase-8.
Bone marrow-derived macrophages (BMDMs) from Casp1/11-/- mice were transduced with retrovirus encoding ASC-GFP. (A) Cells were infected with wild-type L. pneumophila (WT Lp), motility-deficient mutants expressing flagellin (fliI-) or with flagellin-deficient mutants (flaA-) at a MOI of 10. After 1 or 8 hours of infection, the cells were fixed, and the number of transduced cells containing ASC-GFP puncta was determined using an epifluorescence microscope. (B) Representative images of a transduced BMDM infected with fliI- at a MOI of 10. The cultures were stained with anti-caspase-8 (red), cell nuclei were stained with DAPI (cyan) and ASC-GFP is shown in green. The images show the colocalization between ASC-GFP and caspase-8 in Casp1/11-/- BMDMs infected for 8 hours. The images were acquired by multiphoton microscopy with a 63x oil immersion objective and analyzed using ImageJ Software. Scale bar, 10μm. Data show the average ± SD of triplicate wells. *, P<0.05, Student’s t test. NI, uninfected. Data are presented for one representative experiment of three (A) and two (B) experiments with similar results.
The double-stranded DNA sensor AIM2 is known to recruit ASC to trigger puncta formation in response to infection, leading to caspase-1 activation and IL-1β and IL-18 release [31,52–55]. The role of AIM2 inflammasome in the recognition of L. pneumophila has been demonstrated using sdhA- deficient bacteria. In the absence of SdhA, bacteria do not maintain vacuole integrity and localize in the macrophage cytoplasm, triggering activation of the AIM2 inflammasome [56,57]. In addition, the AIM2 inflammasome has been shown to trigger caspase-8 activation independently of caspase-1 [33,34,58]. Thus, we investigated whether AIM2 is present in the NLRC4/ASC/caspase-8 inflammasome that is formed in response to flagellin-positive L. pneumophila. We stained AIM2 in macrophages transduced with retrovirus encoding NLRC4-GFP and found no AIM2 in the NLRC4 puncta (S3A Fig). Moreover, we generated Aim2/Casp1/11-/- mice and found that AIM2 was dispensable for the formation of NLRC4 puncta in response to flagellin-positive bacteria (S3B Fig).
The NLRC4/ASC/caspase-8 inflammasome accounts for flagellin-dependent restriction of Legionella replication in macrophages in the absence of caspase-1/11
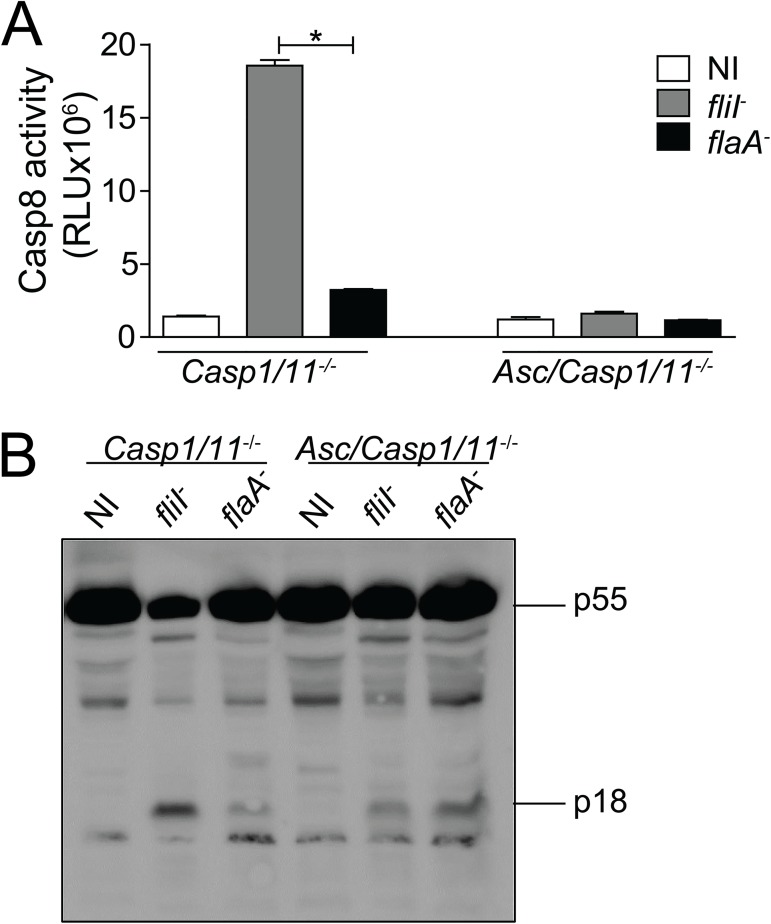
Our data are consistent with the hypothesis that caspase-8 is a part of the inflammasome composed of NLRC4 and ASC. Thus, we investigated whether caspase-8 is activated during infection. We found that caspase-8 was strongly activated in Casp1/11-/- BMDMs in response to fliI- but not flaA- bacteria (Fig 3A). In agreement with the requirement of ASC for the assembly of the NLRC4/ASC/caspase-8 inflammasome, we found that caspase-8 activation was abolished in Asc/Casp1/11-/- cells (Fig 3A). Caspase-8 activation occurred normally in Aim2/Casp1/11-/- cells, indicating that AIM2 was not involved in the activation of caspase-8 through the flagellin/NLRC4/ASC inflammasome (S4 Fig). We also evaluated caspase-8 activation by western blot analysis by measuring the cleavage of p55 and the production of p18 isoforms. We found that flagellated bacteria triggered caspase-8 activation in Casp1/11-/- but not in Asc/Casp1/11-/- cells. This phenomenon was evident by the reduction in p55 and increased production of p18 in Casp1/11-/- BMDMs infected with fliI- but not flaA- bacteria (Fig 3B).
Fig 3. Caspase-8 is activated in response to flagellated bacteria in a process that is ASC-dependent and caspase-1/11-independent.
Bone marrow-derived macrophages (BMDMs) from Casp1/11-/- and Asc/Casp1/11-/- mice were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) or with flagellin-deficient bacteria (flaA-) at a MOI of 10 for 8 hours. The activity of caspase-8 was measured using the Caspase-8 Glo Assay (A) or by western blot analysis (B). The pro-caspase-8 p55 and the processed form of caspase-8 p18 are indicated. Data show the average ± SD of triplicate wells. *, P<0.05, Student’s t test. RLU, relative luminescence units; NI, uninfected. Data are presented for one representative experiment of three (A) and two (B) experiments with similar results.
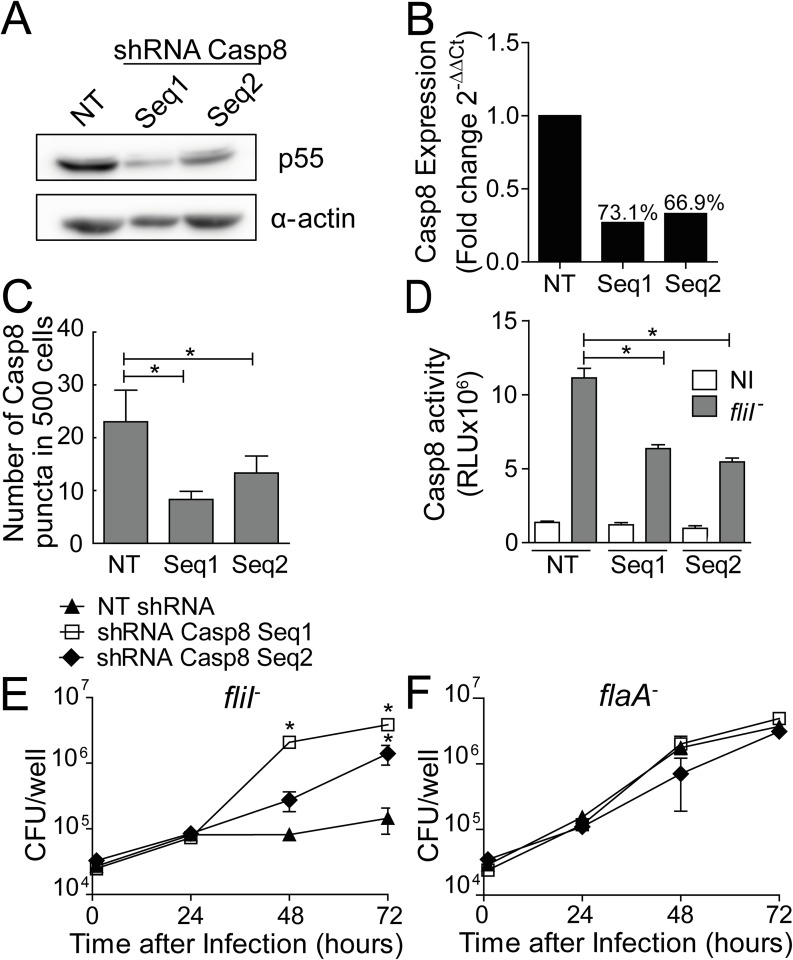
Next, we evaluated the participation of caspase-8 in caspase-1/11-independent restriction of L. pneumophila replication in macrophages, a process that was dependent on flagellin and NLRC4. Endogenous caspase-8 was silenced in Casp1/11-/- BMDMs using two independent retrovirus encoding shRNA to target caspase-8. A non-target sequence was used as a control (NT). By western blotting, we detected reduced caspase-8 expression in Casp1/11-/- transduced cells. The shRNA Casp8 Seq1 was more efficient than Seq2 for silencing caspase-8 as determined by western blot (Fig 4A and S5 Fig) and RT-PCR (Fig 4B). Importantly, complete silencing of caspase-8 cannot be achieved because caspase-8 expression is required for macrophage survival [59,60]. Nonetheless, using the described silencing conditions, we did not detect signs of cell death or LDH in the supernatant of the transduced macrophages. To evaluate the efficiency of caspase-8 silencing, we quantified caspase-8-containing puncta formation and caspase-8 activation in macrophages infected with flagellated L. pneumophila. We found that the frequency of puncta containing caspase-8 and caspase-8 activation was reduced in caspase-8-silenced cells (Fig 4C and 4D). Next, we evaluated the effect of caspase-8 for the restriction of L. pneumophila replication in Casp1/11-/- BMDMs. We found that silencing caspase-8 culminated in increased replication of fliI- but not flaA- bacteria (Fig 4E and 4F). These data indicated that caspase-8 contributed to the restriction of bacterial replication in a process that was dependent on flagellin, supporting the hypothesis that caspase-8 functionally participates in responses that are NLRC4/ASC-dependent and caspase-1/11-independent.
Fig 4. Caspase-8 is important for NLRC4-mediated restriction of L. pneumophila replication independently of caspase-1/11.
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequences to target caspase-8 (Seq1, Seq2) and a non-target shRNA sequence (NT). (A) The silencing was confirmed by western blot analysis and Real Time qPCR. (A) Cell lysates were separated by SDS-PAGE, blotted and probed with anti-caspase-8 (pro-caspase-8 p55) and anti-α-actin. (B) Quantification of the casp8 gene expression by Real Time qPCR. Actin beta gene was used as a control for normalization of expression levels. The number above the bars indicates the percentage of silencing compared to the NT sequence. (C-D) The transduced Casp1/11-/- BMDMs were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 10. After 8 hours the cells were fixed, the number of caspase-8 puncta was quantified using an epifluorescence microscope (C) and the activity of caspase-8 was measured using the Caspase-8 Glo Assay (D). (E-F) The transduced Casp1/11-/- BMDMs were infected with fliI- (E) and flaA- (F) at a MOI of 10 to evaluate bacterial replication. The cells were incubated for 24, 48 and 72 hours for CFU determination. Data show the average ± SD of triplicate wells. *P<0.05, compared with NT. (C-D) Student’s t test, (E-F) ANOVA. RLU, relative luminescence units; NI, uninfected. Data are presented for one representative experiment of three experiments with similar results.
ASC is required for NLRC4/caspase-8-mediated restriction of Legionella replication in the absence of caspase-1/11
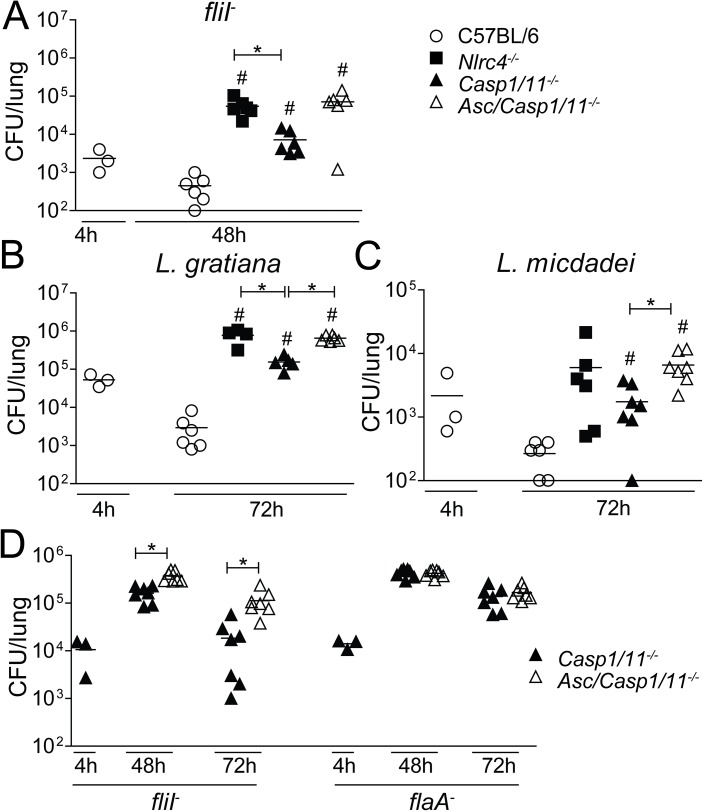
Our data indicate that caspase-8 is part of the NLRC4/ASC inflammasome and that ASC is essential for the assembly of this inflammasome. Thus, we reasoned that in the absence of ASC, the NLRC4/ASC/caspase-8 inflammasome would not be functional. If this hypothesis is correct, Asc/Casp1/11-/- macrophages should be more permissive than Casp1/11-/- and as permissive as Nlrc4-/-. We infected C57BL/6, Casp1/11-/-, Asc/Casp1/11-/- and Nlrc4-/- BMDMs with fliI- and flaA-, and evaluated bacterial replication after 24, 48 and 72 hours. Using flagellin-positive bacteria, we confirmed that C57BL/6 BMDMs were restrictive to bacterial growth, Nlrc4-/- were permissive and Casp1/11-/- were partially restrictive (Fig 5A). Importantly, Asc/Casp1/11-/- cells were highly permissive and phenocopied the Nlrc4-/- cells (Fig 5A). As predicted, flagellin mutants bypassed the NLRC4 and replicated in all macrophages evaluated (Fig 5B). These data further confirmed that Casp1/11-/- cells were more restrictive than Nlrc4-/- macrophages, possibly due to the presence of the NLRC4/ASC/caspase-8 inflammasome. We also used non-pneumophila species to compare bacterial replication in Nlrc4-/- and Asc/Casp1/11-/- macrophages. Infections performed with L. gratiana and L. micdadei indicated that Asc/Casp1/11-/- macrophages were as susceptible as Nlrc4-/-, whereas Casp1/11-/- cells were partially restrictive (Fig 5C and 5D). Similar experiments performed with Aim2/Casp1/11-/- macrophages did not support the role of AIM2 in the NLRC4/ASC-dependent growth restriction that occurred in the absence of caspase-1/11 (S6 Fig). These data indicate that flagellated species of Legionellae trigger NLRC4 responses that are independent of caspase-1/11 but dependent on ASC. To further confirm the participation of caspase-8 in this NLRC4/ASC inflammasome, we silenced caspase-8 in Casp1/11-/- and Asc/Casp1/11-/- macrophages. We confirmed the silencing by western blot analysis (Fig 5E and S7 Fig) and found that the reduction in caspase-8 expression impaired the restriction of bacterial replication in Casp1/11-/- infected with fliI- (Fig 5F). Inhibition of caspase-8 expression affected neither the replication of fliI- in Asc/Casp1/11-/- cells (Fig 5G) nor the replication of flaA- in Casp1/11-/- and in Asc/Casp1/11-/- cells (Fig 5H and 5I). Collectively, these data are consistent with the hypothesis that flagellin activates a response that is dependent on NLRC4, ASC and caspase-8 and occurs in the absence of caspase-1/11.
Fig 5. ASC is important for NLRC4/caspase-8-mediated restriction of L. pneumophila replication independently of caspase-1/11.
(A-D) Bone marrow-derived macrophages (BMDMs) from C57BL/6 (open circles), Nlrc4-/- (closed squares), Casp1/11-/- (closed triangles) and Asc/Casp1/11-/- (open triangles) mice were infected with motility-deficient mutants expressing flagellin (fliI-, A), with flagellin-deficient mutants (flaA-, B), L. gratiana (C) or with L. micdadei (D) at a MOI of 10. The cells were incubated for 24, 48 and 72 hours for CFU determination. Data show the average ± SD of triplicate wells. *, P<0.05 compared with Casp-1/11-/- BMDMs. #, P<0.05 compared with C57BL/6 BMDMs, ANOVA. (E-I) BMDMs from Casp1/11-/- and Asc/Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequences to target caspase-8 (Seq1, Seq2) and a non-target shRNA sequence (NT). (E) Caspase-8 silencing was confirmed by western blot analysis. Cell lysates were separated by SDS-PAGE, blotted and probed with anti-caspase-8 (pro-caspase-8 p55) and anti-α-actin. (F-I) Transduced Casp1/11-/- (F, H) and Asc/Casp1/11-/- (G, I) BMDMs were infected with fliI- (F, G) or flaA- (H, I) at a MOI of 10 and incubated for 24, 48 and 72 hours for CFU determination. Data show the average ± SD of triplicate wells. *, P<0.05 compared with NT shRNA, ANOVA. NT, non-target shRNA. Data are presented for one representative experiment of four (A), two (B-D) and one (F-I) experiments performed with similar results.
Casp8-/- mice are embryonic lethal [59], and we were not able to generate Casp8/1/11-/- mice. Since the deletion of ASC impairs the assembly of the NLRC4/ASC/caspase-8 inflammasome and caspase-8 activation, we used Asc/Casp1/11-/- mice to assess the role of the NLRC4/ASC/caspase-8 inflammasome in the restriction of Legionella replication in vivo. Using flagellin-positive bacteria such as L. pneumophila, L. gratiana and L. micdadei, we demonstrated that Asc/Casp1/11-/- mice were highly permissive to bacterial replication and phenocopied infection of Nlrc4-/- mice (Fig 6A–6C). Casp1/11-/- mice were more permissive than C57BL/6, but they were less permissive than Nlrc4-/- mice (Fig 6A–6C). Experiments performed with fliI- and flaA- indicated that Asc/Casp1/11-/- were more permissive than Casp1/11-/- when infected with fliI- but not flaA- (Fig 6D). To determine whether AIM2 accounted for the restriction of bacterial replication in the absence of caspase-1/11, we compared infection of Aim2/Casp1/11-/- with Casp1/11-/-. We found that Aim2/Casp1/11-/- and Casp1/11-/- supported similar replication levels of fliI- L. pneumophila in the lungs. In contrast, Asc/Casp1/11-/- and Nlrc4-/- mice were significantly more permissive to bacterial replication (S8 Fig). Collectively, these data indicate that AIM2 is dispensable for the functions of the NLRC4/ASC/caspase-8 inflammasome. This molecular platform is assembled in response to flagellin-positive bacteria and operates to restrict bacterial replication in vitro and in vivo in a process that is independent of both caspase-1 and caspase-11.
Fig 6. ASC is essential for NLRC4-mediated restriction of L. pneumophila replication independently of caspase-1/11 in vivo.
C57BL/6 (open circles), Nlrc4-/- (closed squares), Casp1/11-/- (closed triangles) and Asc/Casp1/11-/- (open triangles) mice were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-, A, D), with flagellin-deficient L. pneumophila (flaA-, D), with L. gratiana (B) or with L. micdadei (C) at a dose of 105 bacteria/mice. The mice were euthanized at 4, 48 or 72 hours after infection. Dilutions of the lung homogenates were added to charcoal-yeast extract agar plates for colony-forming unit determination. Each dot represents a single animal, and the horizontal lines represent the averages. *, P<0.05. #, indicates P<0.05 compared with C57BL/6, Mann Whitney test. Data are presented for one representative experiment of five (A), one (B-C) and two (D) experiments performed with similar results.
Activation of the NLRC4/ASC/caspase-8 inflammasome triggers pore formation and cell death
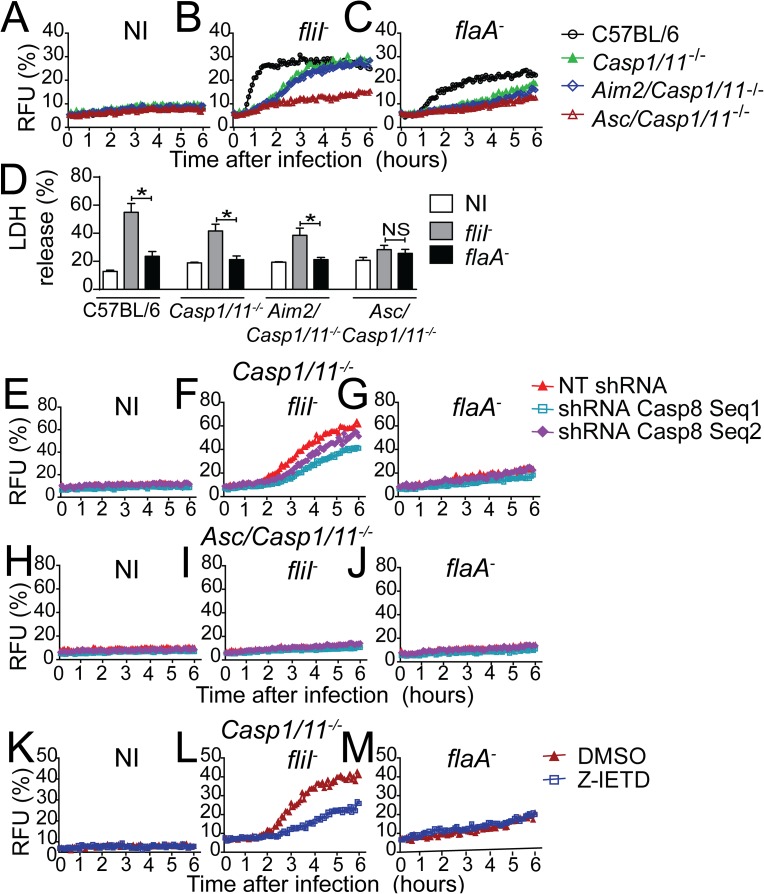
Activation of caspase-1 inflammasomes induces pyroptosis and contributes to the restriction of infection by flagellated bacteria such as L. pneumophila, Salmonella typhimurium and Burkholderia thailandensis [23]. Accordingly, we have previously demonstrated that L. pneumophila trigger pyroptosis in a process mediated by caspase-1 and caspase-11, which are activated in response to flagellin and LPS, respectively [20,24,35,61]. Thus, we investigated whether activation of the NLRC4/ASC/caspase-8 inflammasome could trigger cell death in Casp1/11-/- macrophages. We assessed membrane permeabilization fluorometrically in real time via the uptake of propidium iodide. Macrophages were infected with fliI- or flaA-, and pore formation was monitored in real time for 6 hours. C57BL/6 macrophages triggered robust pore formation in response to infection with fliI- and reduced pore formation in response to flaA- (Fig 7A–7C). The pore formation observed in C57BL/6 macrophages infected with flaA- mutants was dependent on caspase-11 [61] and will not be addressed herein. Importantly, despite the absence of both caspase-1 and caspase-11, we detected significant pore formation in Casp1/11-/- cells infected with fliI- (Fig 7B). This response was not detected in Casp1/11-/- cells infected with flaA- or in Asc/Casp1/11-/- macrophages infected with fliI- or flaA- (Fig 7B and 7C). These data support the hypothesis that NLRC4/ASC/caspase-8 induces pore formation. Experiments performed using Aim2/Casp1/11-/- macrophages corroborate our previous findings, indicating that AIM2 is not required for the activities of the NLRC4/ASC/caspase-8 inflammasome (Fig 7B and 7C). Pore formation induced in response to caspase-1 and caspase-11 activation culminates with the induction of macrophage lysis, a process that can be assessed by the presence of LDH in tissue culture supernatants [24,61,62]. Thus, we measured LDH release in the supernatants of macrophages infected with fliI- and flaA- for 8 hours. By comparing Casp1/11-/- and Asc/Casp1/11-/- cells, we found that macrophage lysis occurred despite the absence of caspase-1/11 (Fig 7D). Cell death was flagellin-dependent because infections with fliI- but not flaA- induced LDH release (Fig 7D). The participation of caspase-8 in pore formation and cell death induced in caspase-1/11-deficient macrophages was evident using both MOI 5 (Fig 7) and MOI 10 (S9 Fig). Importantly, cell death was not observed in Asc/Casp1/11-/-, a feature that corroborates the pore formation studies and indicates that the NLRC4/ASC/caspase-8 inflammasome triggers pore formation and lysis of infected cells. We also assessed whether the NLRC4/ASC/caspase-8 inflammasome was important for the activation of inflammatory cytokines. We found that whereas C57BL/6 macrophages readily triggered the production of IL-1β after 24 hours of infection with flagellated bacteria, the Casp1/11-/- or Asc/Casp1/11-/—deficient cells do not trigger a IL-1β production (S10A Fig). The production of IL-12p40 by these cells confirmed that all macrophages were primed and could respond to L. pneumophila infection (S10B Fig). To evaluate the participation of caspase-8 in cell death induced by the NLRC4/ASC/caspase-8 inflammasome, we silenced endogenous caspase-8 using shRNA. Macrophages that were transduced with retrovirus encoding shRNA did not exhibit pore formation before infection, indicating that transduction itself did not trigger cell death (Fig 7E and 7H). In contrast, pore formation was evident in Casp1/11-/- but not in Asc/Casp1/11-/- macrophages in response to fliI- infection (Fig 7F and 7I). Pore formation induced in Casp1/11-/- was diminished in caspase-8-silenced cells (Fig 7F). In support of the role of flagellin for triggering these responses, we did not detect pore formation in cells infected with flaA- (Fig 7G and 7J). To further evaluate the participation of caspase-8 in pore formation induced by flagellin, we performed pore formation assays using Z-IETD, a cell permeable peptide that binds irreversibly to the catalytic site of caspase-8 [63–65]. We found that treatment of Casp1/11-/- macrophages with DMSO or Z-IETD did not cause pore formation in uninfected cells (Fig 7K). However, Z-IETD treatment reduced the pore formation induced by fliI- (Fig 7L) but not by flaA- (Fig 7M). Collectively, these data indicate that flagellin-positive bacteria trigger pore formation and cell death-independent of caspase-1/11 via a process that requires ASC and caspase-8.
Fig 7. The NLRC4/ASC/caspase-8 inflammasome is important for pore formation and cell death independently of caspase-1/11.
(A-D; K-M) Bone marrow-derived macrophages (BMDMs) were generated from C57BL/6, Casp1/11-/-, Aim2/Casp1/11-/- and Asc/Casp1/11-/- mice and infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) or with flagellin-deficient bacteria (flaA-) at a MOI of 5. (E-J) BMDMs were transduced with retrovirus encoding shRNA sequences to target caspase-8 (Seq1, Seq2) and a non-target shRNA sequence (NT) and infected with fliI- or flaA- at a MOI of 5. (A-C; E-J) Pore formation was assessed fluorometrically in real time by the uptake of propidium iodide. RFU (%) represents the percentage of RFU estimated with cells lysed with Triton X-100. (D) LDH release was measured using the CytoTox 96 LDH-release kit. The LDH release (%) represents the percentage of LDH release estimated with cells lysed with Triton X-100. (K-M) BMDMs were treated with 50 μM of Z-IETD or DMSO for 1 hour and infected with (L) fliI- or (M) flaA- at a MOI of 5. Pore formation was assessed fluorometrically in real time by the uptake of propidium iodide. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. NS, not significant; RFUs, relative fluorescence units; NI, uninfected. Data are presented for one representative experiment of five (A-C), three (E-J) and two (D and K-M) experiments with similar results.
Naip5 is required for NLRC4/ASC/caspase-8 inflammasome activation in response to flagellated Legionella
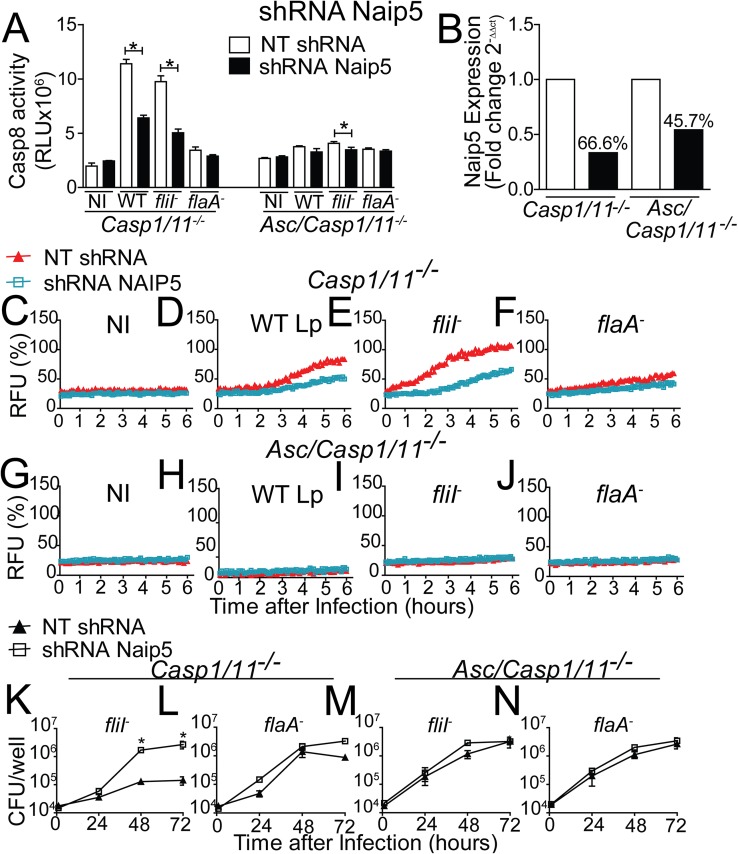
Our data reveal that the NLRC4/ASC/caspase-8 inflammasome is activated in Casp1/11-/- macrophages in response to infection with flagellated Legionella. To evaluate if Naip5 is required for activation of this inflammasome, we used shRNA to silence endogenous Naip5. In Naip5 silenced Casp1/11-/- macrophages, we detected a reduced activation of caspase-8 in response to WT Lp and fliI- bacteria (Fig 8A). Naip5 silencing was confirmed by RT-PCR (Fig 8B). We also tested if Naip5 is important for pore formation induced via caspase-8 in Casp1/11-/- macrophages. By evaluating pore formation, we found that Naip5 is important for efficient pore formation in response to WT Lp and fliI- bacteria (Fig 8D–8E). As previously reported, no pore formation was detected in response to flaA- bacteria (Fig 8F) or in Asc/Casp1/11-/- macrophages (Fig 8G–8J). Finally, we tested if Naip5 is important for restriction of L. pneumophila replication in Casp1/11-/- macrophages. We found that Naip5 is important for restriction of flagellin-positive L. pneumophila replication in Casp1/11-/- macrophages (Fig 8K). Naip5 silencing did not affect the replication of flaA- bacteria in Casp1/11-/- macrophages (Fig 8L). As predicted, Asc/Casp1/11-/- macrophages were permissive to replication of both flaA- and fliI- bacteria and Naip5 did not influenced this process (Fig 8M and 8N). Taken together, these data indicates that Naip5 participate of the NLRC4/ASC inflammasome that trigger caspase-8 activation in the absence of caspase-1/11.
Fig 8. Naip5 is required for the functions of the NLRC4/ASC/Caspase-8 inflammasome independently of caspase-1/11.
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- and Asc/Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequence to target Naip5 and a non-target shRNA sequence (NT). Transduced BMDMs were infected with wild-type L. pneumophila (WT Lp), motility-deficient mutants expressing flagellin (fliI-) or with flagellin-deficient mutants (flaA-) at a MOI of 10. (A) 8 hours after infection the activity of caspase-8 was measured using the Caspase-8 Glo Assay. (B) Quantification of the Naip5 gene expression by Real Time qPCR. Gapdh gene was used as a control for normalization of expression levels. The number above the bars indicates the percentage of silencing compared to the NT shRNA (open bars). (C-J) Pore formation was assessed fluorometrically in real time by the uptake of propidium iodide. The RFU (%) represents the percentage of RFU estimated with cells lysed with Triton X-100. (K-N) The cells were infected with fliI- or flaA- for 24, 48 and 72 hours for CFU determination. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test (A) and ANOVA (K-N). NS, not significant; RFU, relative fluorescence units; NI, uninfected. Data are presented for one representative experiment of two (A-J) and three (K-N) experiments with similar results.
Caspase-8 is recruited to the NLRC4/ASC/caspase-1 inflammasome but it is only activated when caspase-1 or gasdermin-D is suppressed
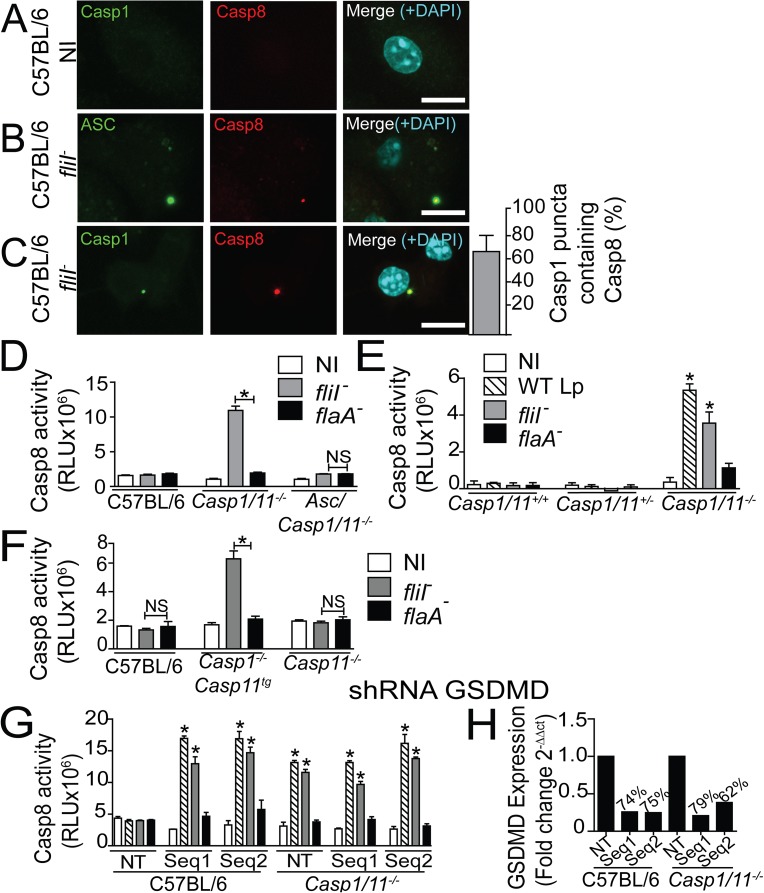
In the experiments shown thus far, we used Casp1/11-/- macrophage as a tool to assess the caspase-8 effects without the interference of caspase-1. However, because caspase-1 is present in natural conditions, we tested if caspase-8 participates in the NLRC4/ASC inflammasome in the presence of caspase-1. First, we infected C57BL/6 macrophages with flagellin-positive L. pneumophila to assess if endogenous caspase-8 colocalizes with the Naip5/NLRC4/ASC inflammasome. In uninfected conditions, we detected no significant puncta formation (Fig 9A). However, in response to fliI- bacteria, caspase-8 colocalizes with ASC (Fig 9B) and caspase-1 (Fig 9C). We determined that caspase-8 is present in more than 60% puncta containing caspase-1 (Fig 9C). These data indicates that regardless to the presence of caspase-1, the caspase-8 is recruited to the inflammasome during activation.
Fig 9. Caspase-8 colocalizes with the NLRC4/ASC/Caspase-1 inflammasome, but it is only activated if caspase-1 or gasdermin-D is inhibited.
(A-C) Bone marrow-derived macrophages (BMDMs) generated from C57BL/6 mice were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 10 for 8 h. The cultures were stained with anti-caspase-1 (green) (A, C) or anti-ASC (green) (B), anti-caspase-8 (red). Cell nuclei were stained with DAPI (cyan). Images were acquired by multiphoton microscopy with a 63x oil immersion objective and analyzed using ImageJ software. The images are the maximal projection of a z project. Scale bars, 10μm. (C) The percentage of the caspase-1 puncta containing caspase-8 was determined using an epifluorescence microscope. (D) BMDMs were generated from C57BL/6, Casp1/11-/- and Asc/Casp1/11-/- mice and infected with fliI- or flaA-. (E) BMDMs were generated from Casp1/11+/+, Casp1/11+/- and Casp1/11-/- littermate control mice and infected with WT, fliI- and flaA-. (F) BMDMs were generated from C57BL/6, Casp1-/-Casp11tg and Casp11-/- mice and infected with WT, fliI- and flaA-. (G-H) BMDMs generated from C57BL/6 and Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequences to target Gasdermin D (GSDMD) (Seq1, Seq2) and a non-target shRNA sequence (NT). (G) Transduced cells were infected with WT Lp, fliI- and flaA-. (D-G) Cells were infected with an MOI of 10 and after 8 hours the activity of caspase-8 was measured using the Caspase-8 Glo Assay. (H) GSDMD silencing was confirmed by Real Time qPCR. Gapdh gene was used as a control for normalization of expression levels. The numbers above the bars indicate the percentage of silencing compared to the NT sequence. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test in relation to flaA-. RLU, relative luminescence units; NI, non-infected. Data are presented for one representative experiment of two (A-C and E-H) and five (D) experiments with similar results.
Next, we evaluated caspase-8 activation in wild-type macrophages. We found that caspase-8 activation does not occur in C57BL/6 macrophages infected with L. pneumophila. As expected caspase-8 is readily activated in Casp1/11-/-, but not in Asc/Casp1/11-/- macrophages infected with fliI- bacteria (Fig 9D). To ensure similar genetic background, we intercrossed a F1 progeny of Casp1/11-/- x C57BL/6 to obtain F2 littermate controls. Infections in macrophages from littermate control mice indicated that caspase-8 activation occur in Casp1/11-/-, but not in Casp1/11+/- and Casp1/11+/+ macrophages (Fig 9E). To further test whether the deficiency in caspase-1 or caspase-11 enable caspase-8 activation, we performed experiments using mice single deficient in caspase-1 or caspase-11 as previously described [35,66]. We found that caspase-11 deficiency alone is not sufficient to enable caspase-8 activation in response to L. pneumophila infection (Fig 9F). In contrast, caspase-1-deficient cells expressing caspase-11 as a transgene (Casp1-/-Casp11tg) effectively trigger caspase-8 activation in response to fliI- L. pneumophila (Fig 9F). These data indicate that caspase-1 but not caspase-11 is required to prevent caspase-8 activation.
Caspase-1 activation via the NLRC4 inflammasome is known to trigger activation of gasdermin-D (GSDMD) to induce cell death [67,68]. Thus, we tested if inhibition of GSDMD is sufficient to enable caspase-8 activation in the presence of caspase-1. To achieve this, we inhibited endogenous GSDMD using shRNA and found that despite the presence of caspase-1, caspase-8 is robustly activated when GSDMD is inhibited (Fig 9G). RT-PCR was used to confirm the silencing of the two different sequences of shRNA used (Fig 9H). To further confirm the GSDMD silencing, we performed pore formation assay in C57BL/6 macrophages infected with L. pneumophila. We found that GSDMD silencing inhibited the caspase-1-mediated pore formation induced by WT Lp and fliI- L. pneumophila (S11A–S11D Fig). However, GSDMD did not participate of pore formation induced via caspase-8 that occurs in Casp1/11-/- macrophages (S11E–S11H Fig). Collectively, these data indicates that despite the presence of caspase-1, caspase-8 activation occur in the Naip5/NLRC4/ASC inflammasome when GSDMD is inhibited.
Discussion
The recognition of Legionella flagellin by the Naip5/NLRC4 inflammasome in macrophages is a major mechanism for the restriction of bacterial replication in mouse cells [17–20,22,69,70]. It is well accepted in the field that not all NLRC4 functions require caspase-1 [29,38,71,72]. This conclusion is supported by the observation that Nlrc4-/- mice (and their macrophages) are significantly more permissive to L. pneumophila replication than Casp1/11-/- mice [29,38]. Here, we unraveled this caspase-1/11-independent pathway and characterized an inflammasome composed of Naip5, NLRC4, ASC and caspase-8, which operates in the absence of caspase-1 and caspase-11. This inflammasome effectively participates in the mechanisms involved in the restriction of bacterial replication in macrophages and in vivo. Previous biochemistry studies using yeast two-hybrid screening showed that NLRC4 is ubiquitinated by Sug1, a process that facilitates the activation of caspase-8 [51]. Thus, it is possible that Sug1 is also a component of this NLRC4 inflammasome. In addition, previous studies using the Salmonella enterica serovar Typhimurium have indicated that both caspase-8 and caspase-1 are recruited to the ASC puncta in response to infection. However, caspase-8 is involved in the synthesis of pro-IL-1β and is dispensable for Salmonella-induced cell death [32]. These data contrast with published data using L. pneumophila, which indicate that in the absence of caspase-1/11, no inflammatory cytokines are produced [20,21,25,30,35,66]. Accordingly, our data indicates that this Naip5/NLRC4/ASC/Caspase-8 inflammasome is very inefficient to trigger IL-1β maturation when caspase-1 is not present.
Importantly, AIM2 is not part of this NLRC4/ASC/caspase-8 inflammasome. AIM2 is well-known to trigger caspase-8 activation via ASC [33,34,58]. Our data unequivocally demonstrate that AIM2 is neither a component of this inflammasome, nor is it required for inflammasome functions. AIM2 did not colocalize with the NLRC4/ASC/caspase-8 puncta, it was dispensable for the activation of caspase-8 in response to flagellated L. pneumophila and for the induction of cell death and restriction of L. pneumophila replication. The pyrin domain of ASC can bind to the death domain of caspase-8 [46]. Thus, it is possible that the interactions of the ASC pyrin domain with the caspase-8 dead domain are critical for the recruitment of caspase-8 to the complex. Consistent with this hypothesis, our studies unequivocally show that ASC is required for the assembly and function of this NLRC4 inflammasome. Importantly, the characterization of this NLRC4/ASC/caspase-8 inflammasome accounted to clarify a controversy in the field concerning the participation of ASC in the NLRC4 inflammasome. Studies utilizing biochemistry and cells from gene-deficient mice have demonstrated that ASC is dispensable for NLRC4 functions, including pyroptosis and the restriction of L. pneumophila replication [20,21,29]. However, ASC is essential for caspase-1 cleavage and the processing of inflammatory cytokines in response to flagellated L. pneumophila, a process that is NLRC4-dependent and NLRP3-independent [20,21,25,30]. In addition, ASC participates in the restriction of intracellular replication of L. pneumophila under certain circumstances [36,37]. Our studies provide data that help to consolidate these data in a cohesive model. NLRC4 can operate to form an inflammasome in absence of ASC that triggers pore formation and the restriction of bacterial replication [20,21,25,30]. This platform does not form puncta and is ineffective for triggering caspase-1 cleavage and processing inflammatory cytokines. When ASC is present, NLRC4 inflammasome associates with ASC and recruit caspase-1 and caspase-8 to the puncta. This inflammasome is very efficient to cleave caspase-1 and inflammatory cytokines such as IL-1β. Interestingly, our data indicate that caspase-8 is not activated when caspase-1 is present. However, when caspase-1 is missing or when Gasdermin-D is inhibited, we detected a robust caspase-8 activation. These data suggest that activation of caspase-8 in the Naip5/NLRC4/ASC inflammasome functions as a backup strategy to guarantee cell death when key components of the pyroptotic cell death are inhibited. Interestingly, our data and previously published data indicate that gasdermin-D is dispensable for caspase-8-induced cell death [73]. Therefore, when gasdermin-D is inhibited, caspase-8 engages gasdermin-D-independent cell death. It is possible that caspase-8 induces caspase-3 and caspase-7 to targed gasdermin-E (also known as DFNA5) to induce pore formation and cell death independent of gasdermin-D [74,75]. This may guarantee appropriate responses to pathogens that inhibit canonical components of pyroptotic cell death such as caspase-1 or gasdermin-D.
Materials and methods
Bacterial culture
The L. pneumophila bacteria used were JR32 and isogenic clean deletion mutants for motility (fliI-) and flagellin (flaA-) [19,38]. L. micdadei (ATCC 33218) and L. gratiana (ATCC 49413) were used to generate streptomycin-resistant strains. RpsL mutants of L. micdadei and L. gratiana were isolated by plating these strains on CYE agar containing 100 μg/ml of streptomycin. All bacteria were grown on buffered charcoal-yeast extract (CYE) agar plates [1% yeast extract, 1% MOPS, 3.3 mM L-cysteine, 0.33 mM Fe(NO3)3, 1.5% Bacto agar and 0.2% activated charcoal, pH 6.9] [76].
Macrophages
Bone Marrow derived macrophages (BMDMs) were generated from mice as previously described [77]. Briefly, bone marrow cells were harvested from femurs and differentiated with RPMI 1640 (Gibco) containing 20% fetal bovine serum (FBS—Invitrogen) and 30% L-929 cell-conditioned medium (LCCM), 2 mM L-glutamine (Sigma-Aldrich), 15 mM Hepes (Gibco) and 100 U/ml penicillin-streptomycin (Sigma-Aldrich) at 37°C with 5% CO2 [77]. BMDMs were seeded at 2 X 105 cells/well in 24-well plates and cultivated in RPMI 1640 medium (Gibco) supplemented with 10% FBS, 5% LCCM, 2 mM L-glutamine and 15 mM Hepes.
In vitro infections and CFU determination
For the in vitro infections, the cultures were infected at a multiplicity of infection of 0.015, 5 or 10 followed by centrifugation for 5 minutes at 300 X g at room temperature and incubation at 37°C in a 5% CO2 atmosphere. In the colony-forming units (CFU) experiments, cultures infected at a MOI of 10 were washed two times with PBS, and 1 ml of medium was added to each well. For CFU determination, the cultures were lysed in sterile water, and the cell lysates were combined with the cell culture supernatant from the respective wells. Lysates plus supernatants from each well were diluted in water, plated on CYE agar plates, and incubated for 4 days at 37°C for CFU determination as described previously [28,38].
Retroviral transduction and quantification of NLRC4-GFP and ASC-GFP puncta
Murine Nlrc4 or Asc were cloned into the pEGFP (N2) vector (Clontech) using XhoI and BamHI restriction sites as previously described [30]. NLRC4-GFP or ASC-GFP and GFP were cloned into the pMSCV2.2 murine-specific retroviral vector (Clontech). The pCL vector system 51 was used to package the retroviruses in transfected monolayers of Hek Peak cells (ATCC CRL-2828), which were maintained in RPMI with 10% FBS. The supernatant from the Hek Peak cells containing retrovirus was collected three days after transfection, filtered using a 0.45-μm filter and used for BMDM transduction. BMDMs were obtained from Casp1/11-/-, Asc/Casp1/11-/- and Aim2/Casp1/11-/- mice and seeded in differentiation medium. On day 3 of differentiation, the supernatants containing retroviral were added to BMDMs in 20% FBS and 25% LCCM. After differentiation, the BMDMs were seeded at 2 X 105 cells/well in 24-well plates containing 12-mm glass cover slides and cultivated in RPMI 1640 medium supplemented with 10% FBS and 5% LCCM. For the caspase colocalization experiments, the cultures were treated with 20 μm of Z-VAD for 1 hour and infected at a MOI of 1, 3 or 10. After infection, the plates were centrifuged for 5 minutes at 300 X g at room temperature and incubated at 37°C in a 5% CO2 atmosphere. At 1, 2, 4 and 8 hours after infection, the cells were fixed with 4% paraformaldehyde, permeabilized with 0.05% saponin, stained with DAPI and mounted on glass slides using Prolong Gold Antifade Reagent (Invitrogen). For the colocalization assay, the cells were stained with rat anti-caspase-8 (Enzo– 1G12; 1:50), rabbit anti-cleaved caspase-8 (Cell signaling- 8592; 1:800), rabbit anti-cleaved caspase-3 (Cell signaling- 9664; 1:400); rabbit anti-cleaved caspase-7 (Cell signaling-8438; 1:400), anti-ASC (Adipogen- AL177; 1:250), goat anti-ASC (Santa Cruz–sc33958; 1:50), rabbit anti-caspase-1 (Santa Cruz- sc514; 1:500) or anti-AIM2 (Cell signaling- 13095; 1:400), followed by Alexa 594-conjugated goat anti-rabbit secondary Ab (Invitrogen; 1:3000), Alexa 594-conjugated goat anti-rat secondary Ab (Invitrogen; 1:3000) or Alexa 647-conjugated chicken anti-rabbit secondary Ab (Invitrogen; 1:2000) and DAPI and mounted on glass slides using Prolong Gold Antifade Reagent (Invitrogen). The images were processed using LAS AF software (Leica Microsystems) and analyzed under fluorescence using a Leica DMI 4000B inverted microscope with a 100X oil objective. The number of NLRC4-GFP or ASC-GFP puncta in the transduced cells and the colocalization were quantified. Bacteria were not stained. Therefore, the whole cell population was scored. Multiphoton microscopy images were acquired using an LSM 780 Zeiss AxioObserver microscope equipped with a 63X oil immersion objective and analyzed using ImageJ software.
Retroviral silencing of caspase-8, Naip5 and GSDMD
For retroviral silencing of caspase-8, Naip5 and GSDMD (Gasdermin D), Hek Peak cells were transfected with lentiviral vectors encoding a small hairpin RNA (shRNA) targeting caspase-8 [Sigma- Seq1: TRCN0000231279 (Sequence- CCGGTCATCTCACAAGAACTATATTCTCGAGAATATAGTTCTTGTGAGATGATTTTTG); Seq2: TRCN0000231281 (Sequence–CCGGTCCTGACTGGCGTGAACTATGCTCGAGCATAGTTCACGCCAGTCAGGATTTTTG)], Naip5 [Sigma- TRCN0000114742 (Sequence—CCGGCGCTTGATTATCTTCTGGAAACTCGAGTTTCCAGAAGATAATCAAGCGTTTTTG)], GSDMD [Sigma- TRCN0000219619 (Sequence—CCGGGATTGATGAGGAGGAATTAATCTCGAGATTAATTCCTCCTCATCAATCTTTTTG); TRCN0000219620 (Sequence—CCGGCCTAAGGCTGCAGGTAGAATCCTCGAGGATTCTACCTGCAGCCTTAGGTTTTTG)] and a negative control vector that included a non-target shRNA sequence (NT). The plates were treated with polyethylenimine (Sigma-Aldrich) (Corning). Transduced cells were maintained in RPMI 1640 medium supplemented with 10% FBS at 37°C and 5% CO2. Lentiviruses expressing shRNAs were collected, filtered using a 0.45-μm filter and added to the BMDMs. After selection with puromycin, the resistant cells were seeded at 2 X 105 cells/well in 24-well plates and infected with fliI- or flaA- for CFU determination. The caspase-8 silencing efficiency was measured by immunoblotting: 1 X 106 cells were lysed in RIPA buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% Nonidet P-40, 1% deoxycholate, and 0.1% SDS) in the presence of a protease inhibitor cocktail (Roche). The lysates were suspended in 4X Laemmli buffer, boiled for 5 minutes, resolved by 15% SDS PAGE and transferred (Semidry Transfer Cell, Bio-Rad) to 0.22-μm nitrocellulose membranes (GE Healthcare). The membranes were blocked in Tris-buffered saline (TBS) with 0.01% Tween-20 and 5% non-fat dry milk for 1 hour. Rat anti-caspase-8 p55 (Enzo– 1G12; 1:500) and anti-rat peroxidase-conjugated antibody (KPL, 1:3000) were diluted in blocking buffer for the incubations (overnight for anti-caspase-8 and 1 hour for the secondary antibody). The ECL luminol reagent (GE Healthcare) was used for antibody detection. For evaluation of caspase-8 silencing by immunoblots, Image J software were used to estimate the ratio of caspase-8 p55 to α-actin.
Caspase-8 activation
To assess caspase-8 activation, BMDMs were infected with fliI- or flaA- for 8 hours, and the activity of caspase-8 was measured using the Caspase-8 Glo Assay (Promega) according to manufacturer´s recommendations. To evaluate caspase-8 activation by western blot analysis, 1 X 107 cells were infected at a MOI of 10 and lysed 8 hours after infection in RIPA buffer with protease inhibitor, as previously described. The lysates were suspended in 4X Laemmli buffer, boiled for 5 minutes, resolved by 15% SDS PAGE and transferred to 0.22-μm nitrocellulose membranes. The membranes were blocked in Tris-buffered saline (TBS) with 0.01% Tween-20 and 5% non-fat dry milk or for 1 hour. The mouse anti-caspase-8 (Enzo– 1G12) and anti-rabbit peroxidase-conjugated antibody (KPL; 1:3000) were diluted in blocking buffer for the incubations (overnight for anti-cleaved caspase-8 and 1 hour for the secondary antibody). ECL luminol reagent (GE Healthcare) was used for antibody detection.
Real Time q-PCR
Total RNA was extracted from 2 X 106 macrophages using total RNA isolation kit (illustra RNAspin, GE Healthcare, UK), according to manufacturer’s instructions. After extraction, an aliquot of 2 μl was used to determine the RNA concentration in NanoDrop (Thermo Fisher Scientific) and 1 μg of the extracted RNA was used for the cDNA conversion using the iScriptTM cDNA Synthesis kit (BIO-RAD) in a thermal cycler. The cDNA (10 ng) was used for the quantification of the Caspase 8 gene expression (TaqMan Assay: Casp 8—Mm01255716_m1) by real-time PCR using TaqMan Fast Advanced Master Mix, according to the manufacturer's instruction (Applied Biosystems). Actin beta (Actb) gene (TaqMan Assay: Actb-Mm00607939_s1) was used as a control for normalization of expression levels. The quantification of GSDMD and Naip5 were performed using 25 ng of cDNA and 10 μM of each primer, 1X SYBR Green (Applied Biosystems), and was normalized using the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The specificity of the PCR products was assessed by melting curve analysis for all samples. The following primers were used: Gapdh, FWD- AGGTCGGTGTGAACGGATTTG, REV–TGTAGACCATGTAGTTGAGGTCA, GSDMD, FWD–TCATGTGTCAACCTGTCAATCAAGGACAT, REV- CATCGACGACATCAGAGACTTTGAAGGA, NAIP5, FWD- GTTGAGATTGGAGAAGACCTCG, REV-CACACGTGAAAGCAACCATGG. The real-time quantitative reaction was performed in the Viia 7 Real-Time PCR System (Applied Biosystems). The results were analyzed using the 2-ΔΔCT method and are expressed in relation to the reference group. The percentage of silencing knockdown was estimated using the [1-(2-ΔΔCT) x 100] equation [78].
Mice and in vivo infections
Mice used in this study were breed and maintained in institutional animal facilities. Mice used were C57BL/6 (Jax 000664), Nlrc4-/- [79], Casp1/11-/- [80], Asc-/- [81], Casp11-/- and Casp1-/-Casp11tg [66] were. Double-deficient mice were generated by intercrossing a F1 progeny of the parental strains. All mice were matched by sex and age (all were at least 8 weeks old at the time of infection) and were in a C57BL/6 mouse genetic background. For the in vivo experiments, approximately 5–7 mice per group were used, as indicated in the figures. For in vivo infections, the mice were anesthetized with ketamine and xylazine (50 mg/kg and 10 mg/kg, respectively) by intraperitoneal injection followed by intranasal inoculation with 40 μl of phosphate-buffered saline (PBS) containing 1 X 105 bacteria per mouse. For CFU determination, the lungs were harvested and homogenized in 5 ml of sterile water for 30 seconds using a tissue homogenizer (Power Gen 125; Thermo Scientific). Lung homogenates were diluted in sterile water and plated on CYE agar plates for CFU determination as previously described [28,38].
Pore formation assay
Pore formation in BMDMs was quantified based on the permeability to propidium iodide (PI) in damaged cells as previously described [61]. BMDMs were seeded in a black, clear-bottom 96-well plate (1 X 105 cells/well). Before infection, the medium was replaced with 10% RPMI without phenol red, 0.038 g/ml NaHCO3, 6 μl/ml PI and anti-L. pneumophila (1:1000). Infected BMDMs were maintained at 37 °C, and PI was excited at 538 nm. The fluorescence emission was read at 617 nm every 5 minutes using a plate fluorometer (SpectraMax i3x, Molecular Devices). Total pore formation was determined by lysing cells with Triton X-100.
Lactate dehydrogenase release assay and ELISA
BMDMs were seeded in 24-well plates (5 X 105 cells/well). Infections were performed in RPMI1640 medium without phenol red, 15 mM HEPES and 2 g/l NaHCO3 supplemented with 10% FBS. After 8 hours of infection, the supernatants were collected for analysis of lactate dehydrogenase (LDH) release. Total LDH was determined by lysing the cultures with Triton X-100. LDH was quantified using the CytoTox 96 LDH-release kit (Promega). For cytokine determination, enzyme-linked immunosorbent assay (ELISA) were used. BMDMs were seeded into 24-well plates (5 X 105 cells/well) and infected with WT Lp, fliI- and flaA- (MOI 10) for 24 hours. BMDMs supernatant was assessed using ELISA kits according to manufacturer´s recommendations (BD Biosciences).
Ethics statement
The care of the mice was in compliance with the institutional guidelines on ethics in animal experiments; approved by CETEA (Comissão de Ética em Experimentação Animal da Faculdade de Medicina de Ribeirão Preto, approved protocol number 218/2014). CETEA follow the Brazilian national guidelines recommended by CONCEA (Conselho Nacional de Controle em Experimentação o Animal). For euthanasia, the mice were treated with ketamine and xylazine (50 mg/kg and 10 mg/kg, respectively) by intravenous injection.
Statistical analysis
The data were plotted and analyzed using GraphPad Prism 5.0 software. The statistical significance was calculated using the Student’s t-test or analysis of variance (ANOVA). Nonparametric test Mann–Whitney U test were used for analysis of in vivo experiments. Differences were considered statistically significant when P was <0.05, as indicated by an asterisk in the figures.
Supporting information
Bone marrow-derived macrophages (BMDMs) from C57BL/6 (open circles), Nlrc4-/- (closed squares), Casp1/11-/- (closed triangles) and Asc-/- (open inverted triangles) mice were infected with L. pneumophila for CFU determination. (A, B) Cells were infected with wild-type L. pneumophila (WT Lp). (C, D) Cells were infected with motility-deficient mutants expressing flagellin (fliI-). (E, F) Cells were infected with flagellin-deficient mutants (flaA-). BMDMs were infected with 3x103 (MOI 0.015) or 2x105 (MOI 10) bacteria per well and incubated for 24, 48, 72 and 96 hours for CFU determination. Data show the average ± SD of triplicate wells. *, P<0.05 compared with Casp1/11-/- BMDMs, ANOVA. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- mice were transduced with retrovirus encoding NLRC4-GFP (A) or ASC-GFP (B) and infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 10 for 8 h. (A-B) The percentage of colocalization of caspase-3, caspase-7 and caspase-8 with NLRC4-GFP and ASC-GFP was determined using an epifluorescence microscope. (C-E) BMDMs generated from Casp1/11-/- mice were infected with fliI- at a MOI of 10 for 8 h. The cultures were fixed and stained with anti-ASC (green), anti-caspase-3 (red) (C), anti-caspase-7 (red) (D), anti-caspase-8 (red) (E). Cell nuclei were stained with DAPI (cyan). Images were acquired by multiphoton microscopy with a 63x oil immersion objective and analyzed using ImageJ software. The images are the maximal projection of a z project. Scale bar, 10μm. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- and Aim2/Casp1/11-/- mice were transduced with retrovirus encoding NLRC4-GFP and infected with wild-type L. pneumophila (WT) or with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 10 for 8 h. (A) The cultures were stained with anti-AIM2 (red), the cell nuclei were stained with DAPI (cyan) and the NLRC4-GFP puncta is shown in green. The percentage of colocalization of AIM2 with NLRC4-GFP is shown. Images were acquired by multiphoton microscopy with a 63x oil immersion objective and analyzed using ImageJ software. Scale bar, 10μm. (B) Quantification of the number of transduced cells containing NLRC4-GFP in response to WT or fliI- infection was estimated in Casp1/11-/- and Aim2/Casp1/11-/- BMDMs. Data show the average ± SD of triplicate wells. NS, not significant, Student´s t test. NI, uninfected. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- and Aim2/Casp1/11-/- mice were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) or with flagellin-deficient bacteria (flaA-) at a MOI of 10 for 8 hours. The activity of caspase-8 was measured using the Caspase-8 Glo Assay. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. NI, uninfected. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequences to target caspase-8 (Seq1, Seq2) and a non-target shRNA sequence (NT). The silencing was confirmed by western blot analysis (Fig 4A). Cell lysates were separated by SDS-PAGE, blotted and probed with anti-caspase-8 (pro-caspase-8 p55) and anti-α-actin. Immunoblots were analyzed in Image J software and the caspase-8 p55 to α-actin ratio is shown.
(TIF)
Bone marrow-derived macrophages (BMDMs) from C57BL/6, Nlrc4-/-, Casp1/11-/- and Aim2/Casp1/11-/- mice were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 0.015. The cultures were incubated for 24, 48, 72 and 96 hours after infection for CFU determination. Data show the averages ± SD of triplicate wells. *, P<0.05, compared with Casp1/11-/- cells. Student´s t test. Data are presented for one representative experiment of three experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- and Asc/Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequences to target caspase-8 (Seq1, Seq2) and a non-target shRNA sequence (NT). The silencing was confirmed by western blot analysis (Fig 5E). Cell lysates were separated by SDS-PAGE, blotted and probed with anti-caspase-8 (pro-caspase-8 p55) and anti-α-actin. Immunoblots were analyzed in Image J software and the caspase-8 p55 to α-actin ratio is shown.
(TIF)
C57BL/6 (open circles), Nlrc4-/- (closed squares), Casp1/11-/- (closed triangles), Aim2/Casp1/11-/- (open diamond) and Asc/Casp1/11-/- (closed triangles) mice were infected intranasally with 1x105 motility-deficient L. pneumophila mutants expressing flagellin (fliI-). The mice were euthanatized at 4 and 48 hours after infection. Dilutions of the lung homogenates were added to charcoal-yeast extract agar plates for colony-forming unit determination. Each dot represents a single animal, and the horizontal lines represent averages. *, P<0.05, Student´s t test. NS, not significant.
(TIF)
Bone marrow-derived macrophages (BMDMs) were generated from C57BL/6, Casp1/11-/- and Asc/Casp1/11-/- mice and infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) or with flagellin-deficient bacteria (flaA-) at a MOI of 10. (A-C) Pore formation was assessed fluorometrically in real time by the uptake of propidium iodide. RFU (%) represents the percentage of RFU estimated with cells lysed with Triton X-100. (D) After 8 hours the LDH release was measured using the CytoTox 96 LDH-release kit. The LDH release (%) represents the percentage of LDH released compared with cells lysed with Triton X-100. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. NS, not significant; RFU, relative fluorescence units; NI, uninfected. Data are presented for one representative experiment of five (A-C) and two (D) experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) were generated from C57BL/6, Casp1/11-/- and Asc/Casp1/11-/- mice and infected with wild-type L. pneumophila (WT Lp), motility-deficient mutants expressing flagellin (fliI-) or with flagellin-deficient mutants (flaA-) at a MOI of 10. The production of IL-1β (A) and IL-12p40 (B) in the tissue culture supernatants was estimated by ELISA at 24 hours after infection. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. nd, not detected; RFU, relative fluorescence units; NI, uninfected. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
BMDMs generated from C57BL/6 (A-D) and Casp1/11-/- (E-H) mice were transduced with a retrovirus encoding shRNA sequence to target Gasdermin D (GSDMD) (Seq1) and a non-target shRNA sequence (NT). Transduced cells were infected with wild-type L. pneumophila (WT Lp) (B and F), motility-deficient mutants expressing flagellin (fliI-) (C and G) or with flagellin-deficient mutants (flaA-) (D and H) at a MOI of 10. Pore formation was assessed fluorometrically in real time by the uptake of propidium iodide. The RFU (%) represents the percentage of RFU compared with cells lysed with Triton X-100. Data show the average ± SD of triplicate wells. RFU, relative fluorescence units; NI, uninfected. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Acknowledgments
We are grateful to Maira Nakamura, Catarina Horta, Leticia Corsi and Victoria dos Santos for their technical assistance. We also thank Roberta Ribeiro Rosales for technical assistance in the institutional facility of the Multiphoton Microscopy. We thank Richard Flavell (Yale University) for providing the Casp1/11-/- mice and Vishva Dixit (Genentech) for providing the Casp11-/- and Casp1-/-Casp11tg mice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants 2012/09363-6, 2013/08216-2 and 2014/04684-4), Conselho Nacional do Desenvolvimento Cientifico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). DSZ is a research fellow from CNPq, Brazil. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fraser DW, Tsai TR, Orenstein W, Parkin WE, Beecham HJ, et al. (1977) Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med 297: 1189–1197. doi: 10.1056/NEJM197712012972201 [DOI] [PubMed] [Google Scholar]
- 2.Horwitz MA (1983) The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med 158: 2108–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, et al. (1977) Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med 297: 1197–1203. doi: 10.1056/NEJM197712012972202 [DOI] [PubMed] [Google Scholar]
- 4.Newton HJ, Ang DK, van Driel IR, Hartland EL (2010) Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev 23: 274–298. doi: 10.1128/CMR.00052-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodr A, Kay E, Gomez-Valero L, Ginevra C, Doublet P, et al. (2016) Molecular epidemiology, phylogeny and evolution of Legionella. Infect Genet Evol 43: 108–122. doi: 10.1016/j.meegid.2016.04.033 [DOI] [PubMed] [Google Scholar]
- 6.Berger KH, Isberg RR (1993) Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol 7: 7–19. [DOI] [PubMed] [Google Scholar]
- 7.Berger KH, Merriam JJ, Isberg RR (1994) Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol 14: 809–822. [DOI] [PubMed] [Google Scholar]
- 8.Isberg RR, O'Connor TJ, Heidtman M (2009) The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol 7: 13–24. doi: 10.1038/nrmicro1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy CR, Berger KH, Isberg RR (1998) Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol 28: 663–674. [DOI] [PubMed] [Google Scholar]
- 10.Swanson MS, Hammer BK (2000) Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu Rev Microbiol 54: 567–613. doi: 10.1146/annurev.micro.54.1.567 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Klein TW, Newton CA, Widen R, Friedman H (1988) Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun 56: 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckers MC, Yoshida S, Morgan K, Skamene E, Gros P (1995) Natural resistance to infection with Legionella pneumophila: chromosomal localization of the Lgn1 susceptibility gene. Mamm Genome 6: 540–545. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich WF, Damron DM, Isberg RR, Lander ES, Swanson MS (1995) Lgn1, a gene that determines susceptibility to Legionella pneumophila, maps to mouse chromosome 13. Genomics 26: 443–450. [DOI] [PubMed] [Google Scholar]
- 14.Diez E, Yaraghi Z, MacKenzie A, Gros P (2000) The neuronal apoptosis inhibitory protein (Naip) is expressed in macrophages and is modulated after phagocytosis and during intracellular infection with Legionella pneumophila. J Immunol 164: 1470–1477. [DOI] [PubMed] [Google Scholar]
- 15.Diez E, Lee SH, Gauthier S, Yaraghi Z, Tremblay M, et al. (2003) Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat Genet 33: 55–60. doi: 10.1038/ng1065 [DOI] [PubMed] [Google Scholar]
- 16.Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, et al. (2003) Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol 13: 27–36. [DOI] [PubMed] [Google Scholar]
- 17.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, et al. (2006) Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem 281: 35217–35223. doi: 10.1074/jbc.M604933200 [DOI] [PubMed] [Google Scholar]
- 18.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, et al. (2006) Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med 203: 1093–1104. doi: 10.1084/jem.20051659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE (2006) Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog 2: e18 doi: 10.1371/journal.ppat.0020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, et al. (2006) The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 7: 318–325. doi: 10.1038/ni1305 [DOI] [PubMed] [Google Scholar]
- 21.Case CL, Shin S, Roy CR (2009) Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun 77: 1981–1991. doi: 10.1128/IAI.01382-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, et al. (2008) Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9: 1171–1178. doi: 10.1038/ni.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, et al. (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11: 1136–1142. doi: 10.1038/ni.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silveira TN, Zamboni DS (2010) Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun 78: 1403–1413. doi: 10.1128/IAI.00905-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, et al. (2013) Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog 9: e1003400 doi: 10.1371/journal.ppat.1003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen I, Lopez JP, Laufer SA, Miao EA (2016) IL-1beta, IL-18, and eicosanoids promote neutrophil recruitment to pore-induced intracellular traps following pyroptosis. Eur J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaRock CN, Cookson BT (2013) Burning down the house: cellular actions during pyroptosis. PLoS Pathog 9: e1003793 doi: 10.1371/journal.ppat.1003793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascarenhas DP, Pereira MS, Manin GZ, Hori JI, Zamboni DS (2015) Interleukin 1 receptor-driven neutrophil recruitment accounts to MyD88-dependent pulmonary clearance of legionella pneumophila infection in vivo. J Infect Dis 211: 322–330. doi: 10.1093/infdis/jiu430 [DOI] [PubMed] [Google Scholar]
- 29.Pereira MS, Marques GG, Dellama JE, Zamboni DS (2011) The Nlrc4 Inflammasome Contributes to Restriction of Pulmonary Infection by Flagellated Legionella spp. that Trigger Pyroptosis. Front Microbiol 2: 33 doi: 10.3389/fmicb.2011.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Case CL, Roy CR (2011) Asc modulates the function of NLRC4 in response to infection of macrophages by Legionella pneumophila. MBio 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, et al. (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518. doi: 10.1038/nature07725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, et al. (2013) Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1beta production. J Immunol 191: 5239–5246. doi: 10.4049/jimmunol.1301581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, et al. (2012) AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 19: 1709–1721. doi: 10.1038/cdd.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, et al. (2013) AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 20: 1149–1160. doi: 10.1038/cdd.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerqueira DM, Pereira MS, Silva AL, Cunha LD, Zamboni DS (2015) Caspase-1 but Not Caspase-11 Is Required for NLRC4-Mediated Pyroptosis and Restriction of Infection by Flagellated Legionella Species in Mouse Macrophages and In Vivo. J Immunol 195: 2303–2311. doi: 10.4049/jimmunol.1501223 [DOI] [PubMed] [Google Scholar]
- 36.Abdelaziz DH, Gavrilin MA, Akhter A, Caution K, Kotrange S, et al. (2011) Apoptosis-associated speck-like protein (ASC) controls Legionella pneumophila infection in human monocytes. J Biol Chem 286: 3203–3208. doi: 10.1074/jbc.M110.197681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdelaziz DH, Gavrilin MA, Akhter A, Caution K, Kotrange S, et al. (2011) Asc-dependent and independent mechanisms contribute to restriction of legionella pneumophila infection in murine macrophages. Front Microbiol 2: 18 doi: 10.3389/fmicb.2011.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira MS, Morgantetti GF, Massis LM, Horta CV, Hori JI, et al. (2011) Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo. J Immunol 187: 6447–6455. doi: 10.4049/jimmunol.1003784 [DOI] [PubMed] [Google Scholar]
- 39.Merriam JJ, Mathur R, Maxfield-Boumil R, Isberg RR (1997) Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect Immun 65: 2497–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, et al. (2006) Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 7: 576–582. doi: 10.1038/ni1346 [DOI] [PubMed] [Google Scholar]
- 41.Stutz A, Horvath GL, Monks BG, Latz E (2013) ASC speck formation as a readout for inflammasome activation. Methods Mol Biol 1040: 91–101. doi: 10.1007/978-1-62703-523-1_8 [DOI] [PubMed] [Google Scholar]
- 42.Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, et al. (1999) ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem 274: 33835–33838. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, et al. (2007) The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14: 1590–1604. doi: 10.1038/sj.cdd.4402194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasegawa M, Imamura R, Kinoshita T, Matsumoto N, Masumoto J, et al. (2005) ASC-mediated NF-kappaB activation leading to interleukin-8 production requires caspase-8 and is inhibited by CLARP. J Biol Chem 280: 15122–15130. doi: 10.1074/jbc.M412284200 [DOI] [PubMed] [Google Scholar]
- 45.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM (2010) Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8: 471–483. doi: 10.1016/j.chom.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vajjhala PR, Lu A, Brown DL, Pang SW, Sagulenko V, et al. (2015) The Inflammasome Adaptor ASC Induces Procaspase-8 Death Effector Domain Filaments. J Biol Chem 290: 29217–29230. doi: 10.1074/jbc.M115.687731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masumoto J, Dowds TA, Schaner P, Chen FF, Ogura Y, et al. (2003) ASC is an activating adaptor for NF-kappa B and caspase-8-dependent apoptosis. Biochem Biophys Res Commun 303: 69–73. [DOI] [PubMed] [Google Scholar]
- 48.Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, et al. (2009) Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog 5: e1000361 doi: 10.1371/journal.ppat.1000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Man SM, Hopkins LJ, Nugent E, Cox S, Gluck IM, et al. (2014) Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci U S A 111: 7403–7408. doi: 10.1073/pnas.1402911111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Motani K, Kushiyama H, Imamura R, Kinoshita T, Nishiuchi T, et al. (2011) Caspase-1 protein induces apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC)-mediated necrosis independently of its catalytic activity. J Biol Chem 286: 33963–33972. doi: 10.1074/jbc.M111.286823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar Y, Radha V, Swarup G (2010) Interaction with Sug1 enables Ipaf ubiquitination leading to caspase 8 activation and cell death. Biochem J 427: 91–104. doi: 10.1042/BJ20091349 [DOI] [PubMed] [Google Scholar]
- 52.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, et al. (2008) The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452: 103–107. doi: 10.1038/nature06664 [DOI] [PubMed] [Google Scholar]
- 53.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, et al. (2009) HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323: 1057–1060. doi: 10.1126/science.1169841 [DOI] [PubMed] [Google Scholar]
- 54.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, et al. (2009) An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 10: 266–272. doi: 10.1038/ni.1702 [DOI] [PubMed] [Google Scholar]
- 55.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES (2009) AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458: 509–513. doi: 10.1038/nature07710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, et al. (2013) Caspase-11 protects against bacteria that escape the vacuole. Science 339: 975–978. doi: 10.1126/science.1230751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge J, Gong YN, Xu Y, Shao F (2012) Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking. Proc Natl Acad Sci U S A 109: 6193–6198. doi: 10.1073/pnas.1117490109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pierini R, Perret M, Djebali S, Juruj C, Michallet MC, et al. (2013) ASC controls IFN-gamma levels in an IL-18-dependent manner in caspase-1-deficient mice infected with Francisella novicida. J Immunol 191: 3847–3857. doi: 10.4049/jimmunol.1203326 [DOI] [PubMed] [Google Scholar]
- 59.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, et al. (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368–372. doi: 10.1038/nature09857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, et al. (1998) Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9: 267–276. [DOI] [PubMed] [Google Scholar]
- 61.Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, et al. (2013) Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A 110: 1851–1856. doi: 10.1073/pnas.1211521110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fink SL, Cookson BT (2006) Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8: 1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x [DOI] [PubMed] [Google Scholar]
- 63.Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, et al. (1997) Substrate specificities of caspase family proteases. J Biol Chem 272: 9677–9682. [DOI] [PubMed] [Google Scholar]
- 64.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, et al. (1997) A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272: 17907–17911. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, et al. (1998) Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 273: 32608–32613. [DOI] [PubMed] [Google Scholar]
- 66.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, et al. (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121. doi: 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- 67.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526: 666–671. doi: 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 68.Shi J, Zhao Y, Wang K, Shi X, Wang Y, et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665. doi: 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 69.Kofoed EM, Vance RE (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477: 592–595. doi: 10.1038/nature10394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, et al. (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 596–600. doi: 10.1038/nature10510 [DOI] [PubMed] [Google Scholar]
- 71.Coers J, Vance RE, Fontana MF, Dietrich WF (2007) Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol 9: 2344–2357. doi: 10.1111/j.1462-5822.2007.00963.x [DOI] [PubMed] [Google Scholar]
- 72.Fortier A, Doiron K, Saleh M, Grinstein S, Gros P (2009) Restriction of Legionella pneumophila replication in macrophages requires concerted action of the transcriptional regulators Irf1 and Irf8 and nod-like receptors Naip5 and Nlrc4. Infect Immun 77: 4794–4805. doi: 10.1128/IAI.01546-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, et al. (2017) NAIP-NLRC4 Inflammasomes Coordinate Intestinal Epithelial Cell Expulsion with Eicosanoid and IL-18 Release via Activation of Caspase-1 and -8. Immunity 46: 649–659. doi: 10.1016/j.immuni.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, et al. (2017) Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8: 14128 doi: 10.1038/ncomms14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Gao W, Shi X, Ding J, Liu W, et al. (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a Gasdermin. Nature. [DOI] [PubMed] [Google Scholar]
- 76.Feeley JC, Gibson RJ, Gorman GW, Langford NC, Rasheed JK, et al. (1979) Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol 10: 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marim FM, Silveira TN, Lima DS Jr., Zamboni DS (2010) A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One 5: e15263 doi: 10.1371/journal.pone.0015263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milstein S, Nguyen M, Meyers R, de Fougerolles A (2013) Measuring RNAi knockdown using qPCR. Methods Enzymol 533: 57–77. doi: 10.1016/B978-0-12-420067-8.00006-4 [DOI] [PubMed] [Google Scholar]
- 79.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, et al. (2006) Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med 203: 1407–1412. doi: 10.1084/jem.20060206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, et al. (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267: 2000–2003. [DOI] [PubMed] [Google Scholar]
- 81.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, et al. (2006) Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24: 317–327. doi: 10.1016/j.immuni.2006.02.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bone marrow-derived macrophages (BMDMs) from C57BL/6 (open circles), Nlrc4-/- (closed squares), Casp1/11-/- (closed triangles) and Asc-/- (open inverted triangles) mice were infected with L. pneumophila for CFU determination. (A, B) Cells were infected with wild-type L. pneumophila (WT Lp). (C, D) Cells were infected with motility-deficient mutants expressing flagellin (fliI-). (E, F) Cells were infected with flagellin-deficient mutants (flaA-). BMDMs were infected with 3x103 (MOI 0.015) or 2x105 (MOI 10) bacteria per well and incubated for 24, 48, 72 and 96 hours for CFU determination. Data show the average ± SD of triplicate wells. *, P<0.05 compared with Casp1/11-/- BMDMs, ANOVA. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- mice were transduced with retrovirus encoding NLRC4-GFP (A) or ASC-GFP (B) and infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 10 for 8 h. (A-B) The percentage of colocalization of caspase-3, caspase-7 and caspase-8 with NLRC4-GFP and ASC-GFP was determined using an epifluorescence microscope. (C-E) BMDMs generated from Casp1/11-/- mice were infected with fliI- at a MOI of 10 for 8 h. The cultures were fixed and stained with anti-ASC (green), anti-caspase-3 (red) (C), anti-caspase-7 (red) (D), anti-caspase-8 (red) (E). Cell nuclei were stained with DAPI (cyan). Images were acquired by multiphoton microscopy with a 63x oil immersion objective and analyzed using ImageJ software. The images are the maximal projection of a z project. Scale bar, 10μm. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- and Aim2/Casp1/11-/- mice were transduced with retrovirus encoding NLRC4-GFP and infected with wild-type L. pneumophila (WT) or with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 10 for 8 h. (A) The cultures were stained with anti-AIM2 (red), the cell nuclei were stained with DAPI (cyan) and the NLRC4-GFP puncta is shown in green. The percentage of colocalization of AIM2 with NLRC4-GFP is shown. Images were acquired by multiphoton microscopy with a 63x oil immersion objective and analyzed using ImageJ software. Scale bar, 10μm. (B) Quantification of the number of transduced cells containing NLRC4-GFP in response to WT or fliI- infection was estimated in Casp1/11-/- and Aim2/Casp1/11-/- BMDMs. Data show the average ± SD of triplicate wells. NS, not significant, Student´s t test. NI, uninfected. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- and Aim2/Casp1/11-/- mice were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) or with flagellin-deficient bacteria (flaA-) at a MOI of 10 for 8 hours. The activity of caspase-8 was measured using the Caspase-8 Glo Assay. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. NI, uninfected. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequences to target caspase-8 (Seq1, Seq2) and a non-target shRNA sequence (NT). The silencing was confirmed by western blot analysis (Fig 4A). Cell lysates were separated by SDS-PAGE, blotted and probed with anti-caspase-8 (pro-caspase-8 p55) and anti-α-actin. Immunoblots were analyzed in Image J software and the caspase-8 p55 to α-actin ratio is shown.
(TIF)
Bone marrow-derived macrophages (BMDMs) from C57BL/6, Nlrc4-/-, Casp1/11-/- and Aim2/Casp1/11-/- mice were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 0.015. The cultures were incubated for 24, 48, 72 and 96 hours after infection for CFU determination. Data show the averages ± SD of triplicate wells. *, P<0.05, compared with Casp1/11-/- cells. Student´s t test. Data are presented for one representative experiment of three experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- and Asc/Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequences to target caspase-8 (Seq1, Seq2) and a non-target shRNA sequence (NT). The silencing was confirmed by western blot analysis (Fig 5E). Cell lysates were separated by SDS-PAGE, blotted and probed with anti-caspase-8 (pro-caspase-8 p55) and anti-α-actin. Immunoblots were analyzed in Image J software and the caspase-8 p55 to α-actin ratio is shown.
(TIF)
C57BL/6 (open circles), Nlrc4-/- (closed squares), Casp1/11-/- (closed triangles), Aim2/Casp1/11-/- (open diamond) and Asc/Casp1/11-/- (closed triangles) mice were infected intranasally with 1x105 motility-deficient L. pneumophila mutants expressing flagellin (fliI-). The mice were euthanatized at 4 and 48 hours after infection. Dilutions of the lung homogenates were added to charcoal-yeast extract agar plates for colony-forming unit determination. Each dot represents a single animal, and the horizontal lines represent averages. *, P<0.05, Student´s t test. NS, not significant.
(TIF)
Bone marrow-derived macrophages (BMDMs) were generated from C57BL/6, Casp1/11-/- and Asc/Casp1/11-/- mice and infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) or with flagellin-deficient bacteria (flaA-) at a MOI of 10. (A-C) Pore formation was assessed fluorometrically in real time by the uptake of propidium iodide. RFU (%) represents the percentage of RFU estimated with cells lysed with Triton X-100. (D) After 8 hours the LDH release was measured using the CytoTox 96 LDH-release kit. The LDH release (%) represents the percentage of LDH released compared with cells lysed with Triton X-100. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. NS, not significant; RFU, relative fluorescence units; NI, uninfected. Data are presented for one representative experiment of five (A-C) and two (D) experiments with similar results.
(TIF)
Bone marrow-derived macrophages (BMDMs) were generated from C57BL/6, Casp1/11-/- and Asc/Casp1/11-/- mice and infected with wild-type L. pneumophila (WT Lp), motility-deficient mutants expressing flagellin (fliI-) or with flagellin-deficient mutants (flaA-) at a MOI of 10. The production of IL-1β (A) and IL-12p40 (B) in the tissue culture supernatants was estimated by ELISA at 24 hours after infection. Data show the average ± SD of triplicate wells. *, P<0.05, Student´s t test. nd, not detected; RFU, relative fluorescence units; NI, uninfected. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
BMDMs generated from C57BL/6 (A-D) and Casp1/11-/- (E-H) mice were transduced with a retrovirus encoding shRNA sequence to target Gasdermin D (GSDMD) (Seq1) and a non-target shRNA sequence (NT). Transduced cells were infected with wild-type L. pneumophila (WT Lp) (B and F), motility-deficient mutants expressing flagellin (fliI-) (C and G) or with flagellin-deficient mutants (flaA-) (D and H) at a MOI of 10. Pore formation was assessed fluorometrically in real time by the uptake of propidium iodide. The RFU (%) represents the percentage of RFU compared with cells lysed with Triton X-100. Data show the average ± SD of triplicate wells. RFU, relative fluorescence units; NI, uninfected. Data are presented for one representative experiment of two experiments with similar results.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.