Abstract
The voluntary use and abuse of alcohol and inhalants is a recognized health problem throughout the world. Previous studies have shown that these agents affect brain function in a variety of ways including direct inhibition of key ion channels that regulate neuronal excitability. Among these, the N-methyl-D-aspartate (NMDA) receptor is particularly important given its key role in glutamatergic synaptic transmission, neuronal plasticity and learning and memory. Previous studies from this laboratory and others have identified key residues within transmembrane (TM) domains of the NMDA receptor that appear to regulate its sensitivity to alcohol and anesthetics. In this study, we extend these findings and examine the role of a TM4 residue in modulating sensitivity of recombinant NMDA receptors to ethanol and toluene. HEK293 cells were transfected with GluN1-1a and either wild-type or tryptophan-substituted GluN2(A–D) subunits and whole-cell currents were recorded using patch-clamp electrophysiology in the absence or presence of ethanol or toluene. Both ethanol (100 mM) and toluene (1 or 3 mM) reversibly inhibited glutamate-activated currents from wild-type NMDARs with GluN2B containing receptors showing heightened sensitivity to either agent. Substitution of tryptophan (W) at positions 825, 826, 823 or 850 in the TM4 domain of GluN2A, GluN2B, GluN2C or GluN2D subunits; respectively, significantly reduced the degree of inhibition by ethanol. In contrast, toluene inhibition of glutamate-activated currents in cells expressing the TM4-W mutants was not different from that of the wild-type controls. These data suggest that despite similarities in their action on NMDARs, ethanol and toluene may act at different sites to reduce ion flux through NMDA receptors.
Keywords: GluN1, GluN2, electrophysiology, alcohol, inhalants
Introduction
N-methyl-D-aspartate (NMDA) receptors are glutamate-activated ion channels and are key regulators of neuronal excitability and brain function. Perturbation of NMDA receptor activity is thought to underlie key aspects of a variety of brain disorders including alcohol and drug abuse disorders. NMDA receptor channel activity in both neurons and recombinant expression systems has been shown to be inhibited by ethanol (Lovinger, White, & Weight, 1989; Peoples & Weight, 1992; Woodward & Gonzales, 1990) and representative members of the large class of agents known as abused inhalants (Cruz, Balster, & Woodward, 2000; Cruz, Mirshahi, Thomas, Balster, & Woodward, 1998). These compounds, that include alkylbenzenes such as toluene, are widely used in household and industrial products and their easy availability likely contributes to their use as intoxicating agents among adolescents and children. The mechanism by which alcohol and inhalants like toluene inhibit NMDAR function is not yet completely clear but these agents do not appear to interfere directly with agonist binding sites or act as channel blockers (Cruz et al., 1998; Mirshahi & Woodward, 1995; Peoples & Weight, 1992).
More recent results from mutagenesis studies of recombinant NMDARs has revealed amino acids located within transmembrane domains of NMDARs that appear to regulate the receptor’s sensitivity to ethanol. One site in particular is located in TM4 and in GluN2A and GluN2B receptors, mutating this residue to tryptophan (W) significantly reduced ethanol inhibition of the receptor (Honse, Ren, Lipsky, & Peoples, 2004; Zhao, Ren, Dwyer, & Peoples, 2015). In this study, we investigated whether tryptophan substitutions at homologous sites in GluN2C and GluN2D subunits would also affect ethanol inhibition and whether TM4-W GluN2 containing receptors would show altered sensitivity to the abused inhalant toluene.
Materials and Methods
Molecular biology
The NMDA receptor cDNAs used in these experiments were kindly provided by Drs. S. Nakanishi (Kyoto University, Kyoto, Japan), P. Seeburg (Max-Planck Institute for Medical Research, Heidelberg, Germany) and D. Lynch (Univ. of Pennsylvania) and were sub-cloned in mammalian expression vectors as needed. Site-directed mutagenesis was performed using the QuikChange XL mutagenesis kit (Agilent Technologies, Santa Clara, CA) and mutants were confirmed by DNA sequencing (GeneWiz, South Plainfield, NJ).
HEK293 cell maintenance and receptor expression
HEK293 cells were obtained from American Type Culture Collection (Manassas, VA) and grown according to the provided protocol. In brief, cells were cultured in Dulbecco’s Minimum Eagle Medium (DMEM, Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (Hyclone, Logan, Utah) and grown at 37°C in a 5% CO2 environment. Twenty-four hours following plating of low-density cultures (approximately 5 X 104 cells per dish) onto 35 mm dishes coated with poly-L-lysine, cells were transfected with equal amounts of subunit cDNA (1 μg) using the Lipofectamine 2000 reagent (Life Technologies, Grand Island, NY). To identify transfected cells, 1 μg of pMAX-GFP (Lonza, Basel, Switzerland) was added to the transfection mixture. After transfection, the NMDA antagonist 2-amino-5-phosphonovaleric acid (AP5; 200 μM) was added to the DMEM media to prevent glutamate-mediated excitotoxicity. Cells were used for electrophysiological recordings 24–48 hours following transfection and media containing AP5 was removed by extensive washing just prior to recording.
Electrophysiological recording conditions
All recordings were performed as previously described (Smothers and Woodward, 2009). Briefly, cells were perfused with an external solution containing (in mM): NaCl 135, KCl 5.4, CaCl2 1.8, HEPES 5, EDTA 0.01 and glucose 10 (pH adjusted to 7.4 with NaOH and osmolarity adjusted to 315–325 mOsm/kg with sucrose). Patch electrodes (tip resistance 3–6 MΩ) were fabricated from thick-walled borosilicate glass (B150; WPI, Sarasota, FL) and filled with internal solution composed of (in mM): CsCl 140, NaATP 2, MgCl2 2, HEPES 10, EGTA 5 (pH was adjusted to 7.2 with CsOH and osmolarity was adjusted to 290–300 mOsm/kg with sucrose). All internal solutions used for each experiment were from frozen stocks. All drug solutions were prepared fresh for each experiment from frozen stocks in external solution. Stock solutions of glycine and glutamate were prepared in water and diluted into external solution. Ethanol was purchased from Pharmco-Aaper (Shelbyville, KY).
Whole-cell voltage-clamp recordings (Hamill, Marty, Neher, Sakmann, & Sigworth, 1981) were performed at room temperature using an Axopatch 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA). Cells were voltage-clamped at −60 mV and current records were filtered at 1 kHz (eight-pole Bessel filter) and digitized at 2 kHz using an ITC-16 interface (Instrutech Corp., Port Washington, NY). Software control of data acquisition was provided by Igor Pro software (WaveMetrics, Lake Oswego, OR). A 3-barrel perfusion apparatus (barrel ID 0.6 mm; Warner Instruments, SF-77B) was used to switch between control and drug-containing solutions. Solution exchange times were determined to be between 6 – 8 ms as calculated by measuring changes in the liquid junction current across an open electrode when switching between solutions with different ionic strength. Responses were evoked by a 5 second agonist (10 μM glutamate, 10 μM glycine) application and were measured at two time points, peak and steady-state. Peak current amplitude was determined as the difference between the current immediately prior to agonist application and at the point where inward current was maximal. Steady-state current amplitude was determined as the difference between current immediately prior to agonist application and the last 500 msec of agonist application where currents were stable. Macroscopic desensitization was calculated as the ratio of the current obtained at the steady-state and the peak current. Drug-induced inhibition of receptor currents (IControl) were calculated using the formula {1− (IAgonist+Drug/IControl)} × 100, where IAgonist+Drug represents the response to co-application of agonist + ethanol or toluene, and IControl represents the mean of two responses to agonist, one before and one after the co-application of ethanol. Leak currents were continually monitored as an indicator of seal and cell integrity. Cells that showed unstable leak currents were not included in the data analysis.
Statistical Analysis
AxographX (AxographX, New South Wales) software was used to measure NMDA receptor current parameters. Data were analyzed with SPSS (v.24 IBM, Armonk, NY) or Prism 6.0 (Graphpad Software Inc., San Diego, CA) software with a significance level of p < 0.05. Where appropriate, the alpha value was corrected for multiple comparisons.
Results
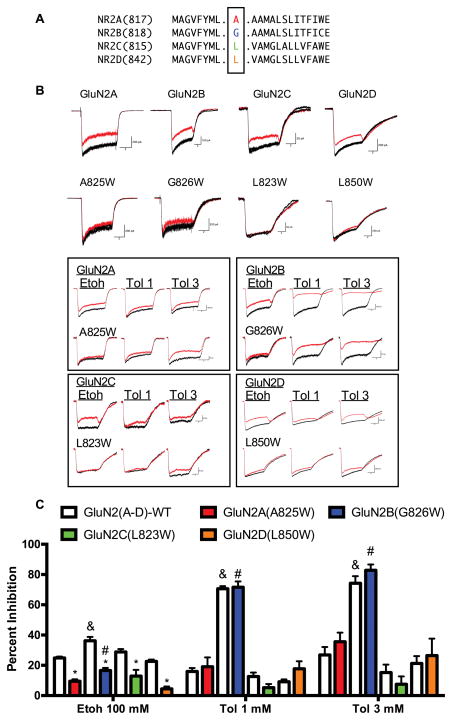
HEK293 cells were transfected with the GluN1-1a subunit and wild-type GluN2 subunits or those where tryptophan (W) was substituted for the TM4 domain residue indicated in in Figure 1A. Currents were elicited in the absence and presence of 100 mM ethanol or toluene (1 or 3 mM); both of which have been previously shown to inhibit native (Bale, Tu, Carpenter-Hyland, Chandler, & Woodward, 2005; Peoples, White, Lovinger, & Weight, 1997) and recombinant (Cruz et al., 1998; Mirshahi & Woodward, 1995) NMDA receptors. Representative examples of currents obtained in wild-type and mutant receptors are shown in Figure 1B and suggest that inhibition of NMDARs by ethanol or toluene is affected in a subunit and mutation dependent manner. To test this hypothesis, the summary data shown in Figure 1C was analyzed with a three factor (Subunit × Mutation × Drug) design using a linear mixed model (SPSS). When appropriate, post-hoc pairwise comparisons were carried out for each factor.
Figure 1.
Effect of TM4 tryptophan mutations on ethanol and toluene inhibition of recombinant NMDARs expressed in HEK293 cells. Cells were transfected with the GluN1-1a subunit and either wild-type or mutant GluN2(A–D) subunits. (A) Sequence alignment of TM4 domain of rat GluN2 subunits. Residues mutated to tryptophan (W) are highlighted in color. (B) Exemplar traces from wild-type and mutant NMDARs currents in the absence (black traces are control and washout) and presence (red trace) of 100 mM ethanol or toluene (1 mM, 3 mM). Scale bars: Upper traces: x-axis 2 sec; y-axis: GluN2A (200 pA), A825W (200 pA); GluN2B (100 pA), G826W (200 pA); GluN2C (50 pA), L823W (50 pA); GluN2D (200 pA), L850W (200 pA). Lower traces (boxed): x-axis 2 sec; y-axis: GluN2A (100 pA), A825W (50 pA); GluN2B (100 pA), G826W (50 pA); GluN2C (50 pA), L823W (50 pA); GluN2D (200 pA), L850W (200 pA). (C) Summary plot showing percent inhibition of steady-state currents by 100 mM ethanol or toluene (1 mM, 3 mM). Data are mean ± SEM (N: GluN2A (43), A825W (10); GluN2B (6), G826W (6); GluN2C (9), L823W (7); GluN2D (4), L850W (4). Symbols: &, value for GluN2B significantly different from GluN2A and GluN2D (Etoh p < 0.004); or GluN2A, 2C and 2D subunits (Toluene p < 0.0001); #value for GluN2B(G826W) significantly different from GluN2D(L850W) (Etoh p < 0.02) or GluN2A(A825W), 2C(L823W) and 2D(L850W) subunits (Toluene p < 0.0001). *: value for TM4-W mutant significantly different from corresponding wild-type control (p < 0.0001).
The mixed model analysis revealed a significant main effect of subunit (F3,202=207.56; p < 0.0001) on drug-induced inhibition of NMDA receptors. For ethanol, post-hoc pairwise comparisons revealed greater inhibition of wild-type GluN2B receptors as compared to that for wild-type GluN2A and GluN2D (both p < 0.004) receptors. In tryptophan-substituted NMDA receptors, post-hoc analysis revealed that ethanol inhibition of GluN2B(G826W) receptors was greater than that of GluN2D(L850W) receptors (p < 0.02) but was not different from that of GluN2A(A825W) or GluN2C(L823W) receptors. Inhibition of wild-type GluN2B-containing receptors by 1 mM or 3 mM toluene was greater than that for GluN2A, 2C and 2D receptors (all p < 0.0001) and this effect was also observed for toluene inhibition of GluN2B(G826W) receptors as compared all other TM4-W mutants (all p < 0.0001).
The mixed model analysis also revealed a main effect of mutation (F1,202=8.38 p < 0.004) on drug-induced inhibition of NMDA currents. Post-hoc pairwise comparisons showed a highly significant difference in the degree of ethanol inhibition between wild-type and the corresponding TM4-W mutant (all p < 0.0001). Compared to the values for the wild-type receptors, the reduction in ethanol inhibition of TM4-W mutants ranged from 54.4% for the GluN2A(A825W) mutant to 80.3% for the GluN2D(L850W) receptor. In contrast to the effect of the TM4-W mutation on ethanol inhibition, post-hoc pairwise comparisons reported no significant difference of toluene inhibition between wild-type and mutant NMDA receptors (all p > 0.05) for either 1 mM or 3 mM toluene.
Finally, there was a main effect of drug (F2, 202=51.56 p < 0.0001) that was largely driven by the enhanced inhibition of both the wild-type and mutant GluN2B receptor by toluene as compared to that produced by ethanol (p < 0.0001). In addition, while there were differences in the degree of ethanol and toluene inhibition among other subunits, we did not carry out a detailed analysis of these findings as this question was not a primary focus of the research design.
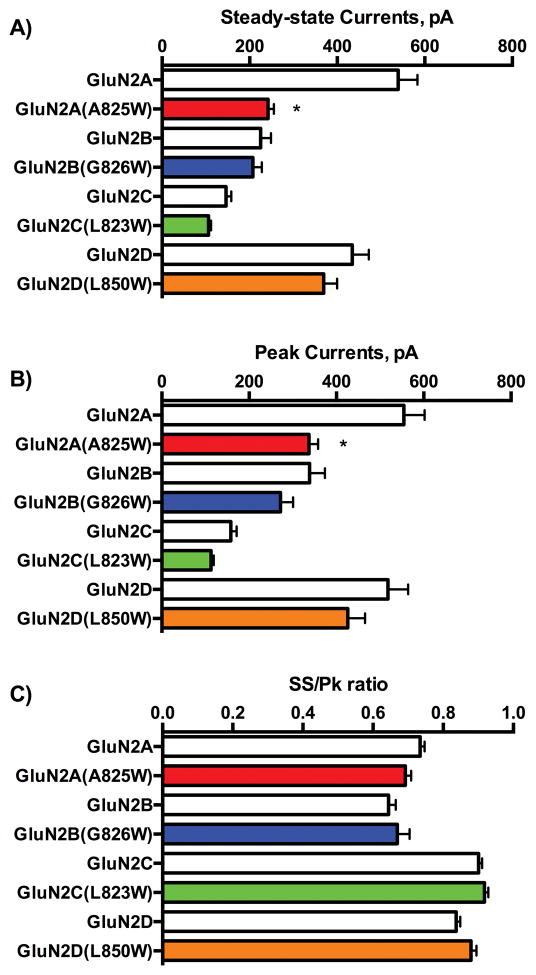
To determine whether the TM4-W mutations also elicited any significant changes on basic receptor properties, we compared steady-state and peak current amplitudes for wild-type and mutant receptors and calculated a measure of macroscopic current kinetics (ratio of steady-state to peak currents). Analysis of these data revealed a main effect of mutation on both steady-state (2-way Anova F1,403=19.57, p < 0.0001; Figure 2A) and peak (2-way Anova F1,403=14.14, p < 0.0002; Figure 2B) currents. Post-hoc pairwise comparisons showed a significant (p < 0.0001) difference in current amplitude between wild-type GluN2A and GluN2A(A825W) receptors but no difference in current amplitude between wild-type and mutant pairs for the remaining GluN2 subunits. Despite the reduction in mean current amplitude for the GluN2A(A825W) mutant, tryptophan substitution had no effect on the SS:Pk ratio (F1,403=0.69, P=0.41) of any subunit (Figure 2C).
Figure 2.
Functional characteristics of wild-type and tryptophan substituted NMDARs. (A) Peak (A) and steady-state (B) currents of wild-type and tryptophan substituted mutants. (C) Steady-state to peak ratio for wild-type and tryptophan substituted mutants. Data are mean ± SEM. (N: GluN2A (87), A825W (48); GluN2B (48), G826W (42); GluN2C (44), L823W (48); GluN2D (52), L850W (44). Symbol: *value significantly different from corresponding wild-type control (steady-state p < 0.0001; peak p < 0.0002).
Discussion
The major finding of this study is that a mutation that significantly reduces ethanol inhibition of NMDARs has little effect on that produced by the abused inhalant toluene. The ability of the TM4-W mutation to reduce ethanol inhibition of GluN2A and GluN2B receptors is consistent with that previously reported in the literature (Honse et al., 2004; Smothers & Woodward, 2006; Xu, Smothers, Trudell, & Woodward, 2012; Zhao et al., 2015) and data from the present study now extends those findings to GluN2C and GluN2D receptors. In addition, we show that these TM4 tryptophan mutations have no significant effect on inhibition produced by the abused inhalant toluene. Although the effects of these mutants on toluene inhibition have not previously been reported, they are consistent with those showing that both wild-type and GluN2A(A825W) receptors were inhibited to a similar degree by the related compound benzene (Ogata et al., 2006).
The Peoples laboratory first reported that tryptophan substitutions in the TM4 domain of the GluN2A subunit could alter the ethanol inhibition of NMDARs (Honse et al., 2004). These findings extended those from our laboratory that identified an ethanol-sensitive site (F639A) in the TM3 domain of the GluN1 subunit (Ronald, Mirshahi, & Woodward, 2001). An extensive series of mutagenesis studies since then has confirmed these findings and suggests that a small number of residues within TM domains of GluN1 and GluN2 subunits regulates much of the receptor’s sensitivity to ethanol (Ren et al., 2008; Ren, Salous, Paul, Lipsky, & Peoples, 2007; Ren, Zhao, Dwyer, & Peoples, 2012; Smothers & Woodward, 2006; Xu et al., 2012; Xu, Smothers, & Woodward, 2015). Interestingly, the contribution that the GluN1 and GluN2 subunits make in determining the overall ethanol inhibition of the tetrameric receptor appears complementary rather than equal as homologous TM mutations made in the opposite subunit generally do not produce the same effect (Ren et al., 2007; Ren et al., 2012; Smothers & Woodward, 2006). This may reflect the differential role that glycine-sensitive GluN1 subunits and glutamate-dependent GluN2 subunits play in activating the receptor (Kussius & Popescu, 2010) and subunit-dependent differences in channel characteristics (e.g. open probability) that appear to influence ethanol inhibition. In the present study, the mean amplitude of GluN2A(A825W) currents was significantly less than that of wild-type GluN2A receptors although there was no change in macroscopic desensitization. When currents from individual cells were examined (data not shown), there was considerable overlap in the amplitude values between wild-type and mutant receptors. In addition, this difference has not been noted in previous studies (Honse et al., 2004; Smothers & Woodward, 2006) and was not accompanied by similar decreases in other TM4-W mutants suggesting that this may have arisen from normal fluctuations in receptor expression that are common in studies of recombinant receptors.
While the actions of ethanol on NMDARs has been relatively well-studied, much less is known regarding the actions of inhalants on receptor function. Previous findings from this laboratory demonstrated that toluene and a series of related alkylbenzenes inhibited GluN2A, 2B and 2C NMDARs expressed in Xenopus oocytes with GluN2B containing receptors showing greater sensitivity (Cruz et al., 2000; Cruz et al., 1998). In this study, we show a similar profile of action for NMDARs expressed in mammalian cells and further demonstrate that GluN2D receptors display a relatively low sensitivity to toluene. As mentioned previously, Ogata et al. found that the A825W mutation did not affect inhibition of GluN2A receptors by benzene. We show here that the homologous mutation in other GluN2 subunits also fails to reduce toluene (methylbenzene) inhibition even in GluN2B receptors that have a high innate sensitivity to alkylbenzenes. Interestingly, while the TM4 tryptophan mutation in GluN2A did not reduce inhibition by benzene, it has been shown to reduce sensitivity to other anesthetics including hexanol, octanol, isoflurane, halothane, chloroform, nitrous oxide and xenon (Ogata et al., 2006) as well as trichloroethanol (Salous et al., 2009). Similar findings were reported for the TM3 site of action where substitution of alanine for phenylalanine at position 639 in the GluN1 subunit reduces inhibition by ethanol and selected volatile anesthetics but not that of benzene or nitrous oxide (Ogata et al., 2006; Ronald et al., 2001). It is unclear at this time how mutations of selective residues within TM3 and TM4 domains of NMDA subunits actually alter sensitivity to alcohols and various anesthetics. One idea is that these sites represent an alcohol/anesthetic site of action that when occupied alters gating of the receptor and that mutating these residues reduces the affinity of the site for the drug. It is also possible that mutations at these sites induces changes in underlying receptor function that then indirectly affects the ability of alcohols/anesthetics to affect channel function. Since mutations that reduce alcohol/anesthetic inhibition of NMDARs typically do not completely eliminate their effect, it is likely that there are multiple sites of action that are controlled by discrete domains of the receptor. Further work using recent high-resolution structural models of NMDARs (Karakas & Furukawa, 2014; Lee et al., 2014) and detailed mechanistic models of NMDAR function (Borschel, Cummings, Tindell, & Popescu, 2015) may help resolve this question.
Highlights.
Recombinant NMDARs are inhibited by alcohol or the abused inhalant toluene
Tryptophan substitution at a TM4 site in GluN2 subunits reduces inhibition by ethanol
Toluene inhibition of NMDARs is unaffected by the TM4 tryptophan mutation
Acknowledgments
This work was supported by grant R01AA009986 and R37AA009986 (JJW).
References Cited
- Bale AS, Tu Y, Carpenter-Hyland EP, Chandler LJ, Woodward JJ. Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons following exposure to the abused inhalant toluene. Neuroscience. 2005;130(1):197–206. doi: 10.1016/j.neuroscience.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Borschel WF, Cummings KA, Tindell LK, Popescu GK. Kinetic contributions to gating by interactions unique to N-methyl-D-aspartate (NMDA) receptors. J Biol Chem. 2015;290(44):26846–26855. doi: 10.1074/jbc.M115.678656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz SL, Balster RL, Woodward JJ. Effects of volatile solvents on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;131(7):1303–1308. doi: 10.1038/sj.bjp.0703666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;286(1):334–340. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Honse Y, Ren H, Lipsky RH, Peoples RW. Sites in the fourth membrane-associated domain regulate alcohol sensitivity of the NMDA receptor. Neuropharmacology. 2004;46(5):647–654. doi: 10.1016/j.neuropharm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344(6187):992–997. doi: 10.1126/science.1251915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussius CL, Popescu GK. NMDA receptors with locked glutamate-binding clefts open with high efficacy. J Neurosci. 2010;30(37):12474–12479. doi: 10.1523/JNEUROSCI.3337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Lu W, Michel JC, Goehring A, Du J, Song X, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511(7508):191–197. doi: 10.1038/nature13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg(2+)-insensitive mutants. Neuropharmacology. 1995;34(3):347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318(1):434–443. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Weight FF. Ethanol inhibition of N-methyl-D-aspartate-activated ion current in rat hippocampal neurons is not competitive with glycine. Brain Res. 1992;571(2):342–344. doi: 10.1016/0006-8993(92)90674-x. [DOI] [PubMed] [Google Scholar]
- Peoples RW, White G, Lovinger DM, Weight FF. Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol. 1997;122(6):1035–1042. doi: 10.1038/sj.bjp.0701483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Salous AK, Paul JM, Lamb KA, Dwyer DS, Peoples RW. Functional interactions of alcohol-sensitive sites in the N-methyl-D-aspartate receptor M3 and M4 domains. J Biol Chem. 2008;283(13):8250–8257. doi: 10.1074/jbc.M705933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Salous AK, Paul JM, Lipsky RH, Peoples RW. Mutations at F637 in the NMDA receptor NR2A subunit M3 domain influence agonist potency, ion channel gating and alcohol action. Br J Pharmacol. 2007;151(6):749–757. doi: 10.1038/sj.bjp.0707254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Zhao Y, Dwyer DS, Peoples RW. Interactions among positions in the third and fourth membrane-associated domains at the intersubunit interface of the N-methyl-D-aspartate receptor forming sites of alcohol action. J Biol Chem. 2012;287(33):27302–27312. doi: 10.1074/jbc.M111.338921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem. 2001;276(48):44729–44735. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]
- Salous AK, Ren H, Lamb KA, Hu XQ, Lipsky RH, Peoples RW. Differential actions of ethanol and trichloroethanol at sites in the M3 and M4 domains of the NMDA receptor GluN2A (NR2A) subunit. Br J Pharmacol. 2009;158(5):1395–1404. doi: 10.1111/j.1476-5381.2009.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Effects of amino acid substitutions in transmembrane domains of the NR1 subunit on the ethanol inhibition of recombinant N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2006;30(3):523–530. doi: 10.1111/j.1530-0277.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Gonzales RA. Ethanol inhibition of N-methyl-D-aspartate-stimulated endogenous dopamine release from rat striatal slices: reversal by glycine. J Neurochem. 1990;54(2):712–715. doi: 10.1111/j.1471-4159.1990.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Xu M, Smothers CT, Trudell J, Woodward JJ. Ethanol inhibition of constitutively open N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2012;340(1):218–226. doi: 10.1124/jpet.111.187179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Smothers CT, Woodward JJ. Cysteine substitution of transmembrane domain amino acids alters the ethanol inhibition of GluN1/GluN2A N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2015;353(1):91–101. doi: 10.1124/jpet.114.222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ren H, Dwyer DS, Peoples RW. Different sites of alcohol action in the NMDA receptor GluN2A and GluN2B subunits. Neuropharmacology. 2015;97:240–250. doi: 10.1016/j.neuropharm.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]