Abstract
The oral cavity is home to unique resident microbial communities whose interactions with host immunity are less frequently studied than those of the intestinal microbiome. We examined the stimulatory capacity and the interactions of two oral bacteria, Porphyromonas gingivalis (P. gingivalis) and Fusobacterium nucleatum (F. nucleatum), on Dendritic Cell (DC) activation, comparing them to the effects of the well-studied intestinal microbe Escherichia coli (E. coli). Unlike F. nucleatum and E. coli, P. gingivalis failed to activate DCs, and in fact silenced DC responses induced by F. nucleatum or E. coli. We identified a variant strain of P. gingivalis (W50) that lacked this immunomodulatory activity. Using biochemical approaches and whole genome sequencing to compare the two substrains, we found a point mutation in the hagA gene. This protein is though to be involved in the alteration of the PorSS/gingipain pathway, which regulates protein secretion into the extracellular environment. A proteomic comparison of the secreted products of the two substrains revealed enzymatic differences corresponding to this phenotype. We found that P. gingivalis secretes gingipain(s) that inactivate several key proinflammatory mediators made by DCs and/or T cells, but spare Interleukin-1 (IL-1) and GM-CSF, which can cause capillary leaks that serve as a source of the heme that P. gingivalis requires for its survival, and GM-CSF, which can cause epithelial-cell growth. Taken together, our results suggest that P. gingivalis has evolved potent mechanisms to modulate its virulence factors and dampen the innate immune response by selectively inactivating most proinflammatory cytokines.
Introduction
Humans harbor diverse microbial communities in various tissues, and these microorganisms can dramatically alter the local and systemic immune functions. The bi-directional interactions between the microbiome and the immune system have been extensively studied in the gut and skin, but relatively little is known about such events in the oral cavity [1–3], which is exposed to an extraordinarily wide variety of external agents. In addition to encounters with food, air and fluids from other individuals, the microbiome of the oral cavity includes over 700 species of microbes, some of which are thought to be involved in pathological conditions ranging from caries to cancer [4–7]. Among the oral microbiota, Porphyromonas gingivalis (P. gingivalis) is relatively well studied because of its association with chronic inflammation of gum tissue (periodontitis) [8, 9]. Although P. gingivalis, by itself is not sufficient to cause gingivitis or bone loss [10], it has been classified as “keystone pathogen” [11] based on the findings that it seems to be the essential component of the microbial community that causes oral disease [10].
An intriguing possibility is that P. gingivalis sets the stage for the entire cascades of disease by altering the local immune microenvironment, thus allowing pathobionts to subsequently colonize or overgrow [12]. There are supportive evidence for this notion that has come from studies on P. gingivalis interactions with cells of the immune system. It had been suggested that P. gingivalis Lipopolysaccharide (LPS) skews the immune responses away from an inflammatory TH1-type response and towards a semi-TH2-like response [13, 14]. There are also data showing that P. gingivalis can directly act on complement system through its arginine-specific gingipains to block antimicrobial response [10, 15–17]. LPS is not the only molecule in a bacterium’s arsenal. We therefore asked if intact P. gingivalis might have even stronger effects on the activation of Dendritic Cells (DCs), one of the earliest immune cells to be activated in an immune response.
Because P. gingivalis has been shown to need companion or “bridge” organisms [10], we also used the oral commensal Gram-negative anaerobe, F. nucleatum that co-aggregates with, and promotes the colonization of periodontitis-associated bacteria such as P. gingivalis [18, 19]. Although F. nucleatum primarily colonizes the human oral cavity, it has been associated with diseases of various other organs, including periodontal, lung, abdominal and gynecological infections [20–23]. We postulated that comparing the two oral microbes for their direct impact on DCs, alone and in combination, might offer some insight into how individual components of the oral microbiota might contribute to control DC activation and its downstream consequences on T cell effector functions.
Using phenotypic and functional assays, we showed that P. gingivalis induced very low levels of inflammatory gene expression in DCs, in contrast to F. nucleatum and E. coli, which were potent activators. In addition, P. gingivalis inactivated a broad assortment of human and mouse cytokines and chemokines that are normally produced by activated DCs, thus further dampening the inflammatory cascade. We compared a mutant strain of P. gingivalis (W50) to the standard strain obtained from American Type Culture Collection (W50-ATCC). We discovered that a mutant strain has markedly stronger immunosuppressive properties compared to W50-ATCC, and was able to degrade a wide variety of mouse and human cytokines and chemokines.
Taken together, our data suggest that P. gingivalis has evolved the ability to operate somewhat under the immunological radar. It is a very weak activator of DCs. It also dampens DC activation by other bacteria, and it backs that up with the ability to degrade almost all of the cytokines and chemokines released by activated DCs and T cells. Such immunomodulatory mechanisms may underlie P. gingivalis ability to operate as a “keystone pathogen” in the oral cavity.
Materials and methods
The authors have declared no conflicts of interest.
Mice
Adult 8-14-wk-old male and female B10.A RAG2-/-, mice and 5C.C7 RAG2-/- TCR transgenic mice [containing T cells specific for peptide 88–103 of moth cytochrome c (MCC)] were purchased from the NIAID/Taconic Farms, Inc. exchange. Mice were housed in the NIH animal facilities (an AAALAC accredited facility) in a specific pathogen-free barrier colony that is certified by the United States Office of Laboratory Animal Welfare (OLAW) under the guidance of the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals. All studies were carried out in accordance with protocols LIG-32 and LCMI-14E approved (5/01/2013) by the Institutional Animal Care and Use Committee (IACUC) of the NIAID, NIH. All experimental protocols involving mice in this paper were specifically stated and approved by the IACUC in LIG-32 or LCMI-14E. The mice euthanasia protocol approved under this protocol involves CO2 asphyxiation followed by cervical dislocation. In general mice are placed in a 20% Co2 displacement unit until they are unconscious and then the Co2 is increased to 100%, followed by cervical dislocation as a secondary method.
Media and reagents
Bacterial Lipopolysaccharide (LPS) was derived from E. coli Serotype 0127:B8 (Sigma Aldrich). All recombinant cytokines were obtained from Peprotech Inc. Cells were cultured in complete medium [(Iscove’s Modified Dulbecco’s Medium (IMDM: GibcoBRL)] supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS: tested to be free of endotoxin, mycoplasma, virus, and bacteriophage; GibcoBRL), L-glutamine, 55 μM 2ß-Mercaptoethanol (GibcoBRL), penicillin, streptomycin, and gentamicin (Biosource). The antibiotics prevented any bacterial growth in our cultures, even when we added large numbers of living bacteria.
Cytokine measurements
Cytokines in the culture supernatant (CSN) were quantitated using SearchLight Multiplex cytokine array (Aushon Biosystems, Billerica, MA). IL-12 heterodimer (IL-12p75) was specifically detected by using the ELISA kit Quantikine (R&D Systems).
Generation of BMDCs
Briefly, bone marrow cells were flushed out of the femurs and tibias of 8-12-wk-old mice into complete medium and pipetted vigorously to make a single cell suspension, then passed through a cell strainer (70-μm Nylon mesh; BD Falcon TM). Erythrocytes were lysed using ACK lysis buffer (Biosource) and cells washed 2x with complete medium and cultured at 1x106 cells in 2ml/well in a 24-well plate with complete medium supplemented with 30U/ml GM-CSF and 60U/ml IL-4 for 6 days as described previously [24].
In vitro generation of antigen-activated/primed 5C.C7 CD4+ T cells
Spleens from B10.A/SgSnAi TCR–Cyt 5C.C7-1 RAG-2-/- mice were dissociated into homogenous single cell suspensions and passed through a cell strainer (70-μm Nylon mesh) from BD FalconTM. Erythrocytes were lysed using ACK lysis buffer (Biosource). Splenocytes were washed 3x in cold complete medium and adjusted to 1x106/ml, then cultured in complete medium plus 1 μM of the Moth Cytochrome C (MCC) 88–103 peptide at 37°C with 5% CO2. Fresh medium was added after 3 days of culture. After 5 days, the cultured cells were harvested, washed 2x with medium, and re-cultured in the absence of peptide with 10–20 U/ml of recombinant mouse (rm) IL-2. The medium was replenished every 5–7 days with fresh medium plus rmIL-2. For T-DC coculture experiments, these CD4+ T cells were purified with a CD4+ T cell isolation kit containing anti-CD8α, CD11b, CD45, DX5, and Ter-119 mAbs for depletion (Miltenyi Biotech.) with the addition of anti-CD11c and anti-class-II (Miltenyi Biotech). We used 3–4 LS columns in tandem instead of using LD columns to deplete the cells. These T cells, which were >95% CD4+, were used at various days of culture as a source of in vitro primed/antigen-activated T cells.
DC/T cell coculture
1x105 bead depleted (CD4+ T cell isolation kit, Miltenyi Biotech) cultured 5C.C7 CD4+ T cells were incubated with 2x104 BMDCs/well from B10.A RAG-/- mice ±0.1μM moth cytochrome c (MCC) peptide 88–103 in triplicate in 96-well u-bottom plates in a final volume of 200 μl/well for 48 h. The supernatants were removed and kept frozen at –30°C before measuring the cytokines by ELISA.
DC/bacteria coculture and treatment
In coculture experiments, 5x107 bacteria were added to the 24-well plate containing BMDCs for 16 h. For bacterial supernatant (SN) preparation, ~1011 bacteria were harvested in the exponential stage of growth, washed twice with PBS, and incubated at 37°C in 5 ml PBS for 1 h. The cells were then spun at 4500 rpm for 15 min and the SN filtered through a 0.22-μm syringe filter before being used (bacteria SN). In order to treat cytokines, 200 μl of the bacteria SN or ~109 whole bacteria were added to 1 ml of complete medium (containing 10% FCS) spiked with various cytokines and kept at 37°C for 24 h before quantitating residual cytokines with cytokine array.
FACS analysis
All steps were done on ice or in 4°C centrifuges to prevent the sudden upregulation of MHC class II caused by handling [25]. Plates containing DC cultures were cooled for 20 min. The cells were then gently harvested, spun, counted and distributed into 96 well plates at about ~106 per well. We added 10 μl “ultrablock” (a mixture of 1:1:1 mouse, rat and goat serum containing 10μg/ml of the monoclonal anti-FcR antibody 2.4G2) for 10 min before adding a master-mix of a combination of antibodies anti-mouse CD80, CD40, CD11c and MHC class II (BD Pharmingen). Each antibody was titrated for optimal signal-to-background before use. After 20 min, the cells were washed and resuspended in PBS containing the viability dye, 7AAD plus 1% FCS, for 5 min, then centrifuged and resuspended in FACS buffer for analysis.
Bacteria culture and strains
P. gingivalis used in this study 53978 (W50), BAA-308 (W83), ATCC33277, and F. nucleatum, were obtained from American Type Culture Collection (ATCC). P. gingivalis W50-NIDCR was a generous gift from Dr. P. Kolenbrander in the National Institute of Dental and Craniofacial Research (NIDCR). All oral bacteria were grown anaerobically (GasPak EZ; BD, Sparks, MD) in BactoTM Brain Heart Infusion (BD, Sparks, MD or Anaerobe Systems Morgan Hill, CA), supplemented with 5 mg/L hemin and 1 mg/L vitamin K. E. coli were grown in Luria Bertani Broth (K.D Medical Columbia, MD). Bacteria were harvested in the exponential stage of their growth and washed 2x with PBS before being used at an O.D. of 600 nm.
Heat-killed P. gingivalis
P. gingivalis was harvested in the exponential stage of growth and immediately incubated in a 65°C water bath for 1 h and washed 2x with PBS before coculturing with BMDCs.
Gingipain enzymatic assay (Rgp)
To assess the gingipain activity of P. gingivalis strains, 20 μl of bacterial supernatant was mixed with Nα-Benzoyl-DL-arginine β-naphthylamide hydrochloride (BANA) substrate and 100 μl of 100 mM Tris-HCl buffer pH 7.4 (with 2mM dithiothreitol), and incubated at 37°C for 2 h. Optical densities at 410 and 600 nm were measured every 5 min. using a PowerWave HT (Biotek) spectrophotometer. Assays were performed three times in triplicate.
Two-dimensional difference in gel-electrophoresis and one-dimensional gel
2.5 ml of cell-free bacterial SN were concentrated (10x) using Amicon Ultra-4 (10,000 MWCO) (Millipore) by spinning at 2500xg for 15 min at 4°C. The concentrated SN was separated by SDS-PAGE for one-dimensional gel and visualized by Coomassie or silver staining and two-dimensional gel was analyzed by a service contract (Applied Biomics, Hayward CA) as previously described [26]. Given the robustness of the biological/enzymatic assays we observed, the proteomic analyses were performed once each.
Quantitative RT-PCR
BMDCs were cultured in a 24-well plate for 6 h with 108 bacteria/well and RNA was isolated from the cells by RNeasy kit (Qiagen). RNA was treated to remove DNA contamination (Invitrogen) and the cDNA was synthesized using random primers, and reverse transcribed using Superscript III (Invitrogen). Real-time PCR analyses were performed for expression of IL-1 α, IL-1β, TNF α, IL-6, IL-12p40, IL-10, IL-23p19, IL-12p35, and using Taqman gene expression primers and probes mix (Applied Biosystems). The mRNA expression levels were calculated as dCt = Target-Actin B/nono, where Actin B/nono was the endogenous control.
Mass spectrometry and data analysis
Peptides were identified from mass spectroscopy (MS) data using SEQUEST algorithms and a minimum of two peptides. Peptide thresholds of 95% were used for identification as previously described [26].
Statistical analysis
All statistical analyses were performed using Prism (GraphPad Software).
Results
P. gingivalis is a poor activator of DCs
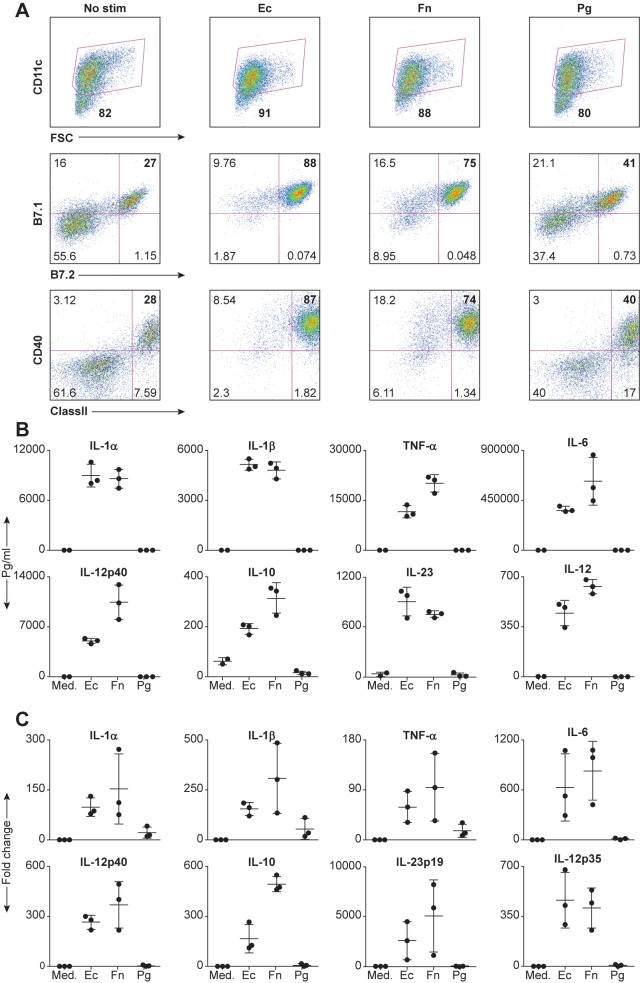
It is well established that DCs respond to various bacterial products from gut bacteria such as E. coli by upregulating cell surface costimulatory molecules and rapidly secreting cytokines and chemokines. To compare the ability of oral bacteria to elicit these functional responses by DCs, we used bone marrow-derived DCs (DCs) obtained from Recombinant Activating Gene-2 deficient mice (RAG2-/-), which lack T and B cells, to eliminate the possibility of B and/or T cell contamination (which might alter the function of the DCs). We stimulated the DCs with P. gingivalis and F. nucleatum, two different oral bacteria species that have been shown to co-aggregate in vitro [18, 27], and with E. coli and assessed their activated status 16 hours later. Fig 1A shows that nearly 100% of the DCs stimulated with E. coli upregulated the co-stimulatory molecule B7.1, and nearly 90% upregulated B7.2, CD40 and the antigen-presenting molecule, MHC class II. The oral anaerobe F. nucleatum was slightly less active, while P. gingivalis stimulated only about 12–15% of the DCs over the background.
Fig 1. Inflammatory cytokine production from DCs is extinguished in the presence of P. gingivalis.
(A) DCs from B10.A-Rag2-/- mice were either unstimulated (medium) or stimulated on the sixth day with 5x107 bacteria in a 24-well plate for 16h at 37°C. Cells were harvested, stained with directly labeled monoclonal antibodies and analyzed by FACS analysis. Cells were gated for the CD11c positive population and analyzed for the costimulatory molecules B7.1, B7.2 and CD40, and the antigen-presenting molecule, MHC class II. Numbers indicate the percentage of the population in the gate or quadrant (B) Same as (A) Culture CSN were analyzed for the presence of various cytokines using Searchlight protein arrays. (C) Same as (B) except the co-culture period was 6h, at which time we isolated total RNA using the RNeasy kit and analyzed it by Real-time RT-PCR. The data are expressed as the mean ± SD of three independent experiments.
We saw dramatic differences when we analyzed the secretion of cytokines by the activated DCs. Fig 1B shows that P. gingivalis failed to induce any of these cytokines from DCs, while both E. coli and F. nucleatum were potent inducers. In some cases, the cytokine levels in P. gingivalis-stimulated cultures were even lower than those secreted by the untreated controls.
To begin to uncover the mechanism by which P. gingivalis blocks cytokines, we started by determining whether it can block transcription. We used qRT-PCR to analyze the amount of cytokine mRNA in DCs after 6 hrs of stimulation with various bacteria. As shown in Fig 1C, both E. coli and F. nucleatum induced greater than 100-fold changes in the mRNA expression of various cytokines in DCs. In contrast, P. gingivalis induced only very low levels of mRNA for IL-1 α, IL-1 β and TNF α, and no mRNA for the other cytokines. Thus, P. gingivalis is a very poor activator of DCs.
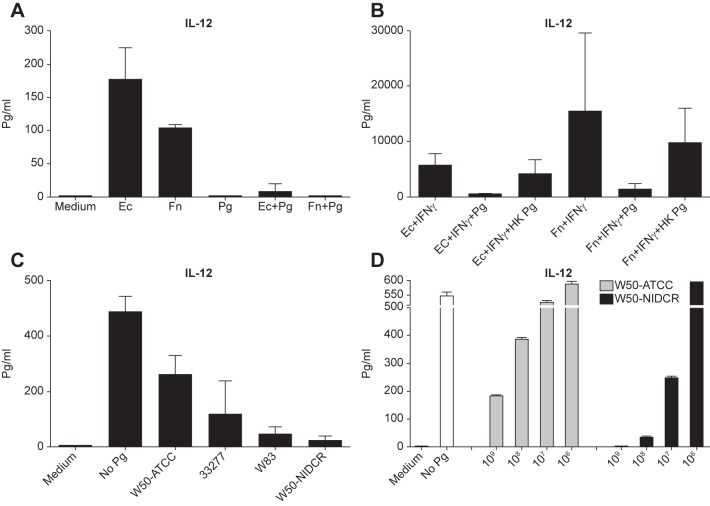
To determine whether P. gingivalis was merely a weak activator of DCs or whether it might actually be suppressive, we asked whether P. gingivalis could modulate cytokine secretion by DCs activated by E. coli or F. nucleatum. We stimulated DCs with E. coli or F. nucleatum, plus IFNγ(strong stimulus for IL-12 production) [28], either alone or in combination with 5x107 P. gingivalis bacteria and found that the addition of P. gingivalis severely impaired the levels of IL-12 produced by the DCs (Fig 2A). The bacterium did not simply kill the DCs, as they remained viable (excluded Trypan blue) by stimulating T helper cells and receive help (S1 Fig). In addition, because it had been shown previously that the LPS of P. gingivalis does not stimulate strong inflammatory responses from DCs [13], we asked if the suppressive activity of P. gingivalis was due to its unique LPS [29] by asking if it was sensitive to heat. Fig 2B shows that the inhibitory activity was markedly reduced in DC cultures given heat-killed P. gingivalis. Thus, in addition to having a unique LPS, P. gingivalis also expresses a suppressive activity that is heat-labile, suggesting that the suppressive activity is protein.
Fig 2. Differential effect of P. gingivalis W50 substrains on extinguishing cytokine production from DCs.
(A) DCs from B10.A-Rag2-/- mice were either unstimulated (medium) or stimulated on the sixth day with 5x107 E. coli, F. nucleatum or P. gingivalis alone or a mixture of two bacteria together in a 24-well plate for 16 h at 37°C. CSN were analyzed for the presence of IL-12p75 using a specific ELISA. (B) Same as (A) except that IFNγ was added to the cultures to enhance IL-12 production. P. gingivalis were heat-killed at 60°C for 1 h in a water bath (HKPg), or not, before being added at 5x107/well to the culture containing F. nucleatum or E. coli. (C) DCs were stimulated with 100 ng/ml of LPS (E. coli) plus 100 ng/ml of IFNγ alone (No Pg) or in combination with various strains of P.gingivalis at 5x107/well for 24 h before testing for the presence of IL-12p75 in the CSN. (D) Same as (C) except various numbers of P. gingivalis W50-NIDCR or -ATCC were added to the DCs in a 24-well plate. (A-D) Data are the mean ± SD of compilation of two independent experiments. (D) The data are expressed as the mean ± SD of duplicates.
Immune modulation by P. gingivalis shows strain-dependent phenotypic polymorphisms
To identify the genetic loci associated with P. gingivalis suppressive function, we examined several strains of P. gingivalis to determine whether there might be genetic variation among them that would allow us to locate relevant gene(s). Thus far, we had used a W50 strain [a gift of Dr. P. Kolenbrander, National Institute of Dental and Craniofacial Research, (NIDCR)], referred to hereafter as W50-NIDCR. To test whether the suppressive activity was unique to this strain, we compared it to three other strains of P. gingivalis (W83, W50, and 33277) from the American Type Culture Collection (ATCC). We stimulated DCs overnight with E. coli LPS plus IFNγ [28], in the absence or presence of each P. gingivalis strain, and measured IL-12 protein in the CSN.
Fig 2C shows that W50 from ATCC (hereafter refer to as W50-ATCC) was considerably less potent than W50-NIDCR at reducing IL-12 production (Fig 2C), while strain W83 was only slightly less inhibitory than W50-NIDCR. Fig 2D shows that, when the two different W50 P. gingivalis were titrated, W50-NIDCR was almost 100 times more potent than W50-ATCC at inhibiting IL-12 production.
Whole genomic sequencing and comparison of P. gingivalis W50-NIDCR and W50-ATCC
The evolutionary adaptation of a microbe to its host often involves multiple genetic modifications. To determine the mechanism by which W50-NIDCR was able to potently modify its virulence factors, we took a genomic approach to compare the four P. gingivalis strains and as a control, we compared them to E coli.
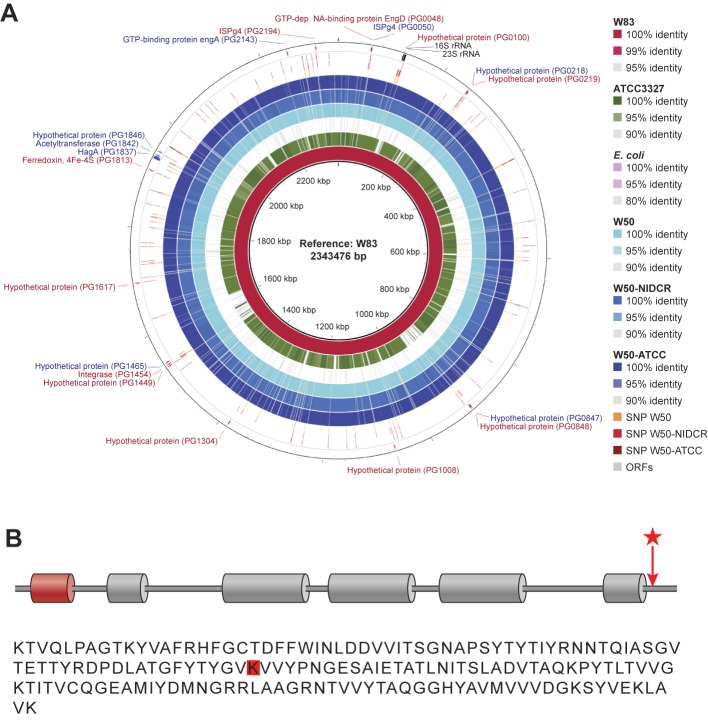
First, we sequenced the whole genomes of W50-NIDCR, W50-ATCC and ATCC 33277, and assembled contigs using the reference genome from the previously sequenced W83 strain (Fig 3A). Across the entire genome of the W50-NIDCR, we were able to identify unique single nucleotide polymorphisms (SNPs) in 20 different loci by BRESEQ analysis. Two of these loci were the 16S and 23S rRNA, while SNPs in 4 of the other loci fell into non-coding or intergenic regions. Thirteen of the remaining 14 loci included a high concentration of SNPs in multiple predicted protein coding regions with yet unknown function.
Fig 3. Mutation in W50-NIDCR HagA causing alteration in the PorSS/gingipain pathway.
(A) Draft genomes of NCBI W50 and two W50 variant strains (W50-ATCC and W50-NIDCR) were mapped to the reference genome of W83 to illustrate the sequence similarities between these two genomes and the reference. As controls, genomes of P. gingivalis 33277 and E. coli were also included in these analyses. The three outer rings (SNP W50, SNP W50-NIDCR, and SNP W50-ATCC) show the SNP loci indentified by the software “breseq.” Those that are unique among the three genomes are shown with the +/- affected genes and their annotations in the outer most ring (ORFs) and their annotations. The map was drawn using “BRIG” genome alignment software. (B) Red cylindrical domain is a peptidase C25 C-terminal domain, grey cylindrical domains are cleaved adhesin domains, and the red star/arrow indicates the location of W50-NIDCR SNP. Not depicted are the Fibronectin Type III domains, which encompass the cleaved adhesin but extend further in the N- and C-terminal directions. K75* in EIW91729.1 (partial PrtH domain) is analogous to K2074* in AAQ66831.1 (HagA). SNP occurs within the Fibronectin Type III domain at N-terminus of the protein. The raw sequences used in this study have been deposited to the NCBI Short Reads Archive (SRA) under the umbrella BioProject ID PRJNA302472 (http://www.ncbi.nlm.nih.gov/bioproject/PRJNA302472).
The 14th was a SNP difference in the coding region of the hagA gene, which encodes hemagglutinin A, a 280-kDa protein encoded by an 8 Kb gene containing multiple large direct repeat units [30, 31]. A single A→T transversion in this locus in W50-NIDCR resulted in a codon change (AAA to TAA) yielding a premature stop codon after amino acid 75 in the open reading frame of the HagA gene (Fig 3B).
The differential proteome of strains W50-NIDCR and W50-ATCC reveal alterations in the proteolytic machinery
HagA is an outer membrane protein that has cysteine proteinase adhesin domains involved in adherence to host cells and to other bacteria [32, 33]. It is also a major virulence determinant in P. gingivalis [34]. Moreover, during periodontal disease, antibodies targeted the protein products of HagA [35–37]. In addition to its role in adhesion, there is some evidence that HagA may have a regulatory effect on the processing and/or secretion of proteins [38]. We therefore used a proteomic approach to look for proteins that might be differentially secreted by W50-NIDCR and W50-ATCC.
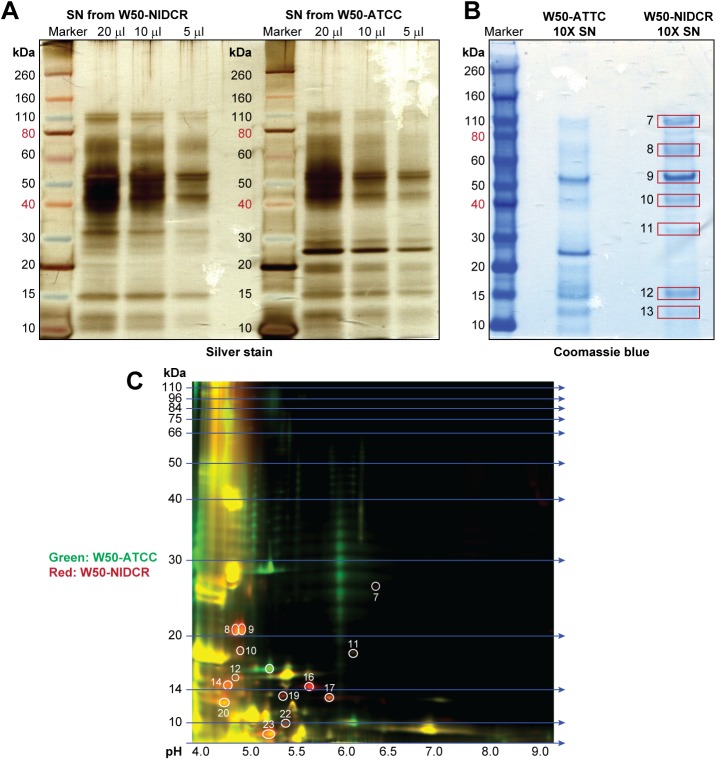
We compared cell-free SNs from each strain, resolved the proteins on one-dimensional SDS-PAGE and revealed by silver staining (Fig 4A). This preliminary analysis suggested that there were indeed unique differences in proteins of different molecular weights secreted by each strain. To further identify these proteins, we concentrated the SNs and visualized the resolved protein bands by Coomassie blue staining (Fig 4B). We excised total of 13 spots (Coomassie gel) from W50-ATCC and W50-NIDCR combined and processed them for identification by liquid chromatography-tandem mass spectrometry (LC-MS). However, we focused only on those proteins that unique to W50-NIDCR in comparison to W50-ATCC, which are marked 7–13 (Fig 4B) while the complete comparison is listed in S2 Fig. These bands contained peptides from both arginine- and lysine-specific gingipains (Table 1), a complex set of “trypsin-like” cysteine and arginine proteases that contribute to the degradation of various host proteins [39, 40]. One of these proteins (immunoreactive 61 kDa antigen PG91) was found in multiple bands (bands 7–13) with no known function. The reason for the presence of this protein in multiple bands is indicative of either posttranslational modification of PG91 or that it might be a target of other active proteolytic enzymes in W50-NIDCR (Table 1). The other non-gingipain protein identified in the W50-NIDCR supernatant was Omp28—an outer membrane protein expressed by a wide variety of Gram-negative bacteria, including P. gingivalis isolates [41]. Many outer membrane proteins are part of the secretory system (small size vesicles 20–500 nm), which are the means by which bacteria disseminate their virulence factors into the extracellular milieu to modulate host immune responses [42], and have been shown to contain gingipain and other proteolytic enzymes [43].
Fig 4. Comparative proteomic of particle-free SN from W50-NIDCR and W50-ATCC.
(A) Various amounts of particle-free SN from W50-ATCC and W50-NIDCR were resolved by one-dimensional SDS-PAGE and protein bands were visualized by Silver staining. (B) Coomassie staining of the same SNs that had been concentrated to 10X as described indicated in the materials and methods. (C) Same as (A) except particle-free bacterial SN from W50-ATCC and W50-NIDCR were labeled green or red, respectively, and resolved by two-dimensional difference in-gel electrophoresis.
Table 1. Summary of proteins identified using Mass Spectrometry of 1D gel bands comparing W50-NIDCR and W50-ATCC. Ranks 3–9 are unique to W50-NIDCR.
| Rank | Accession | Protein | Spots | Total number of Peptides |
|---|---|---|---|---|
| 1 | CAA10226.1 | RagA | 7,8,9,10,11 | 135 |
| 2 | Q51817.1 | Lys-gingipain | 7,8,9,10,11,12,13 | 270 |
| 3 | AAD51076.1 | Immunoreactive 61kD antigen PG91 | 7,8,9,10,11,12,13 | 102 |
| 4 | AAD51843.1 | Outer membrane protein Omp28A | 11 | 18 |
| 5 | AAD51078.1 | Immunoreactive 84kD antigen PG93 | 8 | 8 |
| 6 | P0AA25.2 | Thioredoxin (Trx1) | 8 | 4 |
| 7 | AAF03904.1 | Heme-binding protein FetB | 11 | 2 |
| 8 | Q7MXT8.1 | Bifunctional enzyme LpxC/FabZ | 12 | 2 |
| 9 | AAG24228.1 | Putative outer membrane protein PG57 | 13 | 2 |
We decided to further characterize the proteome of the SN obtained from the two W50 strains at higher resolution using two-dimensional difference in gel-electrophoresis (2D DIGE). Therefore, we analyzed the two W50 SNs by labeling them green for W50-ATCC and red for W50-NIDCR (Fig 4C). The protein spots that showed differential abundance between W50-ATCC and W50-NIDCR were excised and subjected to LC-MS analysis. Table 2 lists a number of distinct proteins (13 spots containing at least 3 peptides. comprising 35 proteins) that were highly enriched in W50-NIDCR (orange and red spots) compared to W50-ATCC (green). Like the 1D analysis, the 2D gel data showed that the supernatant of W50-NIDCR showed more gingipain when compared to the supernatant of W50-ATCC, and that many of the 35 proteins identified from the SN were either gingipain or involved in the processing of gingipain (Table 2). This analysis also revealed an over-expression of several proteases that are associated with the secretory system (PorSS/gingipain pathway). These include multiple gingipains (Rgp and Kgp), PrtH domain proteases, various hemagglutinins, and the majority of these proteins contained C-terminal domains (CTDs) that are usually processed by PorSS/gingipain pathways.
Table 2. Summary of proteins identified by tandem mass spectrometry from 2D gel.
Differential bands were excised from W50-NIDCR (highly enriched red/orange spots) and identified using mass spectrometry (MS). The spots corresponding to each protein, and the total number of peptides identified as belonging to the protein during the MS analyses, are shown in columns 3 and 4.
| Accession | Protein | Spots | Total number of Peptides |
|---|---|---|---|
| WP_005874718.1 | SusC/RagA family TonB-linked outer membrane protein | 9,14,20,23 | 37 |
| AAB49691.1 | hemagglutinin | 22 & 10 | 17 |
| P46071.1 | Protease PrtH | 10 & 22 | 17 |
| AAX47719.1 | RagA3 | 8,9,20 | 14 |
| WP_012457436.1 | T9SS C-terminal target domain-containing protein | 7 | 13 |
| XP_001386199.1 | hypothetical protein PICST_73499 | 19 | 13 |
| WP_005873522.1 | zinc carboxypeptidase | 7 | 12 |
| WP_004583425.1 | membrane protein | 12 & 14 | 11 |
| WP_012457815.1 | collagen-binding protein | 19 | 11 |
| AAX47723.1 | RagA2 | 9, 11, 20 | 11 |
| WP_012457400.1 | SusC/RagA family TonB-linked outer membrane protein | 9,10,20 | 11 |
| AAA69539.1 | Arg-gingipain-1 proteinase | 22 | 10 |
| AAC18876.1 | arginine-specific thiol protease precursor | 22 | 9 |
| WP_010956404.1 | hypothetical protein | 7 | 9 |
| XP_001010368.1 | hypothetical protein TTHERM_00354700 | 20 | 9 |
| WP_012457845.1 | hypothetical protein (old name TPR domain protein) | 16 | 9 |
| WP_012458653.1 | peptidase C25 (old name arginine-specific cysteine proteinase RgpA) | 22 | 8 |
| AAD01810.1 | hemagglutinin/protease | 22 | 8 |
| WP_004585461.1 | hypothetical protein | 16 | 8 |
| WP_010956068.1 | peptidase (old name extracellular protease) | 12 | 7 |
| P72197.1 | Lys-gingipain, catalytic subunit;39 kDa adhesin | 10 | 7 |
| Q51817.1 | Lys-gingipain W83; PrtK48; Lys-gingipain catalytic subunit | 10 | 7 |
| CAA57997.1 | protease precursor | 22 | 7 |
| AAX47715.1 | RagA4 | 12 and 9 | 7 |
| WP_012458254.1 | peptidase | 12 | 6 |
| YP_003987329.1 | putative ankyrin repeat protein | 23 | 6 |
| ZP_04009894.1 | cell wall surface anchor family | 16 | 5 |
| CAN81791.1 | hypothetical protein VITISV_020569 | 20 | 5 |
| Q51817.1 | Lys-gingipain W83; Lys-gingipain catalytic subunit;39 kDa adhesin | 22 | 5 |
| CAA68897.1 | TonB-linked adhesin | 22 | 5 |
| AAR37428.1 | hypothetical protein MBMO_EBAC750 | 20 | 4 |
| AAS68176.1 | Lys-gingipain | 22 | 4 |
| NP_721075.1 | methyltransferase | 23 | 4 |
| WP_015836135.1 | hypothetical protein | 20 | 3 |
| WP_005874011.1 | hypothetical protein | 17 | 3 |
Supernatant from P. gingivalis W50-NIDCR but not W50-ATCC contains high levels of arg-gingipain activities
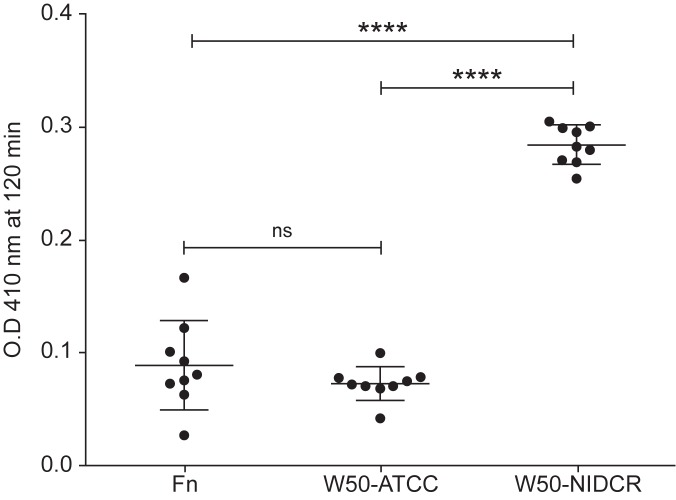
To directly test the levels of gingipain in the supernatants of W50-NIDCR, W50-ATCC, (and F. nucleatum as a control) we used an enzyme assay specific for Arg-gingipains (Rgp) [44] and found that the SN from W50-NIDCR contained significantly more gingipain than the supernatants of W50-ATCC and F. nucleatum (Fig 5). Importantly, various P. gingivalis strains possess membrane-associated gingipain activities on the cell surface, and secretion of gingipain from P. gingivalis has not been widely reported yet [40] except in one strain (HG66) [45, 46]. Collectively, these data suggest that the mutation in W50-NIDCR results in an alteration in PorSS/gingipain secretory pathway and increased secretion or translocation of gingipain from the bacterial outer membrane and its accumulation in the extracellular environment. Alternatively, it is also plausible that this mutation affects gingipain surface attachment. These would need future studies to clarify
Fig 5. Measuring arginine gingipain in the bacterial SN using a gingipain-specific enzyme assay.
20 μl of supernatant from various bacteria were mixed with the substrate Nα-Benzoyl-DL-arginine β-naphthylamide hydrochloride substrate in 100 μl of Tris buffer plus dithiothreitol (D.T.T) and incubated at 37° C for 2 h. Optical density at 410 and 600 nm were measured with a spectrophotometer. These data are expressed as the mean ± SD of multiple wells and are representative of two independent experiments. Significance was tested using a one-way ANOVA. ****P< 0.0001
Supernatants from P. gingivalis cultures degrades mouse and human cytokines and chemokines
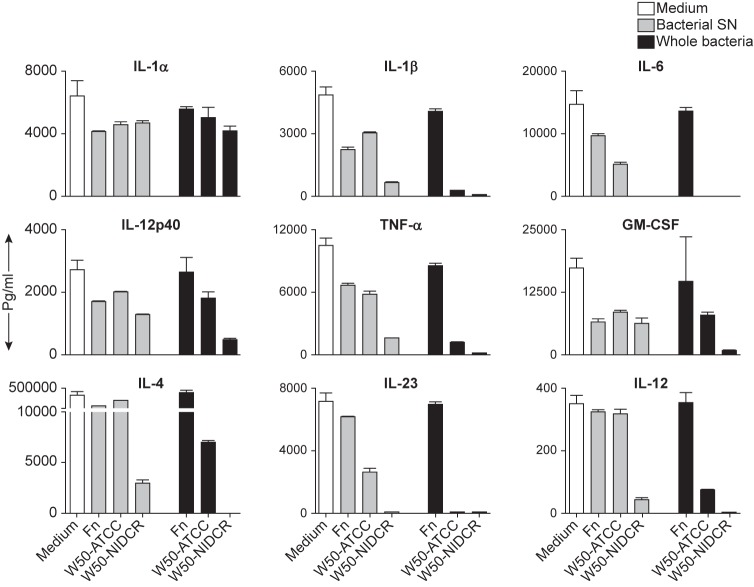
The data so far suggested that the lack of cytokine production we had seen at the mRNA level might not be the only mechanism by which P. gingivalis suppresses immunity, but that P. gingivalis seems to actively secrete proteases that act directly on the cytokines produced by activated immune cells. To test this, we obtained a large set of recombinant mouse cytokines, diluted them to known concentration, pooled them and spiked the sample with either cell-free culture supernatants or intact bacterium from F. nucleatum, W50-ATCC and W50-NIDCR, incubated them for 22 hours at 37°C, and measured the remaining cytokine levels. Fig 6 show that the supernatants from W50-NIDCR destroyed all of the tested cytokines except IL-12p40, GM-CSF and IL-1 α. The intact bacteria were even more potent, destroying both IL-12p40 and GM-CSF while still having little effect on IL-1 α.
Fig 6. Supernatants of P. gingivalis destroy mouse cytokines.
Various recombinant mouse cytokines were pooled and then spiked into a single sample of 800 μl of complete medium (contained 10%FCS) in a 24-well plate in the absence (medium) or presence of 200 μl of particle-free bacteria SN or ~1x108 whole bacteria and incubated at 37°C for 22 h, at which time the CSN was tested for the presence of cytokines using a multiplex cytokine array. These data are expressed as the mean ± SD of duplicates and are representative of two independent experiments.
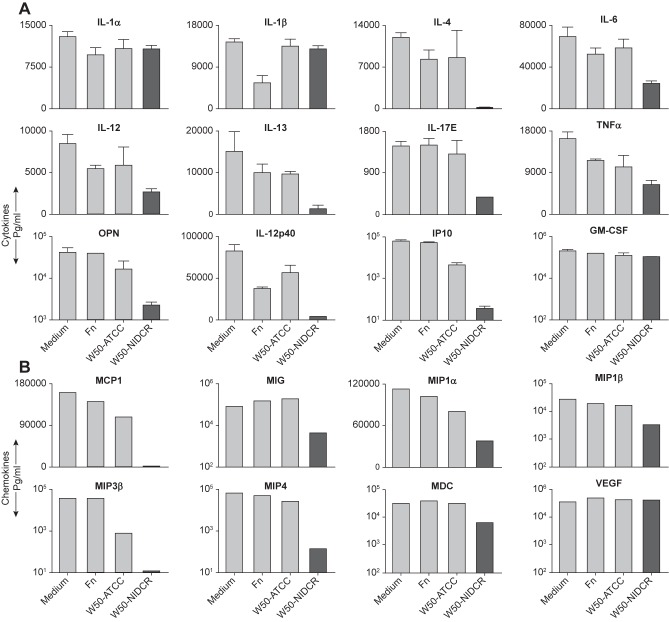
Since P. gingivalis is a human pathogen that does not normally infect mice, we asked if the destructive activity on mouse cytokines was an evolutionary useless feature or whether it would also hold for molecules involved in human immune responses. We tested a wide array of human cytokines and chemokines and found that all of them were sensitive to the action of the bacterial supernatant except IL-1 α, VEGF and GM-CSF (Fig 7). Collectively, these data demonstrate that inactivation of human cytokines are similar to mouse in which the supernatant from W50-NIDCR was more effective than that of W50-ATCC in inactivation of various human cytokines and chemokines.
Fig 7. P. gingivalis supernatants also destroy human cytokines and chemokines.
(A) Recombinant human cytokines were spiked and pooled into a single sample of 800 μl of complete medium (plus 10%FCS) in a 24-well plate in the absence (medium) or presence of 200 μl of particle-free bacterial SN and incubated at 37°C for 22 h, and the CNS were tested using human multiplex cytokine array. (B) Same as (A) except human chemokines were used. These data are expressed as the mean ± SD of representative of two independent experiments.
Discussion
The goal of this study was to examine the broad influence of oral bacteria on DC activation and cytokine production. In this study, we used two oral bacteria, P. gingivalis and F. nucleatum, and compared their properties to those of the well-studied bacterium E. coli. Using assays that directly measure DC functional responses, a series of genomic and proteomic analyses, a gingipain-specific enzyme assay and direct tests on mouse and human cytokines, we found that P. gingivalis dampens innate immune responses. First, P. gingivalis is not a strong activator of DCs. DCs cultured in the presence of P. gingivalis hardly upregulate costimulatory or antigen-presenting MHC molecules. They secreted only small amounts of inflammatory cytokines and did not contain high levels of mRNA for those cytokines in the presence of P. gingivalis. Second, P. gingivalis extinguishes DC activation induced by other bacteria. Because when DCs are activated by E. coli or F. nucleatum (a commensal oral bacterium) in the presence of IFNγ, they generally secrete copious amounts of inflammatory cytokines such as IL-12. However, if P. gingivalis was added to such cultures, the cytokine secretion induced by E. coli or F. nucleatum was suppressed. Because P. gingivalis secretes proteases such as the gingipains, that degrades many of the mouse and human cytokines that we had tested, this will ensure a non-response even if other local oral bacteria (such as F. nucleatum) induce immune responses. In fact, neighboring bacterial populations seem to benefit from the presence of P. gingivalis, as they tend to be increased in number (especially Staphylococcal, Streptococcal and Propionibacterial species), showing that the presence of P. gingivalis is not only a benefit to itself but also to other local bacteria [10].
Intriguingly, we found that P. gingivalis’s secreted products do not affect human IL-1 and GM-CSF (Fig 7A). In contrast, P. gingivalis had strong effects on mouse IL-1 β and GM-CSF (Fig 6). Whereas mouse IL-1 β contains an arginine (a target of gingipain) at position 126 (which would be exposed at the end of the first beta strand in the crystal structure of mouse IL-1 β(PDB id 8I1B), position 126 in the human IL-1 β is changed to threonine. Similarly, there are regions of mouse GM-CSF that contain arginine or lysine residues (also targets of gingipain) that have different amino acids in the respective position of the sequence in the human molecule; and these regions in the structure of mouse GM-CSF are on loops that can be easily targeted by the enzyme. There are several ways to interpret the resistance of the human molecules to the bacterial proteases. One possibility is that the human molecules may have evolved to resist degradation by gingipains as a mechanism to enhance inflammation to remove the bacterium. Another possibility is that the bacterium may have taken an advantage of changes in IL-1 to benefit its survival. Like many pathogenic bacteria, P. gingivalis require iron for its growth and virulence [47, 48], and this is accomplished by hydrolyzing hemoglobin [49, 50]. At high doses, IL-1 α causes capillary leakage [51, 52], which benefits the bacterium that relies on heme for its survival. Indeed, there are data suggesting that P. gingivalis induces high levels of IL-1 from human gingival epithelial cells [53]. Moreover, GM-CSF may also serve the bacterium, as it inhibits terminal differentiation of epithelial and endothelial cells and induces their growth [54, 55] thus, potentially providing a larger subgingival niche for the bacterium to survive. Combined, our data suggest that P. gingivalis evades/inactivate those molecules that will lead to its clearance and at the same time selectively spares those molecules that enhance its survival.
Finally, different studies on P. gingivalis have given different results. For example, it has been reported by Banchereau’s group, that the gums of people with gingivitis contained large numbers of immature/resting Langerhans cells [14], supporting our view that the bacterium is not an strong stimulator of DCs. In contrast, there are studies that have shown that very high doses of P. gingivalis LPS (doses that would have been lethal, had the LPS come from E coli), does stimulate DCs, but seems to prime T cells for the subsequent production of IL-5 rather than IFNγ [13, 14, 56]. In general, bacteria have more weapons that just the LPS. It has been shown that P. gingivalis (inside the cells) secretes a phosphatase (SerB) that prevents translocation of NF-kB to the nucleus (thus hindering cytokine secretion from gingival epithelial cells), several proteases that degrade complement components, and “capturing” ligands that bind complement antagonists [57]. Nevertheless, it has also been shown that in spite of the SerB-mediated suppression, gingival epithelial cells secrete inflammatory cytokines [58]. Our data suggest (perhaps not surprisingly) that the differences found from study to study might be due to the differences in the substrains of P. gingivalis that were used. The W50-NIDCR seems to be particularly effective at evading/dampening the secretion of innate and adaptive immune mediators.
Admittedly, this study has only begun to unravel what seems to be potentially deep nexus of interactions between W50 and DCs. While some of our observations suggest a linear correlation (eg. HagA and degradative ability of P. gingivalis supernatant), it is important to note that the biology of host-microbe interactions are complex. For instance, the composition of the P. gingivalis supernatant itself can be altered by vesiculation resulting from mutations in HagA secretory pathways. Indeed, as a reviewer has correctly pointed out, the mutation in the CTD region of HagA that we observed could also alter its localization. This can potentially short-circuit the ability of the PorSS pathway of CTD protein secretion, which will result in altered localization of gingipain proteinases and adhesins on the surface of bacterial cells. Future studies using more or less suppressive strain(s) isolated from patients may reveal which of these particular attributes correlate best with disease severity.
Supporting information
IL-12 can be elicited from resting or LPS-activated DCs by coculturing them with antigen-activated T cells [24]. To test whether DCs that have been cultured with P. gingivalis are viable or dead, we tested their ability to produce IL-12 when subsequently cocultured with activated 5C.C7 T cells [specific for Moth Cytochrome c (MCC)]. We also tested whether the presence of the bacteria in the DC/T-cell culture would inhibit secretion of IL-12. (A) DCs from B10.A-Rag2-/- mice were pre-activated with F. nucleatum, P. gingivalis or nothing (medium) for 20 h, then washed and cocultured at 2x104 cells/well with 5x105 cell/well antigen-activated 5C.C7 T cells in the presence of 0.1 μM MCC peptide 83–103 in a 96-well plate at 37°C for 48 h. CSN were analyzed for the presence of IL-12 using specific ELISA. (B) Same as (A) except 5x107 F. nucleatum or P. gingivalis were added to the coculture of DCs with T cells. These data are expressed as the mean ± SD of triplicate wells representative of two independent experiments.
(PDF)
(PDF)
Acknowledgments
We thank Drs. Susan Pierce and Thomas van Dyke for comments and critical review of the manuscript; Paul Kolenbrnader for discussion and providing P. gingivalis W50-NIDCR; Ethan Tyler and Alan Hoofring for illustrations.
Data Availability
All relevant data are within the paper.
Funding Statement
The Intramural Research Program at the National Institutes of Health, National Institute of Allergy and Infectious Diseases supported this work. N.J.S. is funded by the NIAID extramural grants R21AI126184 and R01AI110719. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(10):599–608. Epub 2012/08/22. nrgastro.2012.152 [pii] 10.1038/nrgastro.2012.152. doi: 10.1038/nrgastro.2012.152 [DOI] [PubMed] [Google Scholar]
- 2.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70. Epub 2012/03/20. S0092-8674(12)00104-3 [pii] 10.1016/j.cell.2012.01.035. doi: 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. Epub 2012/09/14. nature11552 [pii]10.1038/nature11552. doi: 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 4.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–44. Epub 1998/03/12. . [DOI] [PubMed] [Google Scholar]
- 5.Haffajee AD, Cugini MA, Tanner A, Pollack RP, Smith C, Kent RL Jr., et al. Subgingival microbiota in healthy, well-maintained elder and periodontitis subjects. J Clin Periodontol. 1998;25(5):346–53. Epub 1998/07/03. . [DOI] [PubMed] [Google Scholar]
- 6.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–83. Epub 2001/05/24. doi: 10.1128/JB.183.12.3770-3783.2001 ; PubMed Central PMCID: PMC95255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–32. Epub 2005/11/08. 43/11/5721 [pii]10.1128/JCM.43.11.5721–5732.2005. ; PubMed Central PMCID: PMC1287824. doi: 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–20. Epub 2012/09/01. 0022034512457373 [pii] 10.1177/0022034512457373. doi: 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- 9.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62(4):1244–63. ; PubMed Central PMCID: PMC98945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10(5):497–506. doi: 10.1016/j.chom.2011.10.006 ; PubMed Central PMCID: PMC3221781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darveau RP. The oral microbial consortium's interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28(8):389–95. doi: 10.1089/dna.2009.0864 ; PubMed Central PMCID: PMC2883565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajishengallis G, Lamont RJ. Dancing with the Stars: How Choreographed Bacterial Interactions Dictate Nososymbiocity and Give Rise to Keystone Pathogens, Accessory Pathogens, and Pathobionts. Trends Microbiol. 2016;24(6):477–89. doi: 10.1016/j.tim.2016.02.010 ; PubMed Central PMCID: PMC4874887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167(9):5067–76. Epub 2001/10/24. ; PubMed Central PMCID: PMC3739327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol. 2003;33(11):2980–6. doi: 10.1002/eji.200324392 . [DOI] [PubMed] [Google Scholar]
- 15.Calkins CC, Platt K, Potempa J, Travis J. Inactivation of tumor necrosis factor-alpha by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis. Implications of immune evasion. J Biol Chem. 1998;273(12):6611–4. Epub 1998/04/18. . [DOI] [PubMed] [Google Scholar]
- 16.Palm E, Khalaf H, Bengtsson T. Porphyromonas gingivalis downregulates the immune response of fibroblasts. BMC Microbiol. 2013;13:155 Epub 2013/07/12. 1471-2180-13-155 [pii] 10.1186/1471-2180-13-155. ; PubMed Central PMCID: PMC3717116. doi: 10.1186/1471-2180-13-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajishengallis G, Shakhatreh MA, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J Immunol. 2007;179(4):2359–67. Epub 2007/08/07. 179/4/2359 [pii]. . [DOI] [PubMed] [Google Scholar]
- 18.Kolenbrander PE, Palmer RJ Jr., Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8(7):471–80. Epub 2010/06/02. nrmicro2381 [pii] 10.1038/nrmicro2381. doi: 10.1038/nrmicro2381 [DOI] [PubMed] [Google Scholar]
- 19.Shin JE, Baek KJ, Choi YS, Choi Y. A periodontal pathogen Treponema denticola hijacks the Fusobacterium nucleatum-driven host response. Immunol Cell Biol. 2013;91(8):503–10. doi: 10.1038/icb.2013.35 . [DOI] [PubMed] [Google Scholar]
- 20.Brook I, Frazier EH. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J Med Microbiol. 2000;49(9):827–30. doi: 10.1099/0022-1317-49-9-827 [DOI] [PubMed] [Google Scholar]
- 21.Chaim W, Mazor M. Intraamniotic infection with fusobacteria. Arch Gynecol Obstet. 1992;251(1):1–7. . [DOI] [PubMed] [Google Scholar]
- 22.Koornstra JJ, Veenendaal D, Bruyn GA, de Graaf H. Septic arthritis due to Fusobacterium nucleatum. Br J Rheumatol. 1998;37(11):1249 . [DOI] [PubMed] [Google Scholar]
- 23.Roberts GL. Fusobacterial infections: an underestimated threat. Br J Biomed Sci. 2000;57(2):156–62. . [PubMed] [Google Scholar]
- 24.Abdi K, Singh NJ, Matzinger P. Lipopolysaccharide-activated dendritic cells: "exhausted" or alert and waiting? J Immunol. 2012;188(12):5981–9. Epub 2012/05/09. jimmunol.1102868 [pii] 10.4049/jimmunol.1102868. ; PubMed Central PMCID: PMC3370068. doi: 10.4049/jimmunol.1102868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–55. doi: 10.1038/15200 [DOI] [PubMed] [Google Scholar]
- 26.Abdi K, Singh NJ, Spooner E, Kessler BM, Radaev S, Lantz L, et al. Free IL-12p40 monomer is a polyfunctional adaptor for generating novel IL-12-like heterodimers extracellularly. J Immunol. 2014;192(12):6028–36. Epub 2014/05/14. jimmunol.1400159 [pii] 10.4049/jimmunol.1400159. ; PubMed Central PMCID: PMC4070439. doi: 10.4049/jimmunol.1400159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Periasamy S, Kolenbrander PE. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J Bacteriol. 2009;191(22):6804–11. Epub 2009/09/15. JB.01006-09 [pii] 10.1128/JB.01006-09. ; PubMed Central PMCID: PMC2772475. doi: 10.1128/JB.01006-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdi K, Singh N, Matzinger P. T-cell control of IL-12p75 production. Scand J Immunol. 2006;64(2):83–92. Epub 2006/07/27. SJI1767 [pii] 10.1111/j.1365-3083.2006.01767.x. doi: 10.1111/j.1365-3083.2006.01767.x [DOI] [PubMed] [Google Scholar]
- 29.Rangarajan M, Aduse-Opoku J, Paramonov N, Hashim A, Bostanci N, Fraser OP, et al. Identification of a second lipopolysaccharide in Porphyromonas gingivalis W50. J Bacteriol. 2008;190(8):2920–32. doi: 10.1128/JB.01868-07 ; PubMed Central PMCID: PMC2293225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucht A, Larsson P, Weisbrot L, Thorne C, Pisa P, Smedegard G, et al. Expression of interferon-gamma (IFN-gamma), IL-10, IL-12 and transforming growth factor-beta (TGF-beta) mRNA in synovial fluid cells from patients in the early and late phases of rheumatoid arthritis (RA). Clin Exp Immunol. 1996;103(3):357–67. doi: 10.1111/j.1365-2249.1996.tb08288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozarov E, Whitlock J, Dong H, Carrasco E, Progulske-Fox A. The number of direct repeats in hagA is variable among Porphyromonas gingivalis strains. Infect Immun. 1998;66(10):4721–5. ; PubMed Central PMCID: PMC108580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han N, Whitlock J, Progulske-Fox A. The hemagglutinin gene A (hagA) of Porphyromonas gingivalis 381 contains four large, contiguous, direct repeats. Infect Immun. 1996;64(10):4000–7. ; PubMed Central PMCID: PMC174329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Duncan MJ. Gingipain adhesin domains mediate Porphyromonas gingivalis adherence to epithelial cells. Microb Pathog. 2004;36(4):205–9. doi: 10.1016/j.micpath.2003.12.001 . [DOI] [PubMed] [Google Scholar]
- 34.Belanger M, Kozarov E, Song H, Whitlock J, Progulske-Fox A. Both the unique and repeat regions of the Porphyromonas gingivalis hemagglutin A are involved in adhesion and invasion of host cells. Anaerobe. 2012;18(1):128–34. doi: 10.1016/j.anaerobe.2011.10.005 ; PubMed Central PMCID: PMC3278541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen KA, DeCarlo AA, Paramaesvaran M, Collyer CA, Langley DB, Hunter N. Humoral responses to Porphyromonas gingivalis gingipain adhesin domains in subjects with chronic periodontitis. Infect Immun. 2004;72(3):1374–82. ; PubMed Central PMCID: PMC356009. doi: 10.1128/IAI.72.3.1374-1382.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson FC, 3rd, Savelli J, Van Dyke TE, Genco CA. Gingipain-specific IgG in the sera of patients with periodontal disease is necessary for opsonophagocytosis of Porphyromonas gingivalis. J Periodontol. 2005;76(10):1629–36. doi: 10.1902/jop.2005.76.10.1629 . [DOI] [PubMed] [Google Scholar]
- 37.Yasaki-Inagaki Y, Inagaki S, Yamada S, Okuda K, Ishihara K. Production of protective antibodies against Porphyromonas gingivalis strains by immunization with recombinant gingipain domains. FEMS Immunol Med Microbiol. 2006;47(2):287–95. doi: 10.1111/j.1574-695X.2006.00091.x . [DOI] [PubMed] [Google Scholar]
- 38.Saiki K, Konishi K. Porphyromonas gingivalis C-terminal signal peptidase PG0026 and HagA interact with outer membrane protein PG27/LptO. Mol Oral Microbiol. 2014;29(1):32–44. doi: 10.1111/omi.12043 . [DOI] [PubMed] [Google Scholar]
- 39.Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. Evidence for significant contribution of Arg-gingipain to virulence. J Biol Chem. 1995;270(40):23619–26. . [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick RE, Wijeyewickrema LC, Pike RN. The gingipains: scissors and glue of the periodontal pathogen, Porphyromonas gingivalis. Future Microbiol. 2009;4(4):471–87. doi: 10.2217/fmb.09.18 . [DOI] [PubMed] [Google Scholar]
- 41.Slakeski N, Margetts M, Moore C, Czajkowski L, Barr IG, Reynolds EC. Characterization and expression of a novel Porphyromonas gingivalis outer membrane protein, Omp28. Oral Microbiol Immunol. 2002;17(3):150–6. Epub 2002/05/29. omi170303 [pii]. . [DOI] [PubMed] [Google Scholar]
- 42.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19(22):2645–55. doi: 10.1101/gad.1299905 . [DOI] [PubMed] [Google Scholar]
- 43.Veith PD, Talbo GH, Slakeski N, Dashper SG, Moore C, Paolini RA, et al. Major outer membrane proteins and proteolytic processing of RgpA and Kgp of Porphyromonas gingivalis W50. Biochem J. 2002;363(Pt 1):105–15. ; PubMed Central PMCID: PMC1222457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abe N, Baba A, Takii R, Nakayama K, Kamaguchi A, Shibata Y, et al. Roles of Arg- and Lys-gingipains in coaggregation of Porphyromonas gingivalis: identification of its responsible molecules in translation products of rgpA, kgp, and hagA genes. Biol Chem. 2004;385(11):1041–7. doi: 10.1515/BC.2004.135 . [DOI] [PubMed] [Google Scholar]
- 45.Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995;63(4):1176–82. ; PubMed Central PMCID: PMC173131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269(1):406–11. . [PubMed] [Google Scholar]
- 47.Wooldridge KG, Williams PH. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12(4):325–48. . [DOI] [PubMed] [Google Scholar]
- 48.Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881 . [DOI] [PubMed] [Google Scholar]
- 49.Dashper SG, Cross KJ, Slakeski N, Lissel P, Aulakh P, Moore, et al. Hemoglobin hydrolysis and heme acquisition by Porphyromonas gingivalis. Oral Microbiol Immunol. 2004;19(1):50–6. . [DOI] [PubMed] [Google Scholar]
- 50.Olczak T, Simpson W, Liu X, Genco CA. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev. 2005;29(1):119–44. doi: 10.1016/j.femsre.2004.09.001 . [DOI] [PubMed] [Google Scholar]
- 51.Yi ES, Ulich TR. Endotoxin, interleukin-1, and tumor necrosis factor cause neutrophil-dependent microvascular leakage in postcapillary venules. Am J Pathol. 1992;140(3):659–63. ; PubMed Central PMCID: PMC1886160. [PMC free article] [PubMed] [Google Scholar]
- 52.Smith JW, 2nd, Longo DL, Alvord WG, Janik JE, Sharfman WH, Gause BL, et al. The effects of treatment with interleukin-1 alpha on platelet recovery after high-dose carboplatin. N Engl J Med. 1993;328(11):756–61. doi: 10.1056/NEJM199303183281103 . [DOI] [PubMed] [Google Scholar]
- 53.Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF. Epithelial cell pro-inflammatory cytokine response differs across dental plaque bacterial species. J Clin Periodontol. 2010;37(1):24–9. doi: 10.1111/j.1600-051X.2009.01505.x ; PubMed Central PMCID: PMCPMC2900159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braunstein S, Kaplan G, Gottlieb AB, Schwartz M, Walsh G, Abalos RM, et al. GM-CSF activates regenerative epidermal growth and stimulates keratinocyte proliferation in human skin in vivo. J Invest Dermatol. 1994;103(4):601–4. . [DOI] [PubMed] [Google Scholar]
- 55.Huffman Reed JA, Rice WR, Zsengeller ZK, Wert SE, Dranoff G, Whitsett JA. GM-CSF enhances lung growth and causes alveolar type II epithelial cell hyperplasia in transgenic mice. Am J Physiol. 1997;273(4 Pt 1):L715–25. . [DOI] [PubMed] [Google Scholar]
- 56.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337(6093):431–5. doi: 10.1126/science.1221064 ; PubMed Central PMCID: PMC4078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hajishengallis G, Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol. 2014;44(2):328–38. doi: 10.1002/eji.201344202 ; PubMed Central PMCID: PMC3925422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandros J, Karlsson C, Lappin DF, Madianos PN, Kinane DF, Papapanou PN. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J Dent Res. 2000;79(10):1808–14. doi: 10.1177/00220345000790101301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IL-12 can be elicited from resting or LPS-activated DCs by coculturing them with antigen-activated T cells [24]. To test whether DCs that have been cultured with P. gingivalis are viable or dead, we tested their ability to produce IL-12 when subsequently cocultured with activated 5C.C7 T cells [specific for Moth Cytochrome c (MCC)]. We also tested whether the presence of the bacteria in the DC/T-cell culture would inhibit secretion of IL-12. (A) DCs from B10.A-Rag2-/- mice were pre-activated with F. nucleatum, P. gingivalis or nothing (medium) for 20 h, then washed and cocultured at 2x104 cells/well with 5x105 cell/well antigen-activated 5C.C7 T cells in the presence of 0.1 μM MCC peptide 83–103 in a 96-well plate at 37°C for 48 h. CSN were analyzed for the presence of IL-12 using specific ELISA. (B) Same as (A) except 5x107 F. nucleatum or P. gingivalis were added to the coculture of DCs with T cells. These data are expressed as the mean ± SD of triplicate wells representative of two independent experiments.
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper.