Abstract
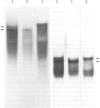
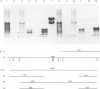
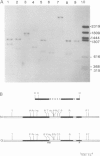
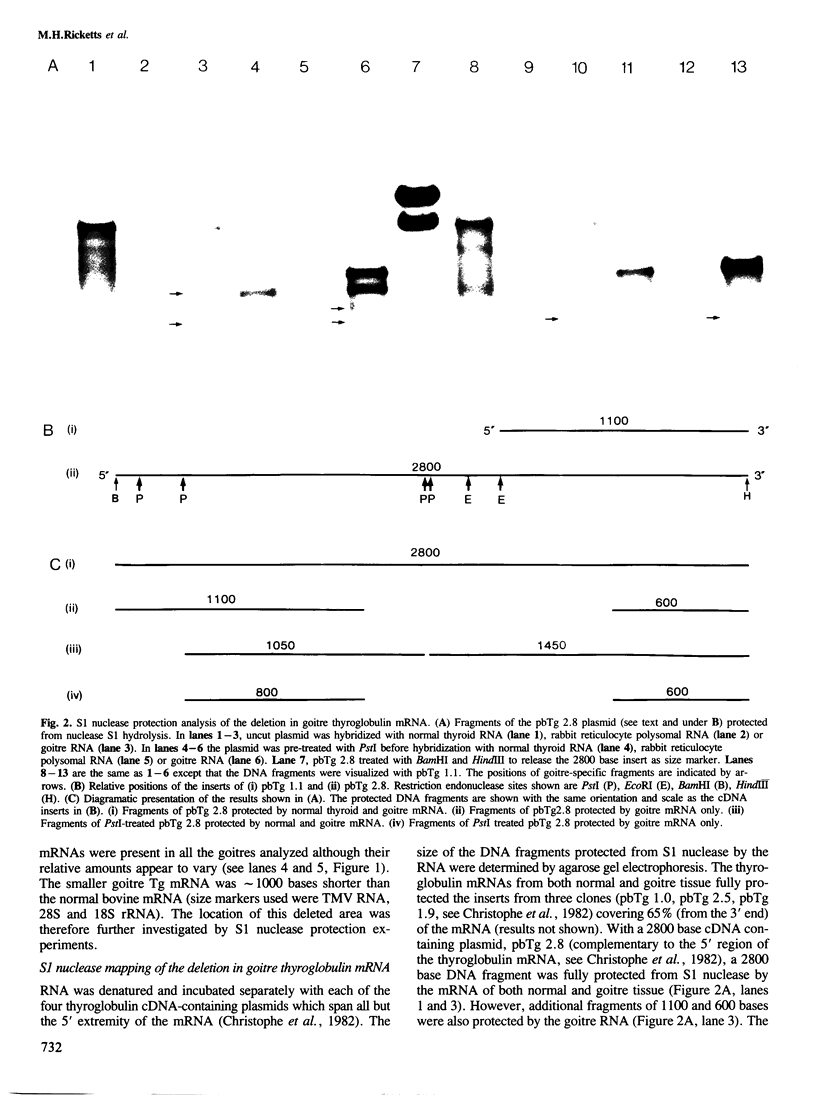
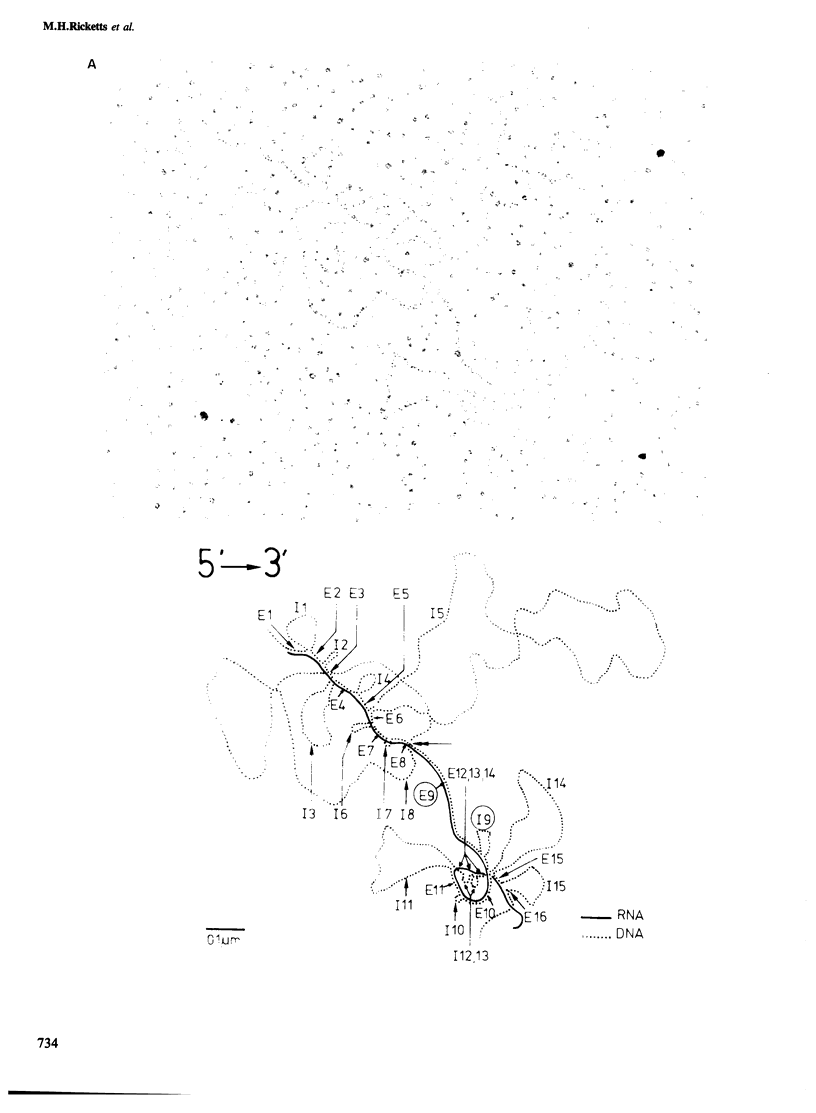
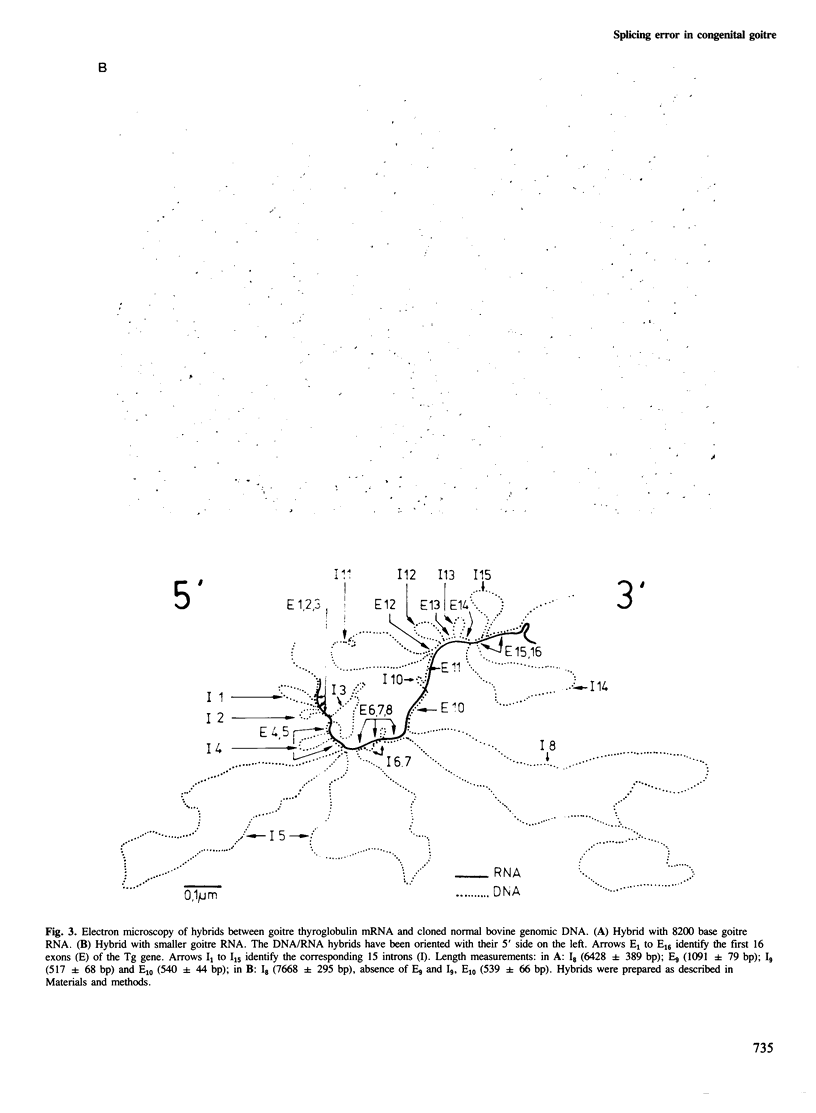
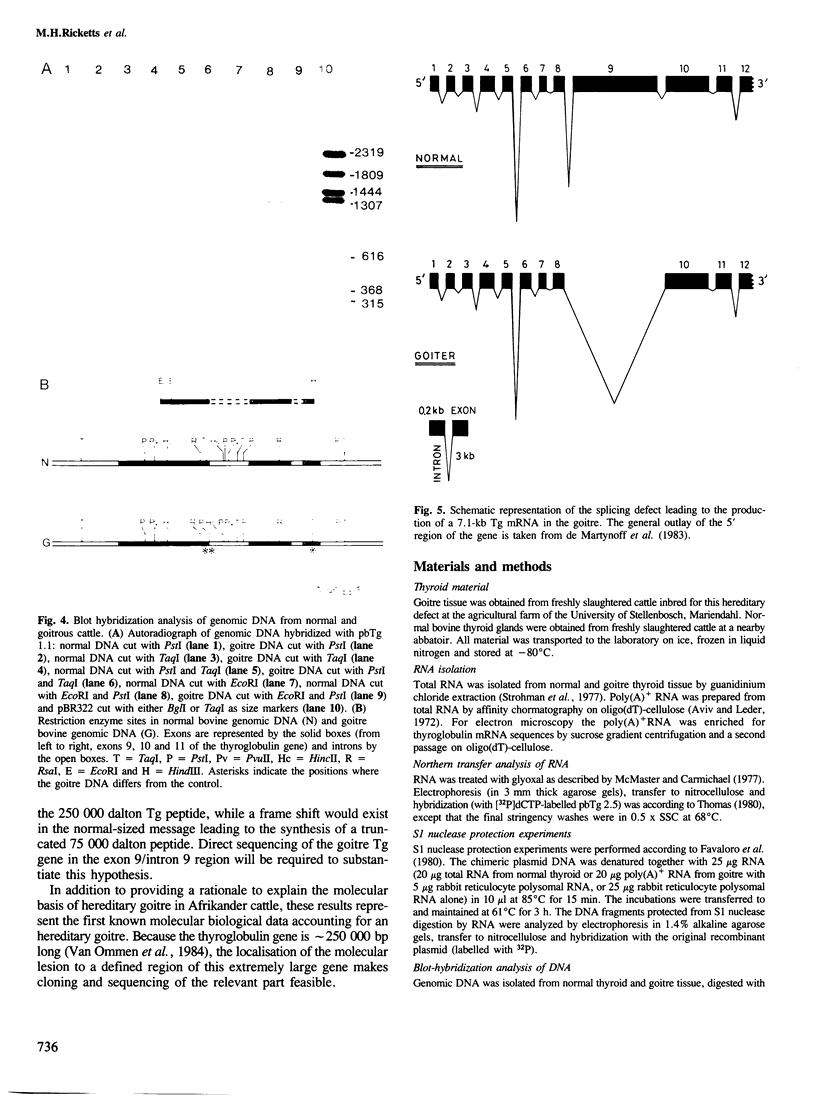
The structure of thyroglobulin mRNA was analyzed in an inbred herd of Afrikander cattle with hereditary goitre. Northern transfer of RNA from affected animals revealed both a shorter (approximately 7100 bases) and a normal-sized (approximately 8200 bases) thyroglobulin mRNA when hybridized to bovine thyroglobulin cDNA clones. S1 nuclease mapping experiments established that 1100 bases are deleted in the 5' region of the smaller mRNA. Electron microscopy of RNA from animals with goitre hybridized to a bovine genomic DNA clone showed that the region deleted corresponds to exon 9 of the thyroglobulin gene. Southern blot analysis of the exon 9 region revealed differences between affected and control animals with the enzymes PstI and TaqI. Although they could reflect a linkage disequilibrium between the mutation and restriction fragment length polymorphism, it is noteworthy that these differences map in the region of the exon 9/intron 9 junction. Our results show that a genetic lesion in the thyroglobulin gene causes aberrant splicing of the pre-mRNA, and suggest that the responsible mutation is at the exon 9/intron 9 junction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvedimento V. E., Musti A. M., Obici S., Cocozza S., Di Lauro R. Structural organization of the 3' half of the rat thyroglobulin gene. Nucleic Acids Res. 1984 Apr 25;12(8):3461–3472. doi: 10.1093/nar/12.8.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Christophe D., Mercken L., Brocas H., Pohl V., Vassart G. Molecular cloning of the 8000-base thyroglobulin structural gene. Eur J Biochem. 1982 Mar 1;122(3):461–469. doi: 10.1111/j.1432-1033.1982.tb06460.x. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Lever E. G., Medeiros-Neto G. A., DeGroot L. J. Inherited disorders of thyroid metabolism. Endocr Rev. 1983 Summer;4(3):213–239. doi: 10.1210/edrv-4-3-213. [DOI] [PubMed] [Google Scholar]
- Lissitzky S., Bismuth J., Jaquet P., Castay M., Michel-Béchet M., Koutras D. A., Pharmakiotis A. D., Moschos A., Psarras A., Malamos B. Congenital goiter with impaired thyroglobulin synthesis. J Clin Endocrinol Metab. 1973 Jan;36(1):17–29. doi: 10.1210/jcem-36-1-17. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Anagnou N. P., Ley T. J. Advances in thalassemia research. Blood. 1984 Apr;63(4):738–758. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robbins J., Van Zyl A., Van der Walt K. Abnormal thyroglobin in congenital goiter of cattle. Endocrinology. 1966 Jun;78(6):1213–1223. doi: 10.1210/endo-78-6-1213. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Targovnik H. M., Pohl V., Christophe D., Cabrer B., Brocas H., Vassart G. Structural organization of the 5' region of the human thyroglobulin gene. Eur J Biochem. 1984 Jun 1;141(2):271–277. doi: 10.1111/j.1432-1033.1984.tb08188.x. [DOI] [PubMed] [Google Scholar]
- Tassi V. P., Di Lauro R., Van Jaarsveld P., Alvino C. G. Two abnormal thyroglobulin-like polypeptides are produced from Afrikander cattle congenital goiter mRNA. J Biol Chem. 1984 Aug 25;259(16):10507–10510. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Van Herle A. J., Vassart G., Dumont J. E. Control of thyroglobulin synthesis and secretion (second of two parts). N Engl J Med. 1979 Aug 9;301(6):307–314. doi: 10.1056/NEJM197908093010605. [DOI] [PubMed] [Google Scholar]
- Vassart G., Verstreken L., Dinsart C. Molecular weight of thyroglobulin 33 S messenger RNA as determined by polyacrylamide gel electrophoresis in the presence of formamide. FEBS Lett. 1977 Jul 1;79(1):15–18. doi: 10.1016/0014-5793(77)80340-2. [DOI] [PubMed] [Google Scholar]
- de Vijlder J. J., van Ommen G. J., van Voorthuizen W. F., Koch C. A., Arnberg A. C., Vassart G., Dinsart C., Flavell R. A. Nonfunctional thyroglobulin messenger RNA in goats with hereditary congenital goiter. J Mol Appl Genet. 1981;1(1):51–59. [PubMed] [Google Scholar]
- van Ommen G. J., Arnberg A. C., Baas F., Brocas H., Sterk A., Tegelaers W. H., Vassart G., de Vijlder J. J. The human thyroglobulin gene contains two 15-17 kb introns near its 3'-end. Nucleic Acids Res. 1983 Apr 25;11(8):2273–2285. doi: 10.1093/nar/11.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]