Abstract
All organisms have evolved pathways to respond to different forms of cellular stress. The Gcn2 kinase is best known as a regulator of translation initiation in response to starvation for amino acids. Work in budding yeast has showed that the molecular mechanism of GCN2 activation involves the binding of uncharged tRNAs, which results in a conformational change and GCN2 activation. This pathway requires GCN1, which ensures delivery of the uncharged tRNA onto GCN2. However, Gcn2 is activated by a number of other stresses which do not obviously involve accumulation of uncharged tRNAs, raising the question how Gcn2 is activated under these conditions. Here we investigate the requirement for ongoing translation and tRNA binding for Gcn2 activation after different stresses in fission yeast. We find that mutating the tRNA-binding site on Gcn2 or deleting Gcn1 abolishes Gcn2 activation under all the investigated conditions. These results suggest that tRNA binding to Gcn2 is required for Gcn2 activation not only in response to starvation but also after UV irradiation and oxidative stress.
Introduction
All cells and organisms are surrounded by a changing and often stressful environment and have developed various signaling pathways to adapt to these changes. An important requirement for maintaining cell homeostasis during stress conditions is the correct regulation of translation. Translational regulation in response to different types of stress involves phosphorylation of serine51 (Ser 52 in S. pombe) of the eukaryotic translation initiation factor 2α (eIF2α) [1]. This phosphorylation is thought to lead to a general downregulation of translation, accompanied by an enhanced translation of specific stress-response mRNAs [2–4]. One of the eIF2α kinases performing this phosphorylation is Gcn2. Gcn2 was first described in budding yeast as a regulator of eIF2α phosphorylation in response to amino-acid starvation. This role is conserved from yeast to human cells and the extent of conservation is such that the human Gcn2 can functionally replace the budding yeast Gcn2 [5]. Fission yeast has several eIF2α kinases and it is GCN2 that is activated in response to nutrient deprivation [6–9].
The mechanism of GCN2 activation in response to amino-acid starvation has been extensively studied through the years, mainly in budding yeast. Under starvation conditions, uncharged tRNAs accumulate and bind a histidyl-tRNA synthetase-like domain (HisRS) in Gcn2. This, in turn, leads to a conformational change and activates the kinase. This model is supported by the findings that (i) mutations leading to amino-acid substitutions close to the predicted active site of the HisRS domain affect Gcn2 activation, either by leading to constitutive activation or abolishing kinase activation [10–12]; (ii) activating mutations that mimic tRNA binding have been identified [13] and (iii) Gcn2 was shown to be associated with the translating ribosome [14], the very site where the absence of charged tRNA-s is most likely to have an effect.
In budding yeast, the association of GCN2 with the ribosome depends on its interaction with GCN1, a cofactor required for Gcn2 activation upon amino-acid starvation. GCN1 binds the ribosome at or near the A site and is thus perfectly placed to ensure the transfer of the uncharged tRNA onto GCN2 [15–19].
In addition to amino-acid starvation various other types of stress can activate Gcn2, including ultraviolet light (UVC), MMS, H2O2, proteasome inhibition, viral infections and serum starvation [20,21]. However, it is not immediately obvious how all these different forms of stress might lead to an accumulation of uncharged tRNAs. Furthermore, accumulation of uncharged tRNAs during starvation is a slow process, whereas the response to for example UV and H2O2 is very fast. Therefore we reasoned that Gcn2 might be activated by other mechanisms.
Here we investigated the mechanism of Gcn2 activation in response to different types of stress. We found that Gcn1 is required for Gcn2 activation after amino-acid starvation also in fission yeast. We show that ongoing translation is not required for UVC-induced activation of Gcn2. However, mutating the tRNA-binding site on Gcn2 or deleting Gcn1 abolishes Gcn2 activation not only in response to starvation but also after UVC irradiation and oxidative stress. These results strongly suggest that tRNA binding is required for Gcn2 activation in response to all these types of stress.
Materials and methods
Strains and media
All strains used are derived from the Schizosaccharomyces pombe L972h- strain, and are listed in Table 1. The growth conditions and media were as described in [22]. The cells were grown in liquid Edinburgh minimal medium (EMM) containing the required supplements, or yeast extract medium (YES) at 25°C, to a cell density of 3–5 X 106 cells/ml (OD595 0,15–0,3).
Table 1. Strains used in this study.
| Strain number | Genotype | Source |
|---|---|---|
| 38 | ura4-D18 leu1-32 h+ | Paul Nurse |
| 1136 | gcn2::ura4+ leu1-32 ura4-D18h- | Ronald Wek |
| 2079 | gcn2-F1066A R1067L leu-32 ura4-D18 h- | This work |
| 2095 | gcn1::kanMX6 ura4-D18 leu1-32 h+ | This work |
| 2120 | gcn1::kanMX6 cdc10-M17 mcm2:GFP:kanR ura4-D18 leu1-32 | This work |
Leucine starvation
Leucine-auxotroph cells were grown in the presence of leucine in EMM medium and washed by filtering with three volumes of EMM lacking leucine. The cells were then resuspended in EMM lacking leucine and samples were collected at different timepoints after leucine removal.
Oxidative stress
Cells grown in EMM to a density of 4X 106/ml were treated with H2O2 at the indicated concentrations, and samples were collected at different timepoints after addition of the oxidative agent.
UVC-irradiation
Cells were grown in EMM medium to early log phase (OD = 0.15 Fission yeast cells suspended in a thin layer (3 mm) of rapidly stirred liquid EMM medium were irradiated with 254-nm UVC light. The dose was measured with a radiometer (Ultraviolet Products, San Gabriel, CA, USA), and a dose of 1100 J/m2 was given at an incident dose rate of approximately 250 J/m2/min [23]. This dose gives a survival >90%.
Pre-RC loading assay
In situ chromatin binding assay was performed as described previously [23,24].
Immunoblots
Samples for immunoblotting were made by the trichloroacetic acid (TCA) protein extraction method [25]. A total of 50–100 μg protein extracts were run on 10% SDS-PAGE, transferred to a PVDF membrane (Immobilon, EMD Millipore Corporation, Billerica, MA, USA) and probed with the following antibodies: anti-phosphorylated eIF2α (Cat. # 44–728G, Life Technologies, Carlsbad, California, USA) 1:3000; anti-α-tubulin (Cat. # T-5168 Sigma) 1:30 000, anti eIF2α (Cat. # sc-11386, Santa Cruz).
Translation assay
Cells were pulse-labelled with the methionine analogue L-Homopropargylglycine (HPG; Life Technologies) at a concentration of 50 μM for 10 minutes. Samples were taken at the indicated timepoints after treatment and fixed in ice-cold 70% ethanol. Newly synthesized proteins were detected by chemoselective fluorescence tagging by means of “click chemistry” [26] using the Click-iT Cell reaction buffer kit (Life Technologies) according to the manufacturer’s protocol. The Alexa Fluor 647- specific fluorescence signal was measured by flow cytometry to detect median fluorescence intensity from 10 000 fission yeast cells. Samples without HPG were used as negative controls.
Gcn2 kinase assays
Gcn2 was immunoprecipitated from exponentially growing unirradiated (“C”) and UV-irradiated “UV” cells, using IP buffer (50 mM Hepes, pH7.5;1 mM EDTA; 20 mM Na-β-glycerolphosphate; 0.1 mM Na3VO4; 50 mM NaF; 75 mM NaCl; 0,1 mM PMSF; 1mM DTT; 0.3% Np40; Roche protease inhibitor cocktail; 1% triton). The immunoprecipiates were resuspended in kinase buffer Tris-HCl pH 7,5; 5 mMMgCl2; 75 mM NaCl DTT 1mM; 0,5% Triton; Protease inhibitor from Roche; 0,1 mM PMSF; 100 μM Na3VO4; 100 μM ATP and mixed with extracts made from an unirradiated culture of gcn2Δ cells. After 30 min incubation EDTA was added to 20 mM, Laemmli sample buffer to 1X and the reaction mix was boiled for 5 min before loading on a gel and immunoblotting for eIF2α-P.
Results
Ongoing translation is not required for the UVC-induced activation of Gcn2
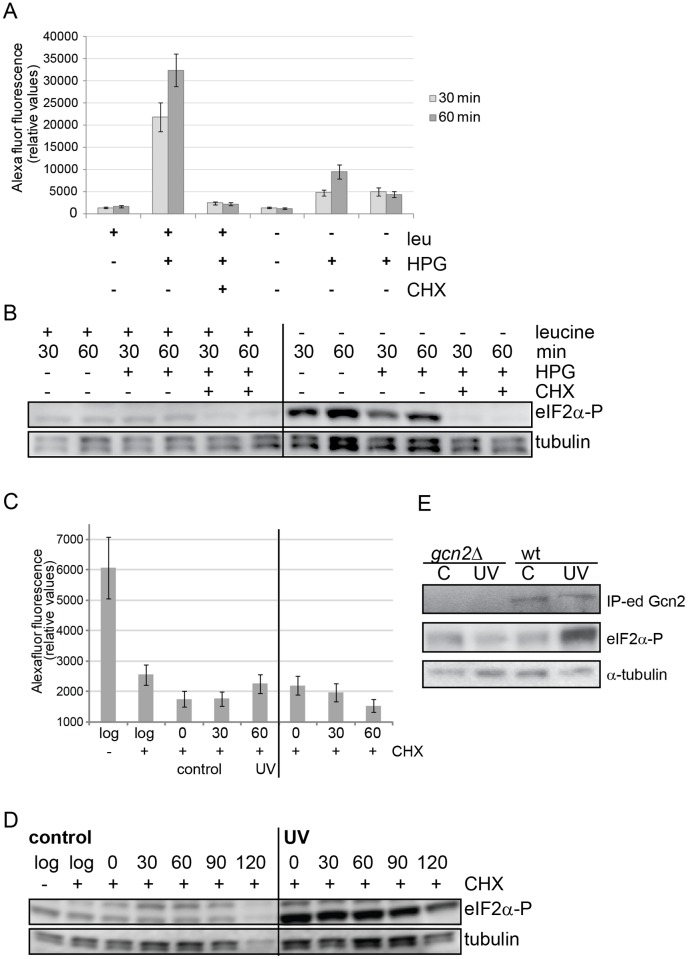
Under starvation conditions uncharged tRNAs accumulate as the existing pool of charged tRNAs is gradually exhausted during protein synthesis. If Gcn2 activation occurs through a similar mechanism of tRNA accumulation also after UV irradiation, one would expect that ongoing translation is also required for activation. To address this, we inhibited translation prior to UVC-irradiation and measured eIF2α phosphorylation to assess Gcn2 activation. To inhibit translation we treated the cells with 100 μg/ml cycloheximide for 10 minutes, under which conditions no further translation could be detected as measured by incorporation of HPG, a methionine analogue (Fig 1A and 1C). To verify that our experimental design can be used to explore the requirement for ongoing translation, we starved fission yeast cells for leucine by withdrawing it from the medium of leucine-auxotroph cells in the presence and absence of cycloheximide. The translation rate was reduced by >80% already after 30 minutes of leucine starvation and comparable to that after cycloheximide treatment (Fig 1A). Notably, the leucine-starvation-induced eIF2α phosphorylation was completely abolished in the presence of cycloheximide (Fig 1B), demonstrating that the approach is suitable to detect a requirement for ongoing translation. In contrast, the phosphorylation of eIF2α after UVC-irradiation was not affected by blocking translation by cycloheximide prior to UVC irradiation (Fig 1C and 1D), suggesting that ongoing translation is not required for Gcn2 activation after UVC irradiation. The amount of total eIF2α did not change in response to UVC irradiation or cycloheximide treatment (S1 Fig).
Fig 1. Inhibition of translation does not prevent Gcn2 activation after UV irradiation in fission yeast.
It cannot be excluded that an eIF2α phosphatase is inactivated upon UVC irradiation in the presence of of cycloheximide and the increased eIF2α phosphorylation we observe is not due to increased Gcn2 activity but rather to reduced phosphatase activity. However, we think this explanation is most unlikely. Frist, we have performed in vitro kinase assays with GCN2 immunoprecipitated from unirradiated control and from UV-irradiated cells. A considerable increase in kinase activity can be clearly observed when GCN2 is immunoprecipitated from irradiated cells (Fig 1E). While this does not exclude a phosphatase being regulated, it demonstrates that the induction of GCN2 kinase activity is a major contributor to the increased eIF2α phosphorylation and supports our conclusion above that Gcn2 activation after UV irradiation does not require ongoing translation. Second, cycloheximide does not induce eIF2α phosphorylation in unirradiated control cells (Fig 1B and 1D), nor is eIF2α phosphorylation increased in cycloheximide-treated and UVC-irradiated gcn2Δ cells (S1 Fig). Interestingly, there is more phosphorylated eIF2α after UV irradiation in the presence of cycloheximide than in the absence (S1 Fig) suggesting that (i) a phosphatase does contribute to the regulation of eIF2α phosphorylation after UV irradiation and (ii) the regulation of the phosphatase involves translational regulation and appears to be dependent on eIF2α phosphorylation. In mammalian cells the inducible eIF2α phosphatase-targeting protein GADD34 (PPP1R15A) is expressed upon eIF2α phosphorylation and limits eIF2α phosphorylation in a negative feedback loop [27]. Although there is no obvious homologue to GADD34 in fission yeast, the increased eIF2α phosphorylation upon cycloheximide treatment after UV irradiation indicates the existence of a similar mechanism in fission yeast.
Cycloheximide was added to 100 μg/ml to inhibit translation for 10 minutes. Half the culture was irradiated with 1100 J/m2 as described [28] and samples were taken at the indicated times after irradiation. Note that c0 and UV0 were taken at the same time after irradiation. Leucine-starved auxotroph cells were grown in medium lacking leucine for the indicated times. At each timepoint a sample was taken to pulse label with HPG for 10 min to measure translation rates (A, C) and a sample was taken to extract proteins and measure eIF2α phosphorylation by immunoblotting (B, D). “log” refers to a sample of exponentially growing cells. On the graphs showing translation rates median fluorescence intensity from 10 000 fission yeast cells and standard deviations are shown. α-tubulin levels are shown to check even loading. E GCN2 was immunoprecipitated from from UV-irradiated “UV” and unirradiated control “C” cells, and incubated with extracts prepared from unirradiated gcn2Δ cells in kinase buffer. eIF2α phosphorylation was measured by immunoblotting. α-tubulin levels are shown to demonstrate that equal amounts of extracts from unirradiated gcn2Δ cells were used as substrate in the kinase assays.
Mutating the tRNA-binding sites of Gcn2 abolishes activation in response to UVC irradiation
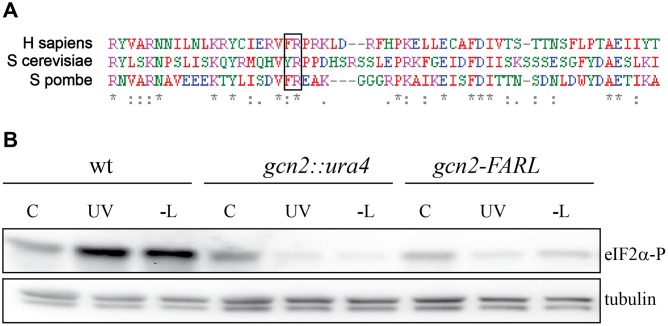
Gcn2p contains a C-terminal domain related to histidyl-tRNA synthetases (HisRS) [29]. This domain includes residues related to the conserved motif 2 sequences that interact with the acceptor stem of the cognate tRNA in authentic class II synthetases [30]. The HisRS-like domain can bind uncharged tRNA in vitro, and mutations in the motif 2 sequence impair tRNA binding and abolish GCN2 activity in budding yeast [31]. The Y1119L R1120L motif 2 mutations rendering GCN2 inactive in budding yeast correspond to F1066A R1067L in fission yeast (Fig 2A). We introduced these two mutations in the genome of fission yeast cells and investigated whether Gcn2 can be activated by starvation. Leucine-auxotroph wild-type, gcn2Δ, and gcn2-FARL cells were starved for leucine and samples were collected at different timepoints. Phosphorylation of eIF2α was measured by immunoblotting. As expected, eIF2α was not phosphorylated after leucine starvation (Fig 2B, “-L”). eIF2 levels were not affected by either starvation or UVC irradiation in any of the strains (S2 Fig). To test whether the mutations affect Gcn2 activation after UV irradiation, we irradiated the mutant strains with UVC and monitored eIF2α phosphorylation. No induction of eFI2α phosphorylation was observed (Fig 2B, UV), suggesting that the ability of the HisRS domain to bind tRNAs is required for Gcn2 activation also after UV irradiation.
Fig 2. Mutating the tRNA-binding sites of Gcn2 abolishes activation in response to UVC irradiation.
A Alignment of the HisRS-like domain of budding yeast and fission yeast Gcn2. B The indicated strains were irradiated with 1100 J/m2 and samples were taken at the indicated times after irradiation. eIF2α phosphorylation was detected by immunoblotting, α-tubulin levels are shown to check even loading.
Gcn1 is required for Gcn2 activation upon amino acid starvation in fission yeast
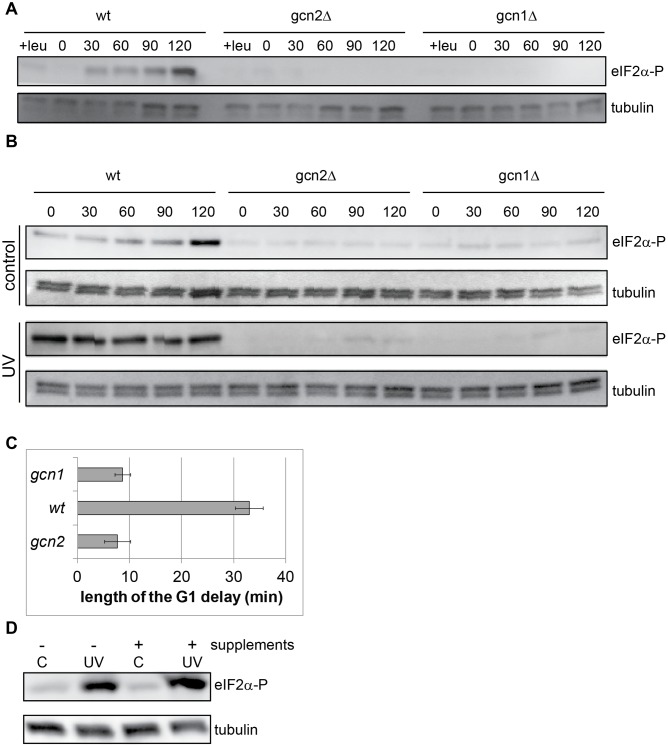
In budding yeast, two proteins have been shown to be involved in GCN2 activation after amino acid starvation, GCN1 and its cofactor GCN20. Both proteins are required to recruit GCN2 to the translating ribosome and transfer uncharged tRNAs to the HisRS domain of GCN2 [15,16,19]. To investigate whether Gcn1 is required for Gcn2 activation also in fission yeast, we deleted the putative GCN1 homologue and investigated whether Gcn2 can be activated in gcn1Δ cells. Leucine was withdrawn from the medium of leucine-auxotroph wild-type, gcn2Δ, and gcn1Δ cells and samples were collected at different timepoints. Phosphorylation of eIF2α was measured by immunoblotting. In wild-type cells, phosphorylation of eIF2α occurred at 30 min after the withdrawal of leucine and persisted for at least one hour (Fig 3A). In a gcn2Δ mutant, eIF2α phosphorylation did not occur, showing that Gcn2 is the sole kinase responsible for the phosphorylation response after leucine starvation. In the leucine-auxotroph gcn1Δ strain the starvation-induced eIF2α phosphorylation was abolished (Fig 3A), demonstrating that the role of Gcn1 in the activation of Gcn2 after amino-acid starvation is conserved between budding and fission yeast.
Fig 3. Gcn1 is required for Gcn2 activation after UVC irradiation.
A The indicated strains were starved for leucine as described in Materials and Methods. eIF2α phosphorylation was detected by immunoblotting, α-tubulin levels are shown to check even loading. B eIF2α phosphorylation after UVC-irradiation in wild-type, gcn2Δ and gcn1Δ cells. Exponentially growing cells of the indicated strains were irradiated as described in Materials and methods and [23]. eIF2α phosphorylation was detected by immunoblotting, α-tubulin levels are shown to check even loading. C preRC loading in gcn1Δ cells. Cells carrying a cdc10-M17 mutation and GFP-tagged Mcm2 were grown in EMM medium, arrested in G1 by shifting them to 36°C for 4 h, released from the G1 block and irradiated with 1100 J/m2 UVC. The percentage of cells containing chromatin-bound Mcm2:GFP was determined. The delay was calculated as the time difference between irradiated and unirradiated cells at reaching 70% of maximal preRC loading. D Prototroph wild-type cells were grown to mid-log phase in EMM medium. Supplements were added to 80 mg/l for 30 min before UV irradiation. Samples were taken immediately after irradiation. eIF2α phosphorylation was detected by immunoblotting, α-tubulin levels are shown to check even loading.
Gcn1 is required for Gcn2 activation after UV irradiation
If the binding of uncharged tRNAs is involved in activation of Gcn2 after other forms of stress one would expect that Gcn1 was also required. We explored whether Gcn1 is involved in the UVC-induced activation of Gcn2. Exponentially growing wild-type, gcn2Δ, gcn1Δ cells were irradiated with UVC light and samples were collected at the indicated timepoints after irradiation. UVC-irradiation-induced phosphorylation of eIF2α was clearly observed in wild-type cells and was abolished in the absence of Gcn2 (Fig 3B) consistent with previous results [23]. In the absence of Gcn1 phosphorylation of eIF2α was abolished (Fig 3B, S2 Fig), demonstrating that Gcn1 is required for the UVC-induced activation of Gcn2.
UVC-irradiation in G1 phase delays the formation of the pre-Replicative Complex (preRC) in a Gcn2-dependent manner [23] and thus delays entry into S phase. The G1 delay correlates with and possibly is caused by the phosphorylation of eIF2α [32,33]. To investigate whether Gcn1 is required for the UVC-induced G1 delay, gcn1Δ cells carrying a GFP-tagged Mcm2 were arrested in G1 using a cdc10 temperature-sensitive mutation, released from the block and irradiated with UVC. Samples were taken at the indicated timepoints and the loading of the MCM complex was assessed using an in situ chromatin binding assay [23,24]. The preRC-loading delay was abolished in the irradiated gcn1Δ cells (Fig 3C), as previously described for gcn2Δ cells [23], suggesting that Gcn1 is required both for the cell-cycle delay and for Gcn2 activation after UVC-irradiation.
Supplementing the medium with all amino acids does not prevent Gcn2 activation after UVC irradiation
The above data suggest that tRNA binding is necessary for GCN2 activation also after UVC irradiation. One plausible mechanism for the accumulation of uncharged tRNA-s is depletion of one (or a few) specific amino-acyl- tRNA due to, for example, a chemical modification or conversion. To test whether such a mechanism might be responsible for the activation of Gcn2, we supplemented the medium with all amino acids as well as adenine and uracil prior to UV irradiation. A prototroph strain was grown in EMM medium and supplements were added 30 min before irradiation. We used concentrations of supplements that were sufficient to allow growth of auxotrophic mutants. The cells were irradiated with UVC and samples were taken for immunoblotting immediately after irradiation. Providing supplements in excess in the medium did not prevent or reduce eIF2α phosphorylation (Fig 3D). These results argue against a model that Gcn2 activation after UVC irradiation is due to a specific amino-acyl- tRNA being depleted.
Gcn1 is required for Gcn2 activation after H2O2- treatment
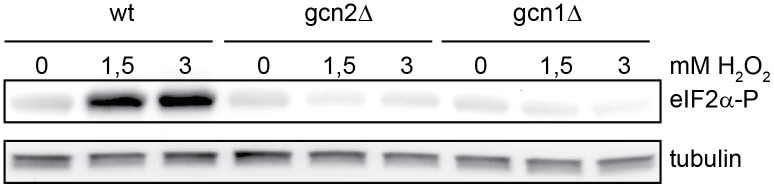
Oxidative stress has also been shown to activate Gcn2 [32,34] and again, it is not obvious how this treatment should lead to an accumulation of uncharged tRNAs. To investigate whether Gcn1 is required for the H2O2-induced activation of Gcn2, wild-type, gcn2Δ and gcn1Δ cells were grown in minimal medium, H2O2 was added to the concentrations shown and samples were collected 15 minutes after the addition of H2O2. In agreement with previous reports, phosphorylation of eIF2α was observed in response to H2O2 in wild-type cells. It should be noted that prolonged oxidative stress or high concentration of H2O2 activates another eIF2α kinase, Hri2, but the initial eIF2α phosphorylation is due to Gcn2 [34 and our unpublished results]. To study the requirement for Gcn1, we used conditions where eIF2α phosphorylation was clearly dependent on Gcn2 (Fig 4). To ensure that the cells of the different strains were exposed to the same level of oxidative stress, both the concentration of H2O2 and cell density were carefully controlled. Interestingly, eIF2α phosphorylation was abolished in the absence of Gcn1. Gcn2 protein levels were not reduced in the gcn1Δ mutant (S3 Fig), thus these results suggest that Gcn1 is required for Gcn2 activation also in response to oxidative stress (Fig 4).
Fig 4. Gcn1 is required for Gcn2 activation after H2O2- treatment.
eIF2α phosphorylation after H2O2 treatment in wild-type, gcn2Δ and gcn1Δ cells. The indicated strains were grown in EMM medium and treated with H2O2 at the concentrations shown, for 15 minutes. eIF2α phosphorylation was detected by immunoblotting, α-tubulin levels are shown to check even loading.
Discussion
The Gcn2 kinase and its regulation attract more and more attention in the field as its role in human diseases such as cancer and neurodegenerative diseases is revealed. Here we investigate mechanisms that activate Gcn2 in response to different stresses.
The molecular mechanism of GCN2 activation was first described in budding yeast after starvation. Under these circumstances GCN2 activation involves binding of uncharged tRNAs and requires the cofactor GCN1. Our findings suggest that also after UV irradiation and oxidative stress tRNA binding is required for Gcn2 activation. It should be noted that the motif 2 mutations in Gcn2 affect not only tRNA binding but also intramolecular interactions between the CTD domain and the HisRS domain, which in turn is required for activation [12]. Thus the finding that the motif 2 mutations abolish Gcn2 activation does not necessarily imply that tRNA binding is required. However, the involvement of Gcn1, thought to transfer uncharged tRNAs to the HisRS domain of GCN2 [15,16,19], strongly suggests that tRNA binding is necessary for Gcn2 activation.
Accumulation of uncharged tRNAs due to starvation is a well-described mechanism that leads to activation of Gcn2. Under these conditions it is obvious that charged tRNAs are depleted and uncharged tRNAs accumulate, but it is not clear how and why the level of charged tRNAs would be reduced in response to stresses like UVC-irradiation or oxidative stress. In fact, both of these treatments are known to reduce overall translation rates [23,35,36], making it even less likely that charged tRNAs would be depleted. Furthermore, we have shown here that ongoing translation is not required for Gcn2 activation after UV irradiation, arguing against a model that tRNA pools are depleted.
However, alternative mechanisms could be activated that would change the balance between charged and uncharged tRNAs and thus lead to Gcn2 activation. In mammalian cells after UVB irradiation nitric oxide is synthesized from Arg, leading to Arg depletion and a starvation response [37]. There is no obvious homologue to nitric oxide synthase in fission yeast, and we have shown here that supplementing the medium with all amino acids does not prevent activation of Gcn2 after UVC irradiation. Thus, it is unlikely that depletion of a specific amino acid is the reason for the UVC-induced activation of Gcn2. The activity of RNA polymerase III is inhibited in response to a number of stresses [38–40], thus it is unlikely that increased transcription of tRNAs would contribute to an increase in uncharged tRNA-s. Recent discoveries have revealed an unexpected complexity of tRNA biogenesis, as well as the role of tRNA-modifications and cleavage fragments in signaling pathways [41,42]. It is plausible that modified tRNAs or even tRNA-derived fragments are responsible for the activation of Gcn2 after UVC.
Alternatively, additional factors might be required for Gcn2 activation after stresses other than starvation. Indeed, some studies reported that Gcn2 activity can be regulated by upstream kinases. In S. cerevisiae a link between the TOR pathway and activation of Gcn2 has been shown [43]. Treating budding yeast with rapamycin, an inhibitor of the TOR kinases, leads to removal of an inhibitory phosphorylation on serine 577 of Gcn2, thought to reduce the threshold of uncharged tRNAs required for activation. However, this phosphorylation site is not conserved in fission yeast or in mammalian cells and even though inhibiting the Tor pathway can lead to increased Gcn2 activity also in fission yeast [9,44], the crosstalk between the two pathways depends on the particular stress applied [9]. In particular, Tor2 does not regulate eIF2α phosphorylation under a number of conditions, including UVC-irradiation and oxidative stress in fission yeast. Furthermore, under leucine starvation the phosphorylation of eIF2α is in fact dependent on maintained TORC1 activity rather than on TORC1 inactivation [9]. Thus it is unlikely that the Tor pathway is the master regulator of Gcn2 after UVC irradiation and oxidative stress.
Another study reported that in response to UVB-irradiation of mammalian cells Gcn2 is activated in a DNA-PK-dependent manner [45]. Thus, Gcn2 activation in human cells might be linked to DNA damage through the activity of DNA-PK, at least in response to some stresses.
It was previously reported that Gcn1 is required for Gcn2 activation after different stresses in mouse embryonic fibroblasts [46]. This conclusion is fully consistent with our results presented here. It should be noted that our experimental design is very different from that of Cambiaghi et al; here we have used loss-of function mutants in fission yeast, while they exploited dominant negative effects of overexpressing either IMPACT, a protein known to interact with Gcn1 and compete with Gcn2 for Gcn1 binding, or a portion of Gcn1mouse that corresponds to the region necessary and sufficient to bind Gcn2 in budding yeast. Collectively, the two studies suggest that the requirement for tRNA binding and for Gcn1 in Gcn2 activation is a conserved feature.
In summary, it appears most likely that Gcn2 is activated by a mechanism(s) involving tRNA binding assisted by Gcn1 in response to stresses other than starvation. However, identification of the mechanisms that alter the balance between charged and uncharged tRNAs and the possible involvement of other upstream regulators requires further studies.
Supporting information
Wild-type and gcn2Δ cells were treated with 100 μg/ml cycloheximide for 10 min as indicated and UV irradiated.
(TIF)
The same samples as shown in (A) Fig 2B and (B) Fig 3B were analyzed by immunoblotting using an antibody against total eIF2α.
(TIF)
Acknowledgments
We thank the Norwegian Research Council and the South-Eastern Norwegian Health Authorities for financial support and Marit Haugli and Lilian Lindbergsengen for excellent technical assistance. Róbert Zach was supported by the Erasmus program.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Norwegian Research Council and the South-Eastern Norwegian Health Authorities supported this work. Róbert Zach was supported by the Erasmus program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kimball SR (1999) Eukaryotic initiation factor eIF2. Int J Biochem Cell Biol 31: 25–29. [DOI] [PubMed] [Google Scholar]
- 2.Hinnebusch AG (1993) Gene-specific translational control of the yeast GCN4 gene by phosphorylation of eukaryotic initiation factor 2. Mol Microbiol 10: 215–223. [DOI] [PubMed] [Google Scholar]
- 3.Gerlitz G, Jagus R, Elroy-Stein O (2002) Phosphorylation of initiation factor-2 alpha is required for activation of internal translation initiation during cell differentiation. Eur J Biochem 269: 2810–2819. [DOI] [PubMed] [Google Scholar]
- 4.Dang Do AN, Kimball SR, Cavener DR, Jefferson LS (2009) eIF2alpha kinases GCN2 and PERK modulate transcription and translation of distinct sets of mRNAs in mouse liver. Physiol Genomics 38: 328–341. doi: 10.1152/physiolgenomics.90396.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dever TE, Chen JJ, Barber GN, Cigan AM, Feng L, Donahue TF, et al. (1993) Mammalian eukaryotic initiation factor 2 alpha kinases functionally substitute for GCN2 protein kinase in the GCN4 translational control mechanism of yeast. Proc Natl Acad Sci U S A 90: 4616–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan K, Narasimhan J, Wek RC (2004) Differential activation of eIF2 kinases in response to cellular stresses in Schizosaccharomyces pombe. Genetics 168: 1867–1875. doi: 10.1534/genetics.104.031443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemoto N, Udagawa T, Ohira T, Jiang L, Hirota K, Wilkinson CR, et al. (2010) The roles of stress-activated Sty1 and Gcn2 kinases and of the protooncoprotein homologue Int6/eIF3e in responses to endogenous oxidative stress during histidine starvation. J Mol Biol 404: 183–201. doi: 10.1016/j.jmb.2010.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udagawa T, Nemoto N, Wilkinson CR, Narashimhan J, Jiang L, Watt S, et al. (2008) Int6/eIF3e promotes general translation and Atf1 abundance to modulate Sty1 MAPK-dependent stress response in fission yeast. J Biol Chem 283: 22063–22075. doi: 10.1074/jbc.M710017200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rødland GE, Tvegård T, Boye E, Grallert B (2014) Crosstalk between the Tor and Gcn2 pathways in response to different stresses. Cell Cycle 13: 453–461. doi: 10.4161/cc.27270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu H, Dong J, Hu C, Francklyn CS, Hinnebusch AG (2001) The tRNA-binding moiety in GCN2 contains a dimerization domain that interacts with the kinase domain and is required for tRNA binding and kinase activation. Embo J 20: 1425–1438. doi: 10.1093/emboj/20.6.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG (2000) Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell 6: 269–279. [DOI] [PubMed] [Google Scholar]
- 12.Lageix S, Zhang J, Rothenburg S, Hinnebusch AG (2015) Interaction between the tRNA-binding and C-terminal domains of Yeast Gcn2 regulates kinase activity in vivo. PLoS Genet 11: e1004991 doi: 10.1371/journal.pgen.1004991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu H, Hu C, Dong J, Hinnebusch AG (2002) Mutations that bypass tRNA binding activate the intrinsically defective kinase domain in GCN2. Genes and Development 16: 1271–1280. doi: 10.1101/gad.979402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez M, Wek RC, Hinnebusch AG (1991) Ribosome association of GCN2 protein kinase, a translational activator of the GCN4 gene of Saccharomyces cerevisiae. Mol Cell Biol 11: 3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattlegger E, Hinnebusch AG (2000) Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. Embo J 19: 6622–6633. doi: 10.1093/emboj/19.23.6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sattlegger E, Hinnebusch AG (2005) Polyribosome binding by GCN1 is required for full activation of eukaryotic translation initiation factor 2{alpha} kinase GCN2 during amino acid starvation. J Biol Chem 280: 16514–16521. doi: 10.1074/jbc.M414566200 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Barrio M, Dong J, Ufano S, Hinnebusch AG (2000) Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation. EMBO J 19: 1887–1899. doi: 10.1093/emboj/19.8.1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marton MJ (1993) GCN1, a translational activator of GCN4 in Saccharomyces cerevisiae, is required for phosphorylation of eukaryotic translation initiation factor 2 by protein kinase GCN2. Molecular and Cellular Biology 13: 3541–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marton MJ, Vazquez de Aldana CR, Vazquez de Aldana CR, Qiu H, Qiu H, Chakraburtty K, et al. (1997) Evidence that GCN1 and GCN20, translational regulators of GCN4, function on elongating ribosomes in activation of eIF2alpha kinase GCN2. Molecular and Cellular Biology 17: 4474–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castilho BA, Shanmugam R, Silva RC, Ramesh R, Himme BM, Sattlegger E (2014) Keeping the eIF2 alpha kinase Gcn2 in check. Biochim Biophys Acta 1843: 1948–1968. doi: 10.1016/j.bbamcr.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 21.Grallert B, Boye E (2013) GCN2, an old dog with new tricks. Biochem Soc Trans 41: 1687–1691. doi: 10.1042/BST20130210 [DOI] [PubMed] [Google Scholar]
- 22.Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823. [DOI] [PubMed] [Google Scholar]
- 23.Tvegård T, Soltani H, Skjølberg HC, Krohn M, Nilssen EA, Kearsey SE, et al. (2007) A novel checkpoint mechanism regulating the G1/S transition. Genes and Development 21: 649–654. doi: 10.1101/gad.421807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearsey SE, Montgomery S, Labib K, Lindner K (2000) Chromatin binding of the fission yeast replication factor mcm4 occurs during anaphase and requires ORC and cdc18. EMBO Journal 19: 1681–1690. doi: 10.1093/emboj/19.7.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caspari T, Dahlen M, Kanter-Smoler G, Lindsay HD, Hofmann K, Papadimitriou K, et al. (2000) Characterization of Schizosaccharomyces pombe Hus1: a PCNA-related protein that associates with Rad1 and Rad9. Mol Cell Biol 20: 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieterich DC, Hodas JJ, Gouzer G, Shadrin IY, Ngo JT, Triller A, et al. (2010) In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat Neurosci 13: 897–905. doi: 10.1038/nn.2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novoa I, Zeng H, Harding HP, Ron D (2001) Feedback Inhibition of the Unfolded Protein Response by <em>GADD34</em>-Mediated Dephosphorylation of eIF2α. The Journal of Cell Biology 153: 1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilssen EA, Synnes M, Kleckner N, Grallert B, Boye E (2003) Intra-G1 arrest in response to UV irradiation in fission yeast. Proceedings of the National Academy of Sciences 100: 10758–10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wek RC, Jackson BM, Hinnebusch AG (1989) Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci U S A 86: 4579–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruff M, Krishnaswamy S, Boeglin M, Poterszman A, Mitschler A, Podjarny A, et al. (1991) Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science 252: 1682–1689. [DOI] [PubMed] [Google Scholar]
- 31.Wek SA, Zhu S, Wek RC (1995) The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol 15: 4497–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krohn M, Skjolberg HC, Soltani H, Grallert B, Boye E (2008) The G1-S checkpoint in fission yeast is not a general DNA damage checkpoint. J Cell Sci 121: 4047–4054. doi: 10.1242/jcs.035428 [DOI] [PubMed] [Google Scholar]
- 33.Grallert B, Boye E (2007) The Gcn2 kinase as a cell cycle regulator. Cell Cycle 6: 2768–2772. doi: 10.4161/cc.6.22.4933 [DOI] [PubMed] [Google Scholar]
- 34.Berlanga JJ, Rivero D, Martin R, Herrero S, Moreno S, de Haro C (2009) The Role of the Mitogen-activated Protein Kinase Sty1 in Regulation of eIF2{alpha} Kinases in Response to Environmental Stress in Schizosaccharomyces pombe. Eukaryot Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D, et al. (2002) Activation of GCN2 in UV-Irradiated Cells Inhibits Translation. Current Biology 12: 1279–1286. [DOI] [PubMed] [Google Scholar]
- 36.Knutsen JHJ, Rødland GE, Bøe CA, Håland TW, Sunnerhagen P, Grallert B, et al. (2015) Stress-induced inhibition of translation independently of eIF2α phosphorylation. Journal of Cell Science 128: 4420–4427. doi: 10.1242/jcs.176545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu W, Laszlo CF, Miao Z, Chen H, Wu S (2009) The role of nitric-oxide synthase in the regulation of UVB light-induced phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Biol Chem 284: 24281–24288. doi: 10.1074/jbc.M109.008821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodfellow SJ, Graham EL, Kantidakis T, Marshall L, Coppins BA, Oficjalska-Pham D, et al. (2008) Regulation of RNA polymerase III transcription by Maf1 in mammalian cells. J Mol Biol 378: 481–491. doi: 10.1016/j.jmb.2008.02.060 [DOI] [PubMed] [Google Scholar]
- 39.Moir RD, Lee J, Haeusler RA, Desai N, Engelke DR, Willis IM (2006) Protein kinase A regulates RNA polymerase III transcription through the nuclear localization of Maf1. Proc Natl Acad Sci U S A 103: 15044–15049. doi: 10.1073/pnas.0607129103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oficjalska-Pham D, Harismendy O, Smagowicz WJ, Gonzalez de Peredo A, Boguta M, Sentenac A, et al. (2006) General repression of RNA polymerase III transcription is triggered by protein phosphatase type 2A-mediated dephosphorylation of Maf1. Mol Cell 22: 623–632. doi: 10.1016/j.molcel.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 41.Kirchner S, Ignatova Z (2015) Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat Rev Genet 16: 98–112. doi: 10.1038/nrg3861 [DOI] [PubMed] [Google Scholar]
- 42.Phizicky EM, Hopper AK (2010) tRNA biology charges to the front. Genes Dev 24: 1832–1860. doi: 10.1101/gad.1956510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherkasova VA, Hinnebusch AG (2003) Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev 17: 859–872. doi: 10.1101/gad.1069003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valbuena N, Rozalen AE, Moreno S (2012) Fission yeast TORC1 prevents eIF2{alpha} phosphorylation in response to nitrogen and amino acids via Gcn2 kinase. Journal of Cell Science 125: 5955–5959. doi: 10.1242/jcs.105395 [DOI] [PubMed] [Google Scholar]
- 45.Powley IR, Kondrashov A, Young LA, Dobbyn HC, Hill K, Cannell IG, et al. (2009) Translational reprogramming following UVB irradiation is mediated by DNA-PKcs and allows selective recruitment to the polysomes of mRNAs encoding DNA repair enzymes. Genes Dev 23: 1207–1220. doi: 10.1101/gad.516509 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Cambiaghi TD, Pereira CM, Shanmugam R, Bolech M, Wek RC, Sattlegger E, et al. (2014) Evolutionarily conserved IMPACT impairs various stress responses that require GCN1 for activating the eIF2 kinase GCN2. Biochem Biophys Res Commun 443: 592–597. doi: 10.1016/j.bbrc.2013.12.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild-type and gcn2Δ cells were treated with 100 μg/ml cycloheximide for 10 min as indicated and UV irradiated.
(TIF)
The same samples as shown in (A) Fig 2B and (B) Fig 3B were analyzed by immunoblotting using an antibody against total eIF2α.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.